Abstract
The rate constant of alkaline fading of brilliant green (BG+) was measured in the presence of non ionic (TX-100), cationic (DTAB) and anionic (SDS) surfactants. This reaction was studied under pseudo first-order conditions at 283–303 K. The rate of reaction showed remarkable dependence on the electrical charge of the used surfactants. It was observed that the reaction rate constant increases in the presence of TX-100 and DTAB (catalytic effect) and decreases in the presence of SDS (inhibitory effect). Binding constants and the related thermodynamic parameters were obtained by the classical model. The results show that binding of BG+ to TX-100 is exothermic and binding of BG+ to DTAB and SDS is endothermic in the used concentration range of surfactants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rates of chemical reactions are known to be changed by self-organized assemblies such as micelles [1–9]. Effects of micelles on these reactions can be attributed to electrostatic and hydrophobic interactions. Brilliant green (BG+) is a triphenylmethane dye. These dyes are aromatic compounds, and are used extensively in the textile industry to dye silk and cotton, and in paper and leather industries [10, 11]. Some of these dyes are used as biological stains and in veterinary medicine [12]. BG+ has been used as an antiseptic. It is used in the laboratory practice for biological staining and as a disinfectant for instruments [13]. Also, BG+ has antifungal properties similar to those of malachite green (MG+) [14]. In continuation of our earlier works [15, 16], we studied the BG+ alkaline fading in the presence of different concentrations of TX-100, DTAB and SDS at 283-303 K. BG+ fading is a one-step reaction and kinetics of these kinds of reactions in the presence of surfactants can be studied using cooperativity and classical models [15, 16]. Also, in this work, substituent effects of ethyl groups of BG+ and methyl groups of MG+ on the fading reaction rates of these two dyes were compared to each other.
Experimental
Materials
Brilliant green (hydrogen sulfate), polyoxyethylene (number of segments 9.5) glycol tert-octylphenyl ether (Triton X-100 or TX-100), dodecyltrimethylammonium bromide (DTAB), sodium dodecyl sulfate (SDS) and NaOH were purchased from Merck.
Methods
The fading of BG+ was followed at its maximum wavelength (λ max) values in a thermostated cell compartment of a Shimadzu UV–1650PC spectrophotometer. The reaction of BG+ with hydroxide ion brings about fading the color of the BG+ and results in the formation of colorless carbinol base, Fig. 1. The experiments were conducted at 283, 293 and 303 K within ± 0.1 K. All the kinetic runs were carried out at least in triplicate. To perform each kinetic run, a 100 μl aliquot of 9.39 × 10−4 M BG+ solution was added by a microsyringe into 2.8 ml of a solution containing 1.42 × 10−3 M sodium hydroxide and a certain concentration of surfactant. To study all interactions occurring between the surfactant and substrate molecules, we carried out the experiments in the surfactant concentration ranges in which the reaction rate finally reaches its maximum value (in the case of the catalytic effect) or becomes very slow (in the case of the inhibitory effect). The reaction between BG+ and hydroxide ion has been found to be bimolecular but pseudo first-order conditions (excess alkali) were used in all cases. We used the second-order reaction rate constants in our calculations.
Results and discussion
Analysis of kinetic data by the classical model
In the classical (or stoichiometric) model [15], it is assumed that in each range of surfactant concentration, the surfactant and substrate can bind together and there is an equilibrium relation between them. A concentration of surfactant is called “substrate-surfactant complex formation point” (or abbreviated as sc point) in which the equilibrium relation between added surfactant and the species already present in the solution ends and a new equilibrium relation between added surfactant and compound resulting from the previous equilibrium relation starts. The critical micelle concentration (cmc) value of a surfactant is also a sc point and there may be some sc points before and after cmc as well. The range of surfactant concentration which covers an equilibrium relation is named “region”. Surfactant molecules can bind to the substrate either monomeric or micellar (by one or more number of their molecules). Thus, we can obtain the stoichiometric ratios and binding constants of interactions of surfactants with substrate molecules in various ranges of surfactant concentrations. For each assumed equilibrium relation, the following equation holds for:
where \( E_{\rm S} = - RTd\ln k^{\prime}{\frac{{d\ln k^{\prime}}}{{d[S]_{t} }}} \) and k′, c, [S] t , R and T are the rate constant in the presence of surfactant, lnk (in the first region) or lnk sc (for other regions), total surfactant concentration, universal gas constant, absolute temperature, respectively. k sc is the k obs in the starting of every region except region one. Eq. 1 can determine the concentration range of each region [15]. If the reaction rate is decreased upon increasing the surfactant concentration, the sign of E S is positive and is called “inhibition energy” and if the reaction rate is increased with increasing the surfactant concentration, the sign of E S is negative and is named “catalytic energy” at constant temperature and various surfactant concentrations. The unit of E s is kJ (mol molar(surfactant))−1.
In this model, it is assumed that one substrate molecule, BG+ in this work, binds to n molecules of surfactant in each region and we have:
where K is the binding constant of the substrate–surfactant interaction in each region. According to the classical model [15], these interactions are of type I or II, in which the surfactant has an inhibitory or catalytic effect on the fading reaction of BG+ and k obs is related to the surfactant concentration as follows [15]:
and
where k and k sc are the reaction rate in the absence of surfactant and at every sc point. k S is the reaction rate in the substrate–surfactant complex and k S = 0 for inhibition. The total binding constant (K itot ) and total stoichiometric ratio (n itot ) values for each substrate, in the ith region, can be obtained from below equations:
Going from one region to the next one, if the K 1/n value (the average binding constant of the interaction between one substrate molecule with one surfactant molecule in each region) increases, the cooperativity of interaction is positive and if the K 1/n value decreases, the cooperativity is negative.
Comparison of substituent effects on the BG+ and MG+ fading rate
As seen in Table 1, fading rate constants of BG+ in water are 7.97, 20.93 and 52.05 M−1 min−1 at 283, 293 and 303 K, whereas the fading rate constants of MG+ in water are 18.89, 46.98 and 118.60 M−1 min−1 at 283, 293 and 303 K, respectively [16]. As we know, the electron-releasing inductive effect of the ethyl groups of BG+ is greater than that of the methyl groups of MG+. This makes the (C2H5)2N+= group of BG+ more stable than the (CH3)2N+= group of MG+ and results in a decrease in the fading rate constant values of BG+ in water compared to those of MG+.
Effect of TX-100 on the BG+ fading
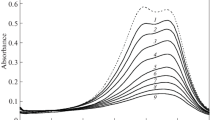
As shown in Figs. 2 and 3, with an increase in TX-100 concentration, the λ max value of BG+ shifts to red and the reaction rate of BG+ fading increases.
As seen in Table 1, the kinetics of BG+ fading in the presence of TX-100 has three regions. The cmc value of TX-100 in pure water is 0.29 mM [17] and the first region is below its cmc point. As given in Table 2, in acidic solution, the interaction of TX-100 with BGH2+ results in the blue shift in λmax value of BGH2+, Fig. 4.
This indicates that a preliminary electrostatic interaction occurs in this region between the (C2H5)2N+= group of BG+ and the oxygen atom of the ethoxy chains of TX-100 molecules which is similar to the interaction of silanol groups of silica gel with TX-100 [17, 18]. This interaction along with further hydrophobic interaction of BG+ with TX-100 molecules results in a sharp red shift in λmax value of BG+. The red shift has been previously reported for other compounds upon going from polar to apolar solvents, as a result of hydrophobic interaction [15, 19–21]. It seems that TX-100 molecules decrease the dielectric constant of the BG+ micro-environment. According to Hughs–Ingold rules for nucleophilic substitution reactions [22, 23], the formation of the neutral carbinol base from two oppositely charged reactants is favorable in lower dielectric constant media and thus with increase in TX-100 concentration, the rate of BG+ fading increases in the first region. In this region, as seen from log K values in Table 1, the interaction of BG+ with TX-100 is exothermic.
In the second region, above the cmc value of TX-100, the reaction rate and λmax value of BG+ fading keep approximately constant (Figs. 2 and 3). In the third region, the reaction rate increases with an increase in TX-100 concentration, but there is no shift in the λmax value of BG+. This indicates that BG/TX-100 forms through hydrophobic association between bound TX-100 molecules. In this region, the interaction is exothermic (Table 1).
As seen in Table 3, the interaction of BG+ with TX-100 molecules throughout the concentration range of TX-100 is exothermic and its ΔS value is negative.
The electron-releasing inductive effect of the ethyl groups of BG+ is greater than that of the methyl groups of MG+. This makes the (C2H5)2N+= group of BG+ more stable than the (CH3)2N+= group of MG+ and results in an increase in K tot and thus fading rate constant values of BG/TX-100 at 283–303 K, Tables 1 and 3, compared to those of MG/TX-100 [16].
Effect of DTAB on the BG+ fading
As seen in Figs. 2 and 5 and Table 4, the kinetics of BG+ fading in the presence of DTAB has three regions.
The cmc value of DTAB in pure water is 13.77 mM [24] and the first region is below the cmc value of DTAB. In this region, with an increase in DTAB concentration, the rate constant of BG+ fading and the λmax value of BG+ keep approximately constant and it seems that there is no interaction between BG+ and DTAB molecules. Obviously, at the beginning of the second region (≈13 mM ≈cmc value of DTAB), a weak electrostatic interaction occurs between the (C2H5)2N– group of BG+ and the positive head group of DTAB molecules and along with hydrophobic interaction of BG+ with nonpolar moiety of DTAB molecules results in the red shift in the λmax value of BG+. Confirming this result, it is seen from Table 2 that 15 mM DTAB acidic solution has no effect on the λmax value of BGH2+. According to Hughs–Ingold rules for nucleophilic substitution reactions, under these conditions, the rate constant of BG+ fading increases with increase in DTAB concentration. Similar results were obtained by Olanrewaju et al. [25]. The positive ΔS value of this interaction (996.6 J mol−1 K−1) shows the increased randomness which suggests that the hydrocarbon chain of DTAB molecules replace some water molecules of solvating BG+, Table 5. These displaced molecules gain more translational entropy than is lost by DTAB molecules, thus allowing the prevalence of randomness in the system.
In the third region, the rate constant of BG+ fading and λmax value of BG+ keep approximately constant and it seems that there is no interaction between BG+ and DTAB molecules (Table 4 and Fig. 2). As given in Table 5, the interaction of BG+ with DTAB molecules throughout concentration range of DTAB is endothermic and its ΔS value is positive.
The electron-releasing inductive effect of the ethyl groups of BG+ is greater than that of the methyl groups of MG+. This makes the (C2H5)2N+= group of BG+ more stable than the (CH3)2N+= group of MG+ and results in a decrease in K tot and thus the fading rate constant values of BG/DTAB compound at 283–303 K (Tables 4, 5) compared to those of MG/DTAB compound [16].
Effect of SDS on the BG+ fading
As seen in Figs. 2 and 6 and Table 6, the kinetics of BG+ fading in the presence of SDS has five regions.
The cmc value of SDS in 0.001 N NaOH is 3.5 mM [26] and the used concentration range of SDS is below its cmc value. It seems that a preliminary electrostatic interaction occurs between the (C2H5)2N+= group of BG+ and the negative charge on the sulfate head group of SDS. As shown in Table 2, in acidic solution, appearance of a peak at 632.5 nm shows that BG/SDS has been formed. This electrostatic interaction along with further hydrophobic interaction of BG+ with the nonpolar moiety of SDS results in the red shift in the λmax value of BG+ and thus a decrease in reaction rate of BG+ fading with an increase in SDS concentration.
The BG/SDS complex has no positive electric charge or may be negatively charged and SDS nonpolar moiety decreases the dielectric constant of BG+ micro-environment. According to Hughs–Ingold rules, under these conditions, formation of the neutral carbinol base is not favorable. Similar results were reported for interaction of MG+ with SDS [16].
It is clear from Fig. 2 that the λmax value of BG+ keeps constant at the end of region 3 (0.93 mM) where the rate constant of fading decreases. This indicates that in regions 2 and 3, BG/SDS complex forms via electrostatic and hydrophobic interactions between BG+ and SDS molecules and in regions 4 and 5, BG/SDS forms through hydrophobic association between free SDS with bound SDS molecules. It is found that the electrostatic interaction between the sodium salt of carboxymethylcellulose (NaCMC) and CTAB increases hydrophobicity of the polymer-surfactant complex [27]. The interaction of BG+ with SDS in regions 2, 3, 4 and 5 is endothermic. As seen in Table 7, throughout the concentration range of SDS, the interaction of BG+ with SDS is endothermic and its ΔS value is positive.
The electron-releasing inductive effect of the ethyl groups of BG+ is greater than that of the methyl groups of MG+. This makes the (C2H5)2N+= group of BG+ more stable than the (CH3)2N+= group of MG+ and results in an increase in K tot values of BG/SDS at 283–303 K, Table 7, compared to those of MG/SDS [16] and thus the fading rate constants of BG/SDS, Table 6, are less than those of MG/SDS [16].
Effect of DTAB and TX-100 mixtures on the BG+ fading rate
This test was carried out to verify the existence of regions and is called Pardis test [15]. Pardis is an abbreviation for the statement “proof of adjacent regions by destructive interaction with surfactant”. In this test, the concentrations of DTAB used were in the range of 1–4 mM (its first region) in which there is no effect on the rate of BG+ fading (Table 4, Fig. 5) and the concentrations of TX-100 were in the range of 1–7 mM (its second region) and 15–23 mM (its third region). In the second region, there is no effect and, in the third region, there is a large effect on the rate of BG+ fading (Table 1, Fig. 3). As seen in Table 8, an increase in DTAB concentration increases the reaction rate of BG+ fading.
In the used concentrations of DTAB and TX-100, mixed micelles of them form [28, 29], which results in a decrease in the apparent dielectric constant values for the different mixed systems compared to that of TX-100 or DTAB solution [30]. It seems that BG/TX100 (or abbreviated as BGTX n ) complexes formed in the second and third region of TX-100, interact with DTAB molecules via hydrophobic interaction between bound TX-100 molecules and DTAB molecules. Confirming this result, the λmax values of BG+ in the mixtures of DTAB and TX-100 depend only on the TX-100 concentration (Fig. 2, Table 8). This interaction is as follows:
and its reaction rate equation is as follows
where k BGTX is the fading rate constant of BGTX n and subscripts t and f represent the total and free concentrations of complexes, respectively. By substituting Eq. 7 into Eq. 8 we have
where
The data fitted in Eq. 10 along with m and K values are given in Table 8. The results show that in the third region of TX-100, above the cmc point of TX-100, with an increase in the number of TX-100 molecules bound to the BGTX n , the m and K values of interaction of DTAB with BGTX n increase. The interaction of BGTX n with DTAB molecules increases the positive charge of this compound and decreases the dielectric constant of its micro-environment and thus highly increases the BG+ fading reaction rate.
Analysis of kinetic data by the cooperativity model
The Piszkiewicz model [31–33; http://en.wikipedia.org/wiki/Hill_equation], analogous to the Hill model applied to the enzyme-catalyzed reactions may be considered here with some modifications. According to this model, it is assumed that substrate molecule, BG+ in this work, associates with n number molecules of surfactant to form micelle S n BG+, which may react as follows:
The model gives the following rate equation:
where K D is the dissociation constant of micellized surfactant–substrate complex back to its components and [S] t gives the total surfactant concentration. Here, k w is the reaction rate without any surfactant, and k m is the reaction rate with the maximum amount of surfactant concentration within the given range and if reaction is inhibited by adding surfactant, k m ≈ 0. n is known as the cooperativity index and is a measure of the association of additional surfactant molecules to an aggregate in the whole surfactant concentration range. If the value of n is greater than one, the cooperativity of interaction is positive and if its value is less than one, the cooperativity of interaction is negative and if its value is equal to 1, the interaction is noncooperative. It is clear that Eq. 14, a two-parameter equation, cannot fit properly the data of different types of surfactant-substrate interactions.
As shown in Figs. 3, 5 and 6, there are positive catalytic effects by TX-100 and DTAB and negative catalytic effect by SDS in the whole range of the reaction. As seen from Table 9, values of n show positive cooperativity in the used concentration range of SDS and DTAB and negative cooperativity for TX-100. Also, the calculated values of K D show that binding of BG+ to TX-100 is exothermic and binding of BG+ to SDS and DTAB is endothermic.
As an important remark, due to various assumptions used in classical and cooperativity models, occurrence of similar results is definitely random.
Conclusions
The rate constant of alkaline fading of brilliant green (BG+) was measured in various concentrations of TX-100, DTAB and SDS. It was observed that the reaction rate constant increases in the presence of TX-100 and DTAB and decreases in the presence of SDS. The rate of fading reaction showed noticeable dependence on the electrical charge of the used surfactants. According to Hughs–Ingold rules for nucleophilic substitution reactions, the electric charge of BG/surfactant complex along with decrease in dielectric constant of micro-environment of BG+ in this compound varies the rate of fading reaction. Binding constants of surfactant molecules to BG+ were obtained using cooperativity and classical models and the related thermodynamic parameters were calculated by classical model. The results show that the binding of BG+ to TX-100 is exothermic and the binding of BG+ to DTAB and SDS is endothermic in the used concentration range of surfactants.
References
Fendler JH, Fendler EJ, Chang SA (1973) J Am Chem Soc 95:3273
Munoz M, Rodriguez A, Del Mar Graciani M, Moya ML (2002) Int J Chem Kinet 34:445
De TK, Maitra A (1995) Adv Colloid Interface Sci 59:95
Tang SS, Chang GG (1995) J Org Chem 60:6183
Liou JY, Huang TM, Chang GG (1999) J Chem Soc Perkin Trans 2:2171
Kambo N, Upadhyay SK (2009) Int J Chem Kinet 41:123
Ghosh KK, Verma SK (2009) Int J Chem Kinet 41:377
Saha B, Sarkar S, Chowdhury KM (2008) Int J Chem Kinet 40:282
Bravo-Diaz C, Pastoriza-Gallego MJ, Lasada-Barreiro S, Sanchez-Paz V, Fernandez-Alonso A (2008) Int J Chem Kinet 40:301
Gregory P (1993) Dye and dye intermediates. In: Kroschwitz JI (ed) Encyclopedia of chemical technology, vol 8. Wiley, Indiana, USA, p 544
Duxbury DF (1993) Chem Rev 93:381
Kingsland GV, Anderson J (1976) Poult Sci 55:852
Balabanova MB, Popova L, Tchipeva R (2003) Clin Dermatol 21:2
Alderman DJ (1982) J Fish Dis 5:113
Samiey B, Alizadeh K, Moghaddasi MA, Mousavi MF, Alizadeh N (2004) Bull Korean Chem Soc 25:726
Samiey B, Raoof Toosi A (2009) Bull Korean Chem Soc 30:2051
Huang Z, Gu T (1987) Colloids Surf 28:159
Parida SK, Mishra BK (1998) Colloids Surf A 134:249
Caetano W, Tabak M (2000) J Colloid Interface Sci 225:69
Welti R, Mulikin LJ, Yoshimura T, Helmkamp JM (1984) Biochemistry 23:6086
Samiey B, Alizadeh K, Mousavi MF, Alizadeh N (2005) Bull Korean Chem Soc 26:384
Hughes ED (1941) Trans Faraday Soc 37:603
Ingold CK (1993) Structure and mechanism in organic chemistry. Bell, London
Chotipong A, Scamehorn JF, Rirksomboon T, Chavadej S, Supaphol P (2007) Colloids Surf A 297:163
Olanrewaju O, Ige J, Soriyan O, Grace O, Segun Esan O, Olanrewaju O (2007) Acta Chim Slov 54:370
Rabiller-Baudry M, Paugam L, Bégion L, Delaunay D, Fernandez-Cruz M, Phina-Ziebin C, Laviades-Garcia de Guadiana C, Chaufer B (2006) Desalination 191:334
Chakraborty T, Chakraborty I, Ghosh S (2006) Langmuir 22:9905
Mandeep MS, Shweta S, Singh K, Shaheen A (2005) J Colloid Interface Sci 286:369
Soboleva OA, Badun GA, Summ BD (2006) Colloid J 68:255
Carnero Ruiz C, Aguiar J (2000) Langmuir 16:7946
Piszkiewicz DJ (1976) J Am Chem Soc 98:3053
Piszkiewicz DJ (1976) J Am Chem Soc 99:7695
Piszkiewicz DJ (1976) J Am Chem Soc 99:1550
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samiey, B., Dargahi, M.R. Kinetics of brilliant green fading in the presence of TX-100, DTAB and SDS. Reac Kinet Mech Cat 101, 25–39 (2010). https://doi.org/10.1007/s11144-010-0208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-010-0208-9