Abstract
Vanadium complexes of N-salicylidene-l-histidine immobilized on Al-MCM-41 designated as VO(Sal-His)/Al-MCM-41, were prepared and characterized by X-ray powder diffraction (XRD), FT-IR, nitrogen adsorption–desorption and chemical analysis techniques. VO(Sal-His)/Al-MCM-41 was found to catalyze the epoxidation of trans-2-hexen-1-ol, cinnamyl alcohol and geraniol with tert-butyl hydroperoxide (TBHP). The successful catalytic behavior of VO(Sal-His)/Al-MCM-41 with 99% conversion and excellent selectivity toward the corresponding epoxides is described herein.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxidation of organic substrates is potentially very useful, but is usually difficult to control. Significant research efforts have focused on the applicability of oxidation reactions by increasing their selectivity [1]. Despite impressive progress in the level of understanding of such reactions, which has led to various industrial applications [2], their practical use is still quite limited. In attempts to borrow know-how from nature, the study of so-called “biomimetic” or “bioinspired” reagents is enjoying increasing popularity [3, 4]. The goal of these reagents is to mimic the high activity and selectivity of enzymes in designing simpler models of their active sites. Many natural oxidizers contain one or more transition metals in the active center; prominent examples are the cytochrome P450 family [5], copper-based metalloproteins [6, 7], and vanadium-dependent haloperoxidases, which are one of the principle areas of the bioinorganic chemistry of vanadium [8–10]. The haloperoxides themselves catalyze a variety of oxidation reactions, such as the oxidation of bromide to hypobromite, the transformation of organic sulfides to sulfoxides, or the epoxidation of olefins, using different oxygen sources, such as air, hydrogen peroxide and other oxygen donors [11]. These transformations are particularly attractive from a synthetic point of view if the stereo control typical for enzymatic reactions can be fully exploited. In an attempt to gain insight into the biological roles of vanadium, many recent studies have focused on the coordination chemistry of this metal in the oxidation states of III, IV and V with biologically relevant ligands bearing oxygen and nitrogen atoms [12].
In the last decade, Schiff base ligands have received much attention, mainly because of their extensive application in the fields of synthesis and catalysis [13]. This attention is still growing; a considerable research effort is devoted to the synthesis of modified and supported reagents for catalysis and materials chemistry [14–16]. The Schiff bases of vanadium complexes are efficient catalysts for oxidation reactions both in homogeneous [17–26] and heterogeneous [27–30] systems. The activity of these complexes varies with the type of ligands, coordination sites and type of support in the heterogeneous catalysts.
Microporous and mesoporous materials that encapsulate metal complexes hold a key place as model compounds for mimicking enzymes. In fact, these supports act as the protein portion of a natural enzyme in the model and also offer the advantages of easy recovery and catalyst reuse.
In continuation of our research on vanadium catalyzed epoxidation reactions [31], herein, we report the effect of immobilized tridentate N-salicylidene-l-histidine Schiff base vanadium complex within the nanoreactors of Al-MCM-41 as catalysts for the epoxidation of geraniol, cinnamyl alcohol and trans-2-hexene-1-ol.
Experimental
All materials were of commercial reagent grade and used without further purification. TBHP (80% in di-tert-butyl peroxide), was purchased from Fluka, vanadyl sulfate and geraniol were obtained from Aldrich. Methyl amine, cetyltrimethylammonium bromide (CTABr), tetraethylorthosilicate (TEOS), aluminum hydroxide, salicylaldehyde, l-histidine, sodium acetate, chloroform, trans-2-hexen-1-ol, cinnamyl alcohol, acetone, commercial reagent grade diethyl ether and ethanol were purchased from Merck Chemical Company. FT-IR spectra were recorded on a Bruker Tensor 27 FT-IR Spectrometer using KBr pellets over the range of 4000–400 cm−1. The UV–Vis measurements were performed on a double beam UV–Vis Perkin Elmer Lambda 35 spectrophotometer. X-ray powder diffraction (XRD) data were recorded on a diffractometer type, Seifert XRD 3003 PTS, using Cu Kα1 radiation (λ = 1.5406 Å). Chemical analysis of samples was carried out with a Perkin-Elmer atomic absorption spectrometer (AAS). Nitrogen sorption studies were performed at liquid nitrogen temperature (77 K) using a Quanta chrome Nova Win 2, version 2.2. Before the adsorption experiments, the samples were out-gassed under nitrogen gas during 7 h at 423 K. TGA/DTA were carried out by Mettler-Toledo TGA/s-DTA-851. Oxidation products were analyzed by GC and GC–MS using an Agilent 6890 Series with a FID detector, HP-5, 5% phenylmethyl siloxane capillary and an Agilent 5973 Network, mass selective detector, HP-5 MS 6989 Network GC system.
Synthesis of Al-MCM-41
The Al-MCM-41 (Si/Al = 50) was prepared according to a previously reported method [32]. Methylamine (2.08 mL) was added to deionized water (42 mL) and the mixture was stirred at room temperature for 10 min. CTABr (1.47 g) was then added and the mixture was stirred for another 60 min until a clear solution formed. Then, a TEOS solution (2.1 mL) was added dropwise to the solution followed by addition of aluminum hydroxide solution (0.03 g in 5 mL deionized water). The molar composition of the resulting mixture was SiO2: 1.6 MA: 0.215 CTMABr: 125 H2O: 0.02 Al2O3. The pH of the reaction mixture was adjusted to 8.5 by the slow addition of a hydrochloric acid solution (1 M). After slow stirring for 2 h, the solid was separated by centrifugation and washed with methanol. Al-MCM-41 was obtained as a white powder after drying the solid at 45 °C for 12 h followed by calcination at 550 °C for 8 h to decompose the surfactant.
Preparation of [VO(Sal-His)(H2O)]·3H2O
[VO(Sal-His)(H2O)]·3H2O was prepared according to a procedure described previously [19]. Salicylaldehyde (10 mmol) in ethanol (20 mL) was introduced into a histidine solution (10 mmol) dissolved in water (20 mL), followed by the addition of an aqueous sodium acetate solution (0.02 mol in 10 ml water). The color of the solution changed to deep yellow after this addition. An aqueous solution of VOSO4·3H2O (8 mmol in 10 mL water) was then added slowly to the mixture while stirring. The resulting solution, whose color changed from deep yellow to dark red, was concentrated slowly with a rotary evaporator. The solid was filtered, washed with a large amount of water, acetone, and diethyl ether, then dissolved in ethanol (20 mL), followed by the addition of water (50 mL). The mixture was slowly concentrated with a rotary evaporator to obtain the complex compound.
Anal. Calc.: for C13H15N3O5V.3H2O (M = 395 gmol−1): V 12.91, C 39.49, H 4.56, N 10.63. Found: V 12.92, C 39.75, H 4.49, N 10.58%. FT-IR(cm−1): 3143–3024(OH), 1629(C=N or COO),1545(C=C), 1470, 1339, 1297(O–Ph), 941(V=O), which are consistent spectral bands with those reported previously [32]. UV–Vis: λmax at 251, 269, 358, 479 nm [33].
Preparation of catalyst
Al-MCM-41 (1 g) was added to the complex (0.25 mmol in 20 mL ethanol). The mixture was refluxed while stirring for 8 h. The solids were filtered, washed with hot ethanol and the resultant VO(Sal-His)/Al-MCM-41 was dried in air at room temperature. The percentage of vanadium present determined by AAS was 0.25%.
Catalytic epoxidation, general procedure
All epoxidation reactions of the allyl alcohol (geraniol, cinnamyl alcohol and trans-2-hexen-1-ol) were carried out in a round bottom flask equipped with a stir bar and a water-cooled condenser under atmospheric pressure. Typically, a mixture of catalyst (100 mg) and substrate (20 mmol, dissolved in 10 mL chloroform) was added to the reaction flask upon slow stirring. After a few minutes, TBHP (24 mmol, 80% in di-tertiary butyl peroxide) was added and the mixture was refluxed for 8 h. The solid was then filtered and washed with fresh chloroform. The filtrate was subjected to GC and GC mass analysis.
Results and discussion
The Schiff base ligand was prepared via condensation of salicylaldehyde and histidine, followed by treatment of the prepared ligand with VO(SO4) under aerobic conditions to give the desired oxovanadium complex (Scheme 1). The FT-IR, UV–Vis and CHN analysis were consistent with those of the authentic sample reported previously [33].
Fig. 1a shows the X-ray powder diffraction pattern of calcined Al-MCM-41, which exhibits one strong and one weak peak. All two XRD reflections were consistent with those previously reported [31] and can be indexed on a hexagonal lattice. The XRD patterns of VO(Sal-His)/Al-MCM-41, shown in Fig. 1b, display a decrease in the peak intensities compared to the calcined Al-MCM-41 peaks. This change indicates that the Al-MCM-41 pore and surface silanol groups reacted with the vanadium complexes.
The N2 adsorption–desorption isotherms of Al-MCM-41 and VO(Sal-His)/Al-MCM-41 are shown in Fig. 2. The type IV isotherms [34] indicate that adsorption takes place as a thin layer on the walls at low relative pressures, P/P0 (monolayer coverage). In addition, the inflection heights of VO(Sal-His)/Al-MCM-41 in the nitrogen adsorption isotherm plots are smaller than those of Al-MCM-41. This phenomenon is attributed to the reduced pore volume that decreases from 1.074 to 0.8862 cm3 g−1, which reflects the decrease in surface area (Table 1). This effect can be due to the inclusion of VO(Sal-His) on the Al-MCM-41.
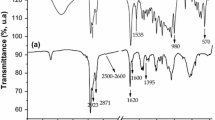
The FT-IR spectra of VO(Sal-His), VO(Sal-His)/Al-MCM-41, Al-MCM-41 and the subtracted spectrum of the immobilized complex within Al-MCM-41 from that of Al-MCM-41 are shown in Fig. 3a, b, c, d and in Table 2. The broad bands appearing from 3424 to 3401 cm−1 may be attributed to the surface silanol and –OH groups of adsorbed water. After the immobilization of the complex within Al-MCM-41 some weak peaks appear at the region 2987–2900 cm−1 due to the C–H and N–H vibrations of the immobilized complex. The bands at 1222, 1219, 1141 and 1133 cm −1 are due to the asymmetric stretching vibrations of Si–O–Si bridges, and those appear at 967–950 cm−1 arise from Si–O–Al, VO–Si and V=O stretching vibrations. However, the band due to the V=O stretching vibration, which appears at 970 cm−1 in the free complex could not be observed probably because it is masked by the strong and broad Si–O–Si and Si–O–Al vibration positioned at 1200–950 cm−1.The the C=N stretching frequency of the immobilized vanadium complex within the Al-MCM-41 appears at 1672 cm−1 [35, 36].
TGA/DTA patterns of VO(Sal-His)/Al-MCM-41 are shown in Fig. 4. The weight loss of the complex immobilized within nanoreactors of Al-MCM-41 takes places at two steps. The first weight loss of 1% is observed below 200 °C, corresponding to the evaporation of water. The second weight loss observed at about 200–550 °C is due to the decomposition of the organic ligand, consistently with DTA results.
Epoxidation of alkenes catalyzed by VO(Sal-His)/Al-MCM-41
The epoxidation reactions were carried out in the presence of 0.1 g of the VO(Sal-His)/Al-MCM-41 as catalyst at different times using geraniol as the representing substrate in order to choose the best reaction time. The results are presented in Fig. 5. As seen, geraniol is mostly oxidized after 4 h, beyond which no considerable oxidation occurs during the next 4 h.
Epoxidation results obtained for geraniol, cinnamyl alcohol and trans-2-hexen-1-ol using TBHP in chloroform in the presence of VO(Sal-His)/Al-MCM-41 are given in Scheme 2 and Tables 3, 4, and 5. The identification of the products was carried out by comparing the mass spectra with those of the authentic samples. We have included the effect of recovered VO(Sal-His)/Al-MCM-41, VO-Al-MCM-41, Al-MCM-41 void of complexes as catalysts and [VO(Sal-His)H2O]·3H2O as a homogeneous catalysis system in Table 3 in order to make the comparisons more convenient.
As seen in Table 3, geraniol is almost quantitatively oxidized to 2,3-epoxygeraniol in the presence of VO(Sal-His)/Al-MCM-41 (Table 3, entry 1) with 99% conversion and 99% selectivity towards the corresponding epoxides. Catalytic efficiencies are observed using recovered VO(Sal-His)/Al-MCM-41 (Table 3, entry 2), VO-Al-MCM-41 (Table 3, entry 3), [VO(Sal-His)H2O]3H2O (Table 3, entry 4), Al-MCM-41 (Table 3, entry 5). Therefore, the key roles of the vanadium complex as well as the heterogeneous character of the catalyst are evidenced (Fig. 5).
The maximum amount of cinnamyl alcohol conversion and selectivity toward the formation of 3-phenyl-oxiran-2-yl methanol occurs in the presence of VO(Sal-His)/Al-MCM-41 as the oxidation catalyst (Table 4, entry 1). Epoxidation reactions proceed less efficiently under VO-Al-MCM-41 (Table 4, entry 2). Therefore, both the presence of the coordinated Schiff base ligand as well as the heterogeneous catalytic character of the system drastically enhances the cinnamyl alcohol conversion percentage and selectivity toward the formation of the corresponding epoxide.
The epoxidation results of trans-2-hexen-1-ol to 2, 3-epoxyhexanol with VO(Sal-His)/Al-MCM-41as catalysts are presented in Table 5. Compared to geraniol and cinnamyl alcohol, the epoxidation reaction of trans-2-hexen-1-ol proceeds less efficiently, but with 100% selectivity to the corresponding epoxide. Utilization of VO-Al-MCM-41 as catalysts decreases the conversion rate from 60 to 32%.
The increasing reactivity trend of geraniol > cinnamyl alcohol > trans-2-hexen-1-ol observed in our reactions may be used as a criterion in gaining insight into the reaction mechanism. This trend reveals that the highly substituted allyl alcohol exhibits the highest epoxidation reactivity. Such observations are reminiscent of the mechanism suggested by Sharpless for the vanadium-catalyzed epoxidations in which the more highly substituted alkenes react faster than the less substituted alkenes [26]. According to this mechanism, both the allylic alcohol and the hydroperoxide initially coordinate to the metal center. Subsequent coordination of the second oxygen of the peroxide generates metal peroxide. Displacement of the epoxide probably proceeds with the loss of the tert-butoxy ligand to regenerate the vanadium catalyst (Scheme 3).
The stability of the VO(Sal-His)/Al-MCM-41 was also studied by recycling the recovered catalyst and through the determination of the metal content using atomic absorption spectroscopy. The vanadium content was shown to be 0.245% with very little change (0.005%,) with respect to the initial catalyst vanadium content (0.25%). As shown in Table 3, entry 2, the recovered VO(Sal-His)/Al-MCM-41 catalyzed the epoxidation of geraniol with a minor decrease in conversion and TON (no. of moles of products/no. of moles of vanadium complex), although with lower selectivity toward the formation of the corresponding epoxide.
The last worthwhile point to emphasize is the highly heterogeneous character of the VO(Sal-His)/Al-MCM-41 catalysts used in this work. The experimental evidence ruled out the effect of the participation of any leaching active species from the solid catalysts in the epoxidation reactions.
Epoxidation of allyl alcohols especially geraniol has been studied previously with homogeneous and heterogeneous catalysts, amongst which VO(acac)2 has proven to be the most active catalysis system. Recently, anchoring of vanadylacetylacetonate onto amine–functionalized clays [37] and mesoporous HMS [38] has been used for the epoxidation of geraniol with high activity and selectivity. On the other hand, immobilization of the catalyst within HMS rather than grafting has provided lower activity with some leaching [38]. We have studied a new Schiff base complex of vanadium VO(Sal-His) immobilized within Al-MCM-4 as catalyst for epoxidation of allyl alchols, especially geraniol in this work. Observation of high activity and selectivity, stability and reusability of the catalyst with no leaching during the course of reactions is promising.
Conclusions
The vanadium complex [VO(Sal-His)(H2O)]·3H2O immobilized within the nanoreactors of Al-MCM-41 was prepared and characterized. It was found that VO(Sal-His)/Al-MCM-41 successfully catalyzes the epoxidation of trans-2-hexene-1ol, cinnamyl alcohol and geraniol with 60–99% conversion and excellent selectivity. Therefore, mesoporous materials with a larger amount of room, such as Al-MCM-41, accommodate both the oxidant and substrates with different sizes in approaching the catalytically active metal centers. The observation of such catalytic behavior with no significant amount of catalyst desorption during the course of the studied reactions as well as almost quantitative epoxidation of geraniol in this study merits consideration by specialists.
References
Shilov AE, Shulpin GB (1997) Chem Rev 97:2879–2932
Bregeault JM (2003) Dalton Trans: 3289–3302
Gamez P, Aubel PG, Driessen WL, Reedijk J (2001) Chem Soc Rev 30:376–385
Lightenbarg AGJ, Hage R, Feringa BL (2003) Coord Chem Rev 237:89–101
Ortiz de Montellano PR (ed) (1995) Cytochrome P450: structure, mechanisms and biochemistry, 2nd edn. Plenum Press, New York
Klinmann JP (1996) Chem Rev 96:2541–2561
Solomon EI, Sundaram UM, Machonkin TE (1996) Chem Rev 96:2563–2606
Rehder D (2008) Bioinorganic vanadium chemistry. John wiley sons & Ltd Press, Chichester, pp 105–128
Rehder D (2003) Inorg Chem Commun 6:604–617
Thomson KH, Mcneill JH, Orvige C (1999) Chem Rev 99:2561–2571
Dembitsky VM (2003) Tetrahedron 59:4701–4720
Rehder D (2008) Bioinorganic vanadium chemistry. John wiley & sons Ltd, Chichester, pp 13–53
Canali L, Sherrington DC (1999) Chem Soc Rev 28:85–93
Kojima M, Taguchi H, Tsuchimoto M, Nakajima K (2003) Coord Chem Rev 237:183–196
Lane BS, Burgress K (2003) Chem Rev 103:2457–2473
Gupta KC, Sutar AK (2008) Coord Chem Rev 252:1420–1450
Boghaei DM, Mohebi S (2002) J Mol Catal A 179:41–51
Kwiatkowski E, Romanowski G, Nowicki W, Kwiatkowski M, Suwinska K (2003) Polyhedron 22:1009–1018
Ando R, Inden H, Sugino M, Ono H, Sakaeda D, Yagyu T, Maeda M (2004) Inorg Chim Acta 357:1337–1344
Kwiatkowski E, Romanowski G, Nowicki W, Kwiatkowski M, Suwinska K (2007) Polyhedron 26:2559–2568
Rayati S, Sadeghzadeh N, Khavasi HR (2007) Inorg Chem Commun 10:1545–1548
Romanowski G, Kwiatkowski E, Nowicki W, Kwiatkowski M, Lis T (2008) Polyhedron 27:1601–1609
Rayati S, Koliaei M, Ashouri F, Mohebbi S, Wojtczak A, Kozakiewicz A (2008) Appl Catal A 346:65–71
Cordelle C, Agustin D, Daran JC, Poli R (2010) Inorg Chim Acta 304:144–149
Yaul AR, Pethe GB, Aswar AS (2010) Russ J Coord Chem 36:254–258
Sharpless KB, Verhoven TR (1979) Aldrichim Acta 12:63–74
Maurya MR, Chandrakar AK, Chand S (2007) J Mol Catal A 270:225–235
Maurya MR, Chandrakar AK, Chand S (2007) J Mol Catal A 274:192–201
Gupta KC, Sutar AK, lin C (2009) Coord Chem Rev 253:1926–1946
Yang Y, Zhang Y, Hao S, Guan J, Ding H, Shang F, Qiu P, Kan Q (2010) Appl Catal A 381:274–281
Farzaneh F, Zamanifar E, Williams CD (2004) J Mol Catal A 218:203–209
Zanjanchi MA, Sh Asgari (2004) Solid State Ionics 171:277–282
Pessoa JC, Cavaco I, Correia I, Duarte MT, Gillard RD, Henriques RT, Higes FJ, Madeira C, Tomaz I (1999) Inorg Chim Acta 293:1–11
Lynch J (2001) Physico–chemical analysis of industrial catalysts. Technip ed., Paris
Selvaraj M, Pandurangan A, Seshadri KS, Sinha PK, Krishnasamy V, Lal KB (2003) J Mol Catal A 192:153–170
Kozlov A, Asakura K, Iwasawa Y (1998) Micropor Mesopor Mater 21:571–579
Pereira C, Silva AR, Carvalho AP, Pires J, Freire C (2008) J Mol Catal A 283:4–14
Jarrais B, Pereira C, Silva AR, Carvalho AP, Pires J, Freire C (2009) Polyhedron 28:994–1000
Acknowledgments
The financial support from the University of Alzahra is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamanifar, E., Farzaneh, F. Immobilized vanadium amino acid Schiff base complex on Al-MCM-41 as catalyst for the epoxidation of allyl alcohols. Reac Kinet Mech Cat 104, 197–209 (2011). https://doi.org/10.1007/s11144-011-0340-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-011-0340-1