Abstract
One pot three-component imino Diels–Alder reactions of various types of aldehydes, aniline and dihydropyrane were studied in the presence of nano sized MCM-41 supported 12-tungstophosphoric acid (TPA) as solid acid catalyst. MCM-41 as solid support was synthesized in nano size and the catalysts with different loading amounts of TPA (5–30 wt%) were prepared and characterized by XRD, FT-IR and SEM techniques. The results confirm a good dispersion of the heteropoly acid on the solid supports and based on the experimental results, the catalyst with 10 wt% heteropoly acid shows the best catalytic activity using 0.5 mol% heteropoly acid relative to the aldehyde. The recovery experiments show that the catalyst is reusable after calcination without significant loss in its activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The compounds with the tetrahydroquinoline scaffold are an important class of heterocyclic compounds with a wide variety of biological activities such as antimalarial [1], antitumor [2], antioxidant [3] and antiallergic [4] activity. Among the various types of heterocyclic compounds with tetrahydroquinoline moieties, pyranotetrahydroquinolines are found in several biologically active naturally occurring alkaloids such as Flindersine, Veprisine and Oricine [5].
The imino Diels–Alder reaction, first explored by Povarov in 1960 [6], is one of the most important synthetic methodologies for the synthesis of six-membered nitrogen containing heterocycles.
This type of Diels–Alder reaction between arylimines and dienophiles is described as inverse-electron-demand hetero Diels–Alder reaction and has been used widely for the synthesis of tetrahydroquinolines. For example, this type of hetero Diels–Alder reaction between arylimines as electron deficient diene and dihydropyrane as electron rich dienophile has been reported for the synthesis of pyranotetrahydroquinolines. A variety of Lewis and Brønsted acidic catalysts have been used for these transformations such as 4-nitrophthalic acid [7], phosphomolybdic acid [8], trifluoroacetic acid [9], triphenylphosphonium perchlorate [10], lanthanide chloride [11], BF3·EtO2 [12], HClO4/SiO2 [13] and I2 [14]. The Povarov reaction using other electron rich dienophiles has also been applied for the synthesis of other quinoline derivatives such as the synthesis of tetrahydroquinolines in the presence of BF3·EtO2 [15], isoindolo[2,1-a]quinoline in the presence of TFA [16], pyrano/thiopyranoquinolines in the presence of InCl3 and InCl3/SiO2 [17], quinazolines in the presence of CuBr2 [18], quinoline-1,2-dicarboxylate derivatives in the presence of Yb(OTf)3 [19]. However, most of these methods involve some drawbacks such as expensive catalysts, excess amounts of Lewis acids due to coordination of Lewis acids to the imine nitrogen, tedious workup and lack of reusability for the catalyst.
The use of solid acid catalysts in organic transformations has attracted considerable attention because of their advantages such as the ease of recovery, reusability, ease of handling and environmental considerations [20]. Apart from various types of solid acids, 12-tungstophosphoric acid (TPA) as a Keggin type heteropoly acid has attracted considerable attention because of its high Brønsted acidity and thermal stability [21, 22]. However, the use of bulk TPA has encountered some difficulties, which have reduced its application in some organic transformations. Some of the major problems associated with TPA in bulk form include low surface area (<10 m2 g−1) [23], production of bulky residue in the reaction media, specially with amino functional groups [20], rapid deactivation and relatively poor stability [24].
The dispersion of TPA on a support having high surface area improves the surface area and improves other features of its solid acid characteristics, which lead to the best catalytic activity. Various mineral oxides such as silica [20], alumina [25], titania [26] and zirconia [27] have been used as the support for TPA in many catalytic reactions. Recent developments on mesoporous molecular sieves provide the possibility of using new high surface area supports for the immobilization of TPA. MCM-41 belongs to the family of mesoporous molecular sieves [28], possesses a large surface area (>1,000 m2 g−1), large pore size (1.5–8 nm) and high thermal stability (ca. 900 °C) [29]. The Keggin type TPA cluster with ~1.2 nm diameter may be introduced inside the MCM-41 pores, thus providing a high surface area solid acid catalyst with high catalytic activities [29]. MCM-41 supported TPA (TPA/MCM-41) has been used as reusable solid acid catalyst in many synthetic methodologies [30–32]. However, less attention has been paid to the use of solid acids as catalyst in the Diels–Alder reaction.
In this paper, we aim to investigate the use of TPA on the nano sized MCM-41 as the solid acid catalyst in the one-pot three-component imino Diels–Alder reaction of aryl aldehydes, anilines and dihydropyrane for the synthesis of pyranotetrahydroquinolines (Scheme 1). To the best of our knowledge, this is the first report of the use of MCM-41 supported TPA as solid acid catalyst in the imino Diels–Alder reaction.
Experimental
Materials and methods
All chemicals were commercial products. All reactions were monitored by TLC and all yields refer to isolated products. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker (DRX-500 AVANCE) spectrometer at 500 MHz, pulse length 3.5 μs and 125.72 MHz, pulse length 2.5 μs. Infrared spectra of the catalysts and reaction products were recorded on a Bruker FT-IR Equinox-55 spectrophotometer in KBr. XRD patterns were recorded on a Bruker D8 ADVANCE X-ray diffractometer using nickel filtered Cu K α radiation. The morphology was studied using a Philips XL30 scanning electron microscopy. The electrode potential variation was measured with a pH/mV meter, Metrohm model 961 using a double junction electrode.
Synthesis of nano sized MCM-41 spheres at room-temperature
The synthesis of nanosized MCM-41 was carried out using tetraethyl orthosilicate (TEOS) as the Si source, cetyltrimethylammonium bromide (CTAB) as the template and ammonia as the pH control agent with the gel composition of SiO2:CTAB:NH4OH:H2O = 22.5:2.74:53.5:11.11.
In a typical procedure, CTAB (1 g, 2.74 mmol) was dissolved in deionized water (200 mL) and the pH of the solution was adjusted to 10.5 by adding 25 wt% ammonia solution (~4 mL). After 10 min, TEOS (5 mL) was slowly added to the solution under continuous stirring for 1 h at 60 °C. The mixture was allowed to cool to room temperature and was stirred for 12 h. The gel was recovered by centrifuging and washed with distilled water (2 × 2 mL). The obtained solid was dried in an oven at 120 °C for 2 h and calcined in air at 550 °C for 4 h.
Preparation of 10 wt% TPA/MCM-41
10 wt% TPA/MCM-41 catalyst was prepared by mixing of TPA (10 mg), MCM-41 (90 mg) and deionized water (15 mL). The resulting suspension was stirred for 12 h followed by evaporation to dryness. The solid was dried at 100 °C for 4 h, powdered and calcined at 550 °C in air for 4 h.
General procedure for the synthesis of pyranoquinolines
A mixture of aldehyde (1 mmol), aniline (1.1 mmol), 3,4-dihydro-2H-pyrane (2 mmol) and CH3CN (1 mL) in the presence of 10 wt% TPA/MCM-41 (150 mg, 0.5 mol% TPA relative to aldehyde) was stirred at room temperature for an appropriate time. The progress of the reaction was followed by TLC using 25% EtOAc in n-hexane as eluent. After the completion of the reaction, the catalyst was recovered and washed with EtOH (2 × 2 mL). The solvent was evaporated and the crude product purified by column chromatography on silica gel using EtOAc:n-hexane, 20:80 as eluent. The pure tetrahydroquinolines as diastereomeric mixtures were obtained in 78–95% yields.
Spectroscopic data for isomers of selected compound
Trans-5-phenyl-3,4,4a,5,6,10b-hexahydro-2H-pyrano[3,2-c]quinoline (4a)
White solid, mp 129–130 °C (Lit. [8] 128–129 °C) IR (KBr): υ max : 3371, 2941, 2872, 1608, 1482, 1085 cm−1. 1H NMR (500 MHz, CDCl3): δ = 1.38 (m, 1H), 1.53 (m, 1H), 1.70 (m, 1H), 1.89 (m, 1H), 2.15 (m, 1H), 3.78 (dt, 1H, J = 2.5, 11.6 Hz), 4.15 (m, 2H, CH, NH), 4.45 (d, 1H, J = 2.72 Hz), 4.77 (d, 1H, J = 10.8 Hz), 6.58 (d, 1H, J = 7.7 Hz), 6.75 (dt, 1H, J = 1, 7.5 Hz), 7.14 (dt, 1H, J = 1.5, 7.7 Hz), 7.28 (dd, 1H, J = 1.4, 7.6 Hz), 7.35–7.48 (m, 5H). 13C NMR (125.7 MHz, 1H-decoupled, CDCl3): δ = 18.45, 25.85, 39.38, 59.77, 61.08, 73.21, 114.82, 118.72, 120.33, 127.24, 127.94, 128.07, 128.51, 128.80, 141.54, 145.60.
Cis-5-phenyl-3,4,4a,5,6,10b-hexahydro-2H-pyrano[3,2-c]quinoline (5a)
Viscous liquid; IR (KBr): υ max : 3417, 2934, 2854, 1611, 1487, 1070 cm−1. 1H NMR (500 MHz, CDCl3): δ = 1.36 (m, 1H), 1.47–1.62 (m, 3H), 2.22 (m, 1H), 3.48 (dt, 1H, J = 2.2, 11.5 Hz), 3.64 (m, 1H), 3.92 (br s, 1H, NH), 4.74 (d, 1H, J = 2.3 Hz), 5.38 (d, 1H, J = 5.5 Hz), 6.65 (d, 1H, J = 7.7 Hz), 6.84 (t, 1H, J = 7.6 Hz), 7.14 (t, 1H, J = 7.8 Hz), 7.35 (t, 1H, J = 7.1 Hz), 7.41–7.48 (m, 5H). 13C NMR (125.7 MHz, 1H-decoupled, CDCl3): δ = 22.48, 24.57, 39.33, 55.27, 69.06, 74.98, 114.56, 117.89, 121.08, 128.25, 128.34, 129.08, 129.80, 131.34, 142.77, 145.18.
Results and discussion
Catalyst characterization
Although the structure of TPA/MCM-41 is well known and its characterization has been reported in many papers, the small difference in the procedure of the preparation of the support and catalysts still requires some characterization experiments.
The morphology of the prepared MCM-41 was studied by SEM (Fig. 1). The SEM image shows agglomerated MCM-41 particles with the size range of <100 nm.
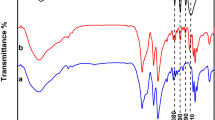
The FT-IR spectra of MCM-41, TPA and MCM-41 supported TPA with different loading amounts of TPA are shown in Fig. 2. As shown in Fig. 2d, the bulk TPA shows peaks at 1,080, 981, 887 and 806 cm−1 which are assigned to the stretching vibrations of P–Oa, W–Od, W–Ob–W and W–Oc–W, in order, associated to the Keggin ions [33]. FT-IR spectrum of MCM-41 (Fig. 2a) shows two characteristic peaks at 812 and 1,084 cm−1. To confirm the presence of TPA on the MCM-41, FT-IR spectra of 10 and 30 wt% TPA/MCM-41 are shown in Figs. 2b and 2c. As shown in these spectra, two characteristic peaks of TPA (887, 1,080 cm−1) overlap over the main peaks of MCM-41. However, the peak at 806 cm−1 was observed in the TPA/MCM-41 spectrum without any change compared to bulk TPA, and the peak at 960 cm−1 was observed in the TPA/MCM-41 spectrum corresponding to the peak at 981 cm−1 for the bulk TPA spectrum. These results confirm that TPA preserved its Keggin structure when supported on the MCM-41.
In order to evaluate the presence and dispersion state of TPA on the support, the X-ray diffraction patterns of the catalysts with various loading amounts of TPA were obtained and were compared with MCM-41 and bulk TPA. Fig. 3 shows the low angle XRD patterns of the MCM-41 and TPA/MCM-41 with 5, 10, 15, 20 and 30 wt% TPA. The characteristic peaks of the MCM-41 are at 2θ = 2.3, 4 and 4.6°. As shown in Fig. 3b–f, supporting with TPA can affect the width and intensity of these peaks. Increase in loading of TPA makes these main peaks broader and weaker. These results suggest that the long-range order of MCM-41 was decreased when increasing the loading amount of TPA.
The high angle XRD patterns of bulk TPA and the catalysts with 10, 15, 20 and 30 wt% loading amount of TPA are shown in Fig. 4. As shown in Fig. 4a, no diffraction peaks of TPA appear in the spectra of the catalyst of 10 wt% TPA/MCM-41. This indicates that the TPA is highly dispersed on the MCM-41 surface. In the pattern of 15 wt% TPA/MCM-41, some characteristic peaks of TPA with very weak intensity appeared (Fig. 4b) and the intensity of these peaks increased with increasing loading amount of TPA up to 30 wt% (Fig. 4d). However, the XRD pattern of the 30 wt% TPA/MCM-41 shows all of the characteristic peaks of the bulk TPA. These results indicate formation of crystalline TPA on the support from 15 wt% up to higher loading of TPA.
The particle size of TPA crystallites on the 20 and 30 wt% catalyst was calculated by the Scherrer equation regarding to peaks at 2θ = 10, 25 and 30°. The mean diameter of TPA crystallites were 25 and 28 nm for 20 and 30 wt% TPA/MCM-41.
A serious problem to heteropoly acid catalysts is their low thermal stability and thus, difficulty of regeneration of solid heteropoly acid catalysts (decoking) [34]. The temperature at which H3PW12O40 starts loss of all acidic protons is 465 °C and complete decomposition to the constituent oxides occurs at 610 °C. Catalyst regeneration (decoking) is usually carried out by coke combustion in oxygen containing atmosphere at 450–550 °C [35]. However, as shown in high angle XRD patterns of TPA/MCM-41 there is not any additional peaks related to decomposition products of TPA in crystalline form on the support in 20 and 30 wt% catalyst. This result suggests that partial decomposition may be take place after calcinations. However, it may be too small that is not detectable by XRD experiment.
The catalyst acidity characters, including the acidic strength and the total number of acid sites were determined by potentiometric titration. According to this method, the initial electrode potential (Ei) indicates the maximum acid strength of the surface sites and the range where a plateau is reached (meq/g solid) indicates the total number of acid sites [22]. Therefore, a suspension of the catalyst in acetonitrile was potentiometrically titrated with a solution of 0.02 M n-butylamine in acetonitrile. As shown in Fig. 5, the acid strength of the supported catalyst was increased with an increasing loading amount of TPA from 5 up to 10 wt% and is constant for higher loading amounts. These results confirm maximum dispersion of TPA on the MCM-41 for the 10 wt% catalyst which is in agreement with the XRD results. In the pattern of 5a, the very low initial potential shows that MCM-41 is very weak acid relative to bulk TPA and any supported catalyst. The former pattern also shows that the initial potential of 140 mV reaches the value of −79 mV for 0.08 meq/g. This confirms the presence of low number of acid sites on the MCM-41.
The distribution of both Brønsted and Lewis acid sites was confirmed by FT-IR spectroscopy by means of pyridine absorption. Fig. 6 shows the pyridine adsorbed FT-IR spectra of the catalyst with 10 wt% loading of TPA at various temperatures from room temperature to 400 °C. The spectra show contribution of pyridine adducts in the region 1,400–1,650 cm−1. The spectrum of the pyridine adsorbed catalyst (Fig. 6b) shows sharp pyridine absorption peaks at 1,597 and 1,446 cm−1. This result confirms that pyridine molecules bonded to Lewis acid sites of the catalyst and is in agreement with results previously reported by others [30, 32, 36–39]. Brønsted acid sites are created due to the presence of small clusters of TPA, while the Lewis acid sites might be due to the interaction of dispersed TPA with MCM-41 framework [36, 39]. The weak peak appearing at 1,543 cm−1 is responsible for Brønsted acid sites (pyridinum ion) [40]. The peak at 1,635 cm−1 in the spectrum of the catalyst before treatment with pyridine (Fig. 6a) is due to the presence of water on the preparation of the pellet sample [41]. This sharp peak may overlap with another weak peak responsible for Brønsted acid sites at 1,635 cm−1. The weak peak at 1,487 cm−1 is the combination band of pyridine bonded to both Brønsted and Lewis acid sites [41]. However, these results confirm the existence of both Brønsted and Lewis acid sites on the catalyst. The peaks characteristic of the Lewis acid sites (1,597 and 1,446 cm−1) show higher intensity than that of the Brønsted acid sites (1,543 cm−1). This result suggests that Lewis acid sites have dominant role in the catalytic activity.
Imino Diels–Alder reaction
A one-pot, three-component imino Diels–Alder reaction of an aldehyde, arylamine and dienophile, first introduced by Povarov [6], has been performed in the presence of various acid catalysts [7–11]. However, less attention has been paid to the performance of this type of reaction in the presence of supported solid acid catalysts. In order to investigate the catalytic activity of TPA supported on the nano sized MCM-41 as solid acid catalyst in the imino Diels–Alder reaction, the three-component reaction of various types of arylaldehydes, anilines and dihydropyrane were studied in the presence of TPA/MCM-41.
Initially, the optimization experiments were performed in the reaction of benzaldehyde (1 mmol), aniline (1.1 mmol) and dihydropyrane (2 mmol) as the model reaction and the results are shown in Table 1.
To investigate the effect of the loading amount of TPA on the catalytic activity of TPA/MCM-41 in the imino Diels–Alder reaction, the model reaction was performed in the presence of 150 mg of catalysts with 5–30 wt% TPA (Table 1, entries 1–5). The results show that the catalyst with 10 wt% TPA has the best catalytic activity. This result is in agreement with acid strength measurement obtained by potentiometric titration (Fig. 5).
In order to optimize the amount of 10 wt% TPA/MCM-41 in the model reaction, various amounts of the catalyst were used in the reaction and the best result in terms of catalyst loading amount and the yield of the product was obtained using 150 mg (0.5 mol%) of the catalyst for the reaction of 1 mmol benzaldehyde in the model reaction with a mole ratio of 1:1.1:2 for benzaldehyde:aniline:dihydropyrane (Table 1, entries 2, 6–8).
To investigate the effect of the solvent on the catalytic reaction, the model reaction in the presence of 10 wt% TPA/MCM-41 was carried out in various solvent media (Table 1, entries 2, 9–10). The results show that the CH3CN is the best solvent with the best yield and time for the reaction.
The scope and generality of this catalytic reaction is illustrated with respect to the reaction of different arylaldehydes with aniline and dihydropyrane and the results are summarized in Table 2. As shown in Table 2, the reaction of benzaldehyde, aniline and dihydropyrane were carried out in the presence of a catalytic amount of 10 wt% TPA/MCM-41 (0.5 mol% TPA per 1 mol aldehyde) at room temperature in CH3CN as solvent. The corresponding pyranoquinolines were obtained as two isomeric products in an overall yield of 88%. The ratio of diastereomers was determined from the 1H NMR spectra of the product mixture and the results show a ratio of 66:34 for trans:cis isomers.
The diastereomers were separated by thin layer chromatography on silica gel (eluent; EtOAc:n-hexane, 20:80) and their structure was established from the spectral data. Various types of arylaldehydes with both electron-donating and electron-withdrawing substituents were reacted under the same reaction conditions and the corresponding pyranoquinolines were obtained in high yields with different diastereoselectivity. This difference is not completely clear. However, a plausible explanation is that aldehydes with various substituents can interact with different orientation in mesoporous channels of MCM-41.
The stereochemistry of the products was assigned on the basis on coupling constant between protons H-4a and H-5. In the products 4a–i, the coupling constant of J(4a–5) = 9.3–11 indicates anti orientation of protons H-4a and H-5. This orientation is in accordance with a trans relationship between the pyrane ring (C4a) and the phenyl group (C5). In the products 5a–i, the coupling constant of J(4a–5) = 5.45–5.5 Hz is significantly smaller and indicates a gauche conformation. This conformation indicates cis orientation of pyrane ring and phenyl group. In all products, J4a–10b was found to be 1.55–3.3 Hz indicating the cis junction between pyrane ring and quinoline moiety which is in accordance with literature values.
The workup and catalyst recovery is very simple. After the completion of the reaction (monitored by TLC, eluent; EtOAc:n-hexane, 20:80), the catalyst was separated by centrifuging and washed with EtOH. The solvent was evaporated and the crude product was subjected to column chromatography using silica gel and EtOAc:n-hexane 15:85 as an eluent.
One of the important features of the solid acids is the reusability. To study the reusability of TPA/MCM-41 in the imino Diels–Alder reaction, the recovered catalyst from the model reaction was washed with EtOH and dried in an oven at 120 °C for 1 h. The recovered catalyst was used in the same reaction. The results show that the catalyst activity decreased after the first run in comparison to the fresh catalyst. This result suggests that deactivation is due to the blockage of active sites of the catalyst during the reaction.
In order to clean of the surface of the deactivated catalyst, the recovered catalyst after a second run was calcined at 550 °C for 2 h. The catalyst was then applied in the same reaction (run 3) and the result shows that the catalytic activity increased to nearly the same value as the fresh catalyst (Table 3).
In conclusion, we have demonstrated that TPA on the nano-sized MCM-41 can be used as an efficient, reusable and stereoselective catalyst for imino Diels–Alder reaction of imines and dihydropyranes. Mild reaction conditions, easy workup, high yield and simple purification make this method attractive for the synthesis of tetrahydroquinolines.
References
Jacquemond-Collet I, Benoit-Vical F, Mustofa V, Valentin A, Stanislas E, Mallié M, Fourasté I (2002) Planta Med 68:68
Wallace OB, Lauwers KS, Jones SA, Dodge JA (2003) Bioorg Med Chem Lett 13(11):1907
Dorey G, Lockhart B, Lestage P, Casara P (2000) Bioorg Med Chem Lett 10(9):935
Yamada N, Kadowaki S, Takahashi K, Umezu K (1992) Biochem Pharmacol 44(6):1211
Ramesh M, Mohan PS, Shanmugam P (1984) Tetrahedron 40(20):4041
Povarov LS (1967) Russ Chem Rev 36(9):656
Srinivasa A, Mahadevan KM, Hosamani KM, Hulikal V (2008) Monatsh für Chem 139(2):141
Nagaiah K, Sreenu D, Rao RS, Vashishta G, Yadav JS (2006) Tetrahedron Lett 47(26):4409
Xing X, Wu J, Dai W-M (2006) Tetrahedron 62(48):11200
Nagarajan R, Chitra S, Perumal PT (2001) Tetrahedron 57(16):3419
Ma Y, Qian C, Xie M, Sun J (1999) J Org Chem 64(17):6462
Zubkov FI, Zaitsev VP, Piskareva AM, Eliseeva MN, Nikitina EV, Mikhailova NM, Varlamov AV (2010) Russ J Org Chem 46(8):1192
Kamble VT, Davane BS, Chavan SA, Muley DB, Atkore ST (2010) Chin Chem Lett 21(3):265
Jin GF, Zhao JW, Han JW, Zhu SZ, Zhang JM (2010) Tetrahedron 66(4):913
Kouznetsov VV, Merchan Arenas DR, Romero Bohórquez AR (2008) Tetrahedron Lett 49(19):3097
Khadem S, Udachin KA, Enright GD, Prakesch M, Arya P (2009) Tetrahedron Lett 50(48):6661
Ramesh E, Vidhya TKS, Raghunathan R (2008) Tetrahedron Lett 49(17):2810
Chen XM, Wei H, Yin L, Li XS (2010) Chin Chem Lett 21(7):782
Wang XS, Zhou J, Yang K, Yao CS (2010) Tetrahedron Lett 51(43):5721
Abdollahi-Alibeik M, Mohammadpoor-Baltork I, Zaghaghi Z, Yousefi BH (2008) Catal Commun 9(15):2496
Dias AS, Pillinger M, Valente AA (2006) Microporous Mesoporous Mater 94(1–3):214
Pizzio LR, Vázquez PG, Cáceres CV, Blanco MN (2003) Appl Catal A Gen 256(1–2):125
Okuhara T (2002) Chem Rev 102(10):3641
Yadav GD (2005) Catal Surv Asia 9(2):117
Abdollahi-Alibeik M, Zaghaghi Z, Mohammadpoor-Baltork I (2008) J Chin Chem Soc 55(1):1
Edwards JC, Thiel CY, Benac B, Knifton JF (1998) Catal Lett 51(1):77
Devassy BM, Lefebvre F, Halligudi SB (2005) J Catal 231(1):1
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359(6397):710
Juan JC, Zhang J, Yarmo MA (2007) J Mol Catal A Chem 267(1–2):265
Rabindran Jermy B, Pandurangan A (2008) Catal Commun 9(5):577
Jin D, Hou Z, Luo Y, Zheng X (2006) J Mol Catal A Chem 243(2):233
Ajaikumar S, Pandurangan A (2008) J Mol Catal A Chem 286(1–2):21
Vázquez P, Pizzio L, Cáceres C, Blanco M, Thomas H, Alesso E, Finkielsztein L, Lantaño B, Moltrasio G, Aguirre J (2000) J Mol Catal A Chem 161(1–2):223
Kozhevnikov IV (2007) J Mol Catal A Chem 262(1–2):86
Kozhevnikov IV (2009) J Mol Catal A Chem 305(1–2):104
Karthikeyan G, Pandurangan A (2009) J Mol Catal A Chem 311(1–2):36
Udayakumar S, Ajaikumar S, Pandurangan A (2007) Catal Commun 8(3):366
Carriazo D, Domingo C, Martín C, Rives V (2008) J Solid State Chem 181(8):2046
Xia QH, Hidajat K, Kawi S (2002) J Catal 209(2):433
López-Salinas E, Hernández-Cortéz JG, Cortés-Jácome MA, Navarrete J, Llanos ME, Vázquez A, Armendáriz H, López T (1998) Appl Catal A Gen 175(1–2):43
Dias JA, Caliman E, Dias SCL, Paulo M, de Souza ATCP (2003) Catal Today 85(1):39
Acknowledgment
We are thankful to the Yazd University Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdollahi-Alibeik, M., Pouriayevali, M. 12-Tungstophosphoric acid supported on nano sized MCM-41 as an efficient and reusable solid acid catalyst for the three-component imino Diels–Alder reaction. Reac Kinet Mech Cat 104, 235–248 (2011). https://doi.org/10.1007/s11144-011-0345-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0345-9