Abstract
Purpose
Comparative assessment of the HRQL of paediatric survivors of brain tumours (BT) and of acute leukaemia against a population of their healthy peers.
Methods
The study consisted of patients who had completed treatment for BT (n = 36) or acute leukaemia (n = 35) and were aged between 8 and 19. Healthy children (n = 60) were selected from among pupils of schools. HRQL was evaluated directly and indirectly on the basis of the Polish language version of the PedsQL™ 4.0 Generic Core scales. The influence of selected factors (sex, age, time from the end of treatment and type of treatment) on the HRQL result was analysed.
Results
In all the aspects analysed (total, physical, psychosocial, emotional, social and school functioning), the HRQL of BT and leukaemia survivors was significantly lower in comparison to their healthy peers. The HRQL of patients after BT treatment was also significantly lower than that of the survivors of leukaemia. The parent-proxy reported HRQL was consistent with the children’s self-assessment. Patients treated with radiotherapy presented a significantly lower evaluation of life quality in the physical sphere.
Conclusions
Evaluation of HRQL should be treated as an additional independent parameter in an assessment of the long-term results of oncological treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The developments in oncological therapy during the last 20–30 years have significantly improved the results of paediatric neoplasm treatment. Currently, the rate of 5-year survival is 75% in terms of all neoplasm types at developmental age, over 80% among children with acute leukaemia and almost 70% among patients with CNS tumours [1, 2]. A result of this is that in the population, there are constantly more people who have successfully completed their therapy and remain in a long-lasting remission of neoplastic disease. Although impressive gains in survival have been achieved, late effects of treatment remain significant for the growth and development of these children and for the health of the adults that they become. Studies of very large cohorts of survivors of childhood cancers have reported that they are five times more likely to suffer from severe or life-threatening health problems than their healthy siblings and finally demonstrated more premature mortality compared with the general population [3, 4]. Therefore, there is more need to monitor the long-term consequences of oncological therapies and assess their influence on quality of life. The results of such monitoring should be helpful in developing new therapeutic strategies.
An assessment of a patients’ quality of life after having undergone oncological treatment in childhood has recently been the focus of many researchers [5–9]. A direct comparison of their results is not always possible, since they used different methods and quality of life assessment criteria [7, 8, 10–12]. However, irrespective of the questionnaires used, the majority of researchers found significantly lower figures for quality of life evaluation among survivors when compared with groups of healthy peers. Within the whole group of convalescents, the biggest problems were reported by patients with a record of CNS neoplasm treatment [5, 6, 9, 10, 13, 14]. In the literature, there are no similar analyses considering Polish paediatric patients.
The aim of this paper is a comparative assessment of the quality of life of survivors of primary brain tumours and acute leukaemia against a population of their healthy peers.
Patients and methods
An assessment of health-related quality of life (HRQL) was conducted at the Department of Paediatric Haematology and Oncology, College of Medicine of the Nicolaus Copernicus University among patients who had been treated for primary brain tumours or acute leukaemia (acute lymphoblastic leukaemia, ALL or acute non lymphoblastic leukaemia, ANLL) during the years 1995–2005. The research qualified all patients who had been in remission for at least 3 months after the completion of their treatment and who had not turned 19 before the end of the research (Table 1). The control group consisted of healthy children selected at random from among pupils of state primary schools, junior high schools and grammar schools in Bydgoszcz.
Questionnaire surveys of patients who had completed cancer treatment were carried out during routine health check-ups in the period from January to November of 2006. The examination of healthy children was carried out from April to June of 2006.
Before the tests, voluntary permits were obtained from the patients themselves (youth above 13) and/or their parents and a permit was issued from the Bioethical Committee at Ludwik Rydygier College of Medicine in Bydgoszcz, University of Nicolaus Copernicus, Torun (No. KB/66/2005).
The evaluation of quality of life was performed on the basis of the Polish language version of the Paediatric Quality of Life Inventory (PedsQL™) 4.0 Generic Core questionnaire by James W. Varni. Approval for using the questionnaires was obtained through the MAPI Research TRUST (Lyon, France). The validity and the reliability of this measuring tool in assessing the quality of life of children have been confirmed in earlier studies [15–17]. In each examination, a form for self-assessment was used (filled in by the participant) as well as a parent-proxy assessment (filled in by one of the parents) adapted for 3 age groups: 5–7 years, 8–12 years and 13–18 years. Among children who had completed treatment for CNS neoplasm, there were only 2 patients below the age of 7, and that is why, finally, HRQL was not evaluated in the 5–7 age category.
On the basis of replies given by participants of the study and their parents, a comprehensive assessment of the quality of life and functional ability in the following spheres—physical, psychosocial, emotional, social and school performance—was established. Seeking potential factors influencing the quality of life of patients after completion of cancer treatment, the HRQL results were analysed depending on the sex, the age at the time of the examination, the age at the time of cancer diagnosis and finally by the time that had passed from completion of the cancer treatment. Additionally, within the group of children that had had CNS tumours, HRQL was evaluated in regard to the location of the tumour in the CNS structures (subtentorial vs. supratentorial tumours) and the type of treatment applied (with radiotherapy versus without radiotherapy; radiotherapy versus radiotherapy associated with chemotherapy). Patients with primary brain tumours subjected to radiotherapy had received doses of radiation of 35 Gy on the entire CNS and up to 54 Gy on the site of the tumour. Amongst patients who had completed leukaemia treatment, none had undergone irradiation of the central nervous system in the course of their treatment (although they have had intensive intrathecal therapy including methotrexate).
Obtained scores were compared among individual groups of participants with the t-Student’s test. For comparing results of the self-assessment and the parent-proxy assessment of the quality of life, a correlation analysis and the Pearson’s test were applied. Statistical calculations were carried out with the help of the SPSS programme version 13.0 (SPSS Inc., Chicago, IL) and of package v.11.65 EDU Microsoft Office.
Results
One hundred and thirty-one children participated in the survey examining the health-related quality of life, 60 healthy children and 71 patients—36 after treatment of primary CNS tumours (low-grade glioma n = 17, medulloblastoma/PNET n = 7, ependymoma n = 4, high-grade glioma n = 1, others with low-grade neoplasm n = 7) and 35 after treatment of acute leukaemia (ALL n = 32, ANLL n = 3). The surveyed groups did not differ in respect of sex distribution. The time between the end of treatment and the beginning of the survey was similar for both groups of patients with neoplastic diseases. At the time of the assessment, the children with CNS tumours were older than both the children with leukaemia (P = .013) and the healthy children (P = .024), however, there was no significant difference between the age of children with leukaemia and healthy children. At the time of cancer diagnosis, the children with CNS tumours were also older than the children with leukaemia (P = .005) (Table 1).
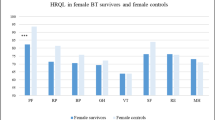
The direct assessment based on the self-report form showed that in comparison with their healthy peers, the patients who had completed oncological treatment evaluated their quality of life as being significantly worse (P < .01) in all the subscales of the questionnaire. Moreover, it was stated that the quality of life of patients who had been treated for CNS tumours was significantly lower (P = .017) in comparison with children who had been treated for leukaemia. Only in two areas, emotional and school performance, were the observed differences in results statistically insignificant (Fig. 1a). The parent-proxy report of the quality of life was consistent with the self-assessment of the children. Both the parents of children with CNS tumours and the parents of children with leukaemia evaluated their children’s quality of life significantly lower than the parents of healthy children (P < .0001). At the same time, parents’ evaluation of the quality of life of children with CNS tumours was significantly lower than parents’ evaluation of children who had been treated for leukaemia (P = .004). This referred to all considered elements except for the emotional sphere (Fig. 1b).
The agreement between the final results of the assessments of the quality of life made by children and by their parents, both in individual examined groups as well as in the subscales of the test, was proved by moderate and high coefficients of correlation that reached values from .403 (the emotional sphere) to .818 (physical functioning).
It was observed that parents of children who had undergone oncological treatment (for either CNS tumours or leukaemia) assessed the quality of life of their children slightly lower than the children assessed themselves (on average lower by 3.5 ± 1.6 points), although the differences were not significant in any of the analysed elements of the final assessment.
In both analysed groups of survivors, the sex, age at the time of diagnosis and the age at the time of making the quality of life assessment had no influence either on the final result of the general HRQL assessment or on its components. In the group of children with CNS tumours, the location of the tumour also proved statistically insignificant for the final HRQL results, although the results were better for children with subtentorial tumours than those with supratentorial tumours (68.3 vs. 64.3; P = .07). During analyses of the treatment that had been applied for children with CNS tumours, the factor that significantly influenced the result of the assessment of the physical functioning turned out to be radiotherapy. Patients treated without the application of irradiation (n = 12, including 9 treated surgically only and 3 with post-operative chemotherapy) definitely evaluated their physical functioning better than the remaining children on whom radiotherapy of the CNS had been applied (78.9 vs. 60.2; P = .014). There was no difference in the assessment of the psychosocial functioning amongst all the patients. Different values of their general HRQL assessment (73.6 vs. 63.6) did not differ in a statistically significant way (P = .065), however, the assessment was higher in the group of children without irradiation. Among the 24 patients treated with radiotherapy, where in the case of 9 patients, it was the only form of oncological treatment after the operation, and in the case of 15 children, radiotherapy and chemotherapy were elements of a combined treatment, the comparison of the HRQL assessment results did not show any significant differences. In the group of patients with CNS tumours, it was observed that the assessment of the quality of life was influence by the criterion of time: the more time that had passed since the end of the treatment, the better the questioned patients evaluated their quality of life on the general (P = .016), physical (P = .015) and psychosocial (P = .027) scales. This relationship was shown when the critical point was assumed as 3 years from treatment completion. In the group of patients with leukaemia, only the evaluation of physical functioning improved with the passage of time from the end of treatment (P = .049), no significant differences were observed in the psychosocial and general assessment of HRQL (Table 2).
Discussion
An assessment of the quality of life based on the general questionnaire PedsQL™ 4.0 showed significantly lower HRQL in the group of patients after a completed oncological treatment in comparison with the group of their healthy peers. A significantly lower assessment of the quality of life was observed in all subscales of the survey. Other authors in their research obtained similar results [6, 8, 15]. With reference to children with CNS tumours, almost identical results have been presented by Bhat et al. [7] and Palmer et al. [18]. They also compared the quality of life of patients with CNS tumours and healthy children on the basis of PedsQL™ 4.0. tests. Our own examination additionally showed that the quality of life of children with CNS tumours is lower not only than the quality of life of healthy children, but also than that of patients who had undergone leukaemia treatment. Similar results have been published by Eiser et al. [9]. The differences referred to all aspects of life except for the emotional sphere and school performance. This data suggest that despite relatively good physical health of leukaemia survivors, their psychosocial functioning remains compromised. During the course of treatment, sick children experience lengthy school absence and limitations in their family and social activities. Survivors of both leukaemia and brain tumour harbour a number of worries about current and future health so adverse experiences during their childhood can influence vulnerability to emotional difficulties in their future life. The stress connected with life-threatening hospitalization and long-term treatment causes increase in the level of fear, whereas isolation from peer groups as well as loss of active involvement in school life results in difficulties in re-adapting to the lifestyle from before the illness. Thus, it can be concluded that, irrespective of their form and location, oncological diseases at developmental age negatively influence emotional development and the implementation of educational tasks. These observations are similar to the results of Meeske et al. [5], who, while making a comparison of the quality of life of children with CNS tumours and leukaemia, noted significant differences in physical functioning, whereas, in the emotional sphere, both groups of patients presented a similar assessment. However, they observed a statistically significantly poorer school performance in survivors of brain tumours than in survivors of ALL—which is different to our findings. In this respect, our results indicate that clinicians and researchers in paediatric oncology should be concerned with the psychosocial functioning of survivors who received potentially neurotoxic CNS-directed treatment. With this aim in mind, general programs for facilitating school integration and social competence have been prepared for children with cancer [19]. Evaluation of social skills interventions to enhance the peer relationships of isolated and rejected children has only met with limited success [20]. However, new approaches should also be developed.
In this study, the assessment of quality of life was based on the results obtained from self-assessment questionnaires and parent-proxy reports. Comparative analysis of them showed their moderate or high level of congruence in all subscales of the test, and this is consistent with study of Russel et al. [8] and Varni et al. [15]. Generally, greater agreement is observed when a child has a diagnosed health problem, as parental involvement around issues of health increases so that their understanding of the child’s well-being is heightened [21]; this usually was not observed in group of healthy children. Moreover, PedsQL™ is a tool in which the majority of items (18 of 23) concern what a child can do, rather than how they feel. Thus, it may be that good agreement reflects the observable nature of the items [21]. Another tool to analyse internalizing and externalizing symptoms is Child Behaviour Checklist (CBCL). However, CBCL was not analysed in our study as some previous studies have demonstrated that the PedsQL™ Emotional Functioning Scale is significantly correlated with the Child Behaviour Checklist Anxious-Depressed Scale and Internalizing Behaviour Scale for patient-proxy reports [22]. Significant correlations of the PedsQL™ physical Functioning and Social Functioning Scales with the Child Behaviour Checklist subscales and the Social Support Scale for Children and Adolescents have also been shown [22, 23].
On this basis, we can conclude that in the case of young children or other patients unable to fill in forms by themselves that the assessment of the quality of life can be based exclusively on the evaluation given by parents. In the assessment carried out in such a way, one may have to take into consideration an error that results from a tendency observed among parents of ill children to underrate the evaluation of the quality of life while parents of healthy children tend to overrate it [8]. In our own examination, there were no statistical differences observed in this respect, nevertheless, the average assessment by parents of ill children was indeed lower in all subscales of the test, and lack of statistical difference may result from the small number of members in the tested groups.
Similarly to other authors [7–10, 24], no relationship has been proved between the assessment of the quality of life and factors like sex, age at the time of the test, age when cancer was diagnosed or location of the tumour. However, a significantly lower assessment of the quality of life in the physical sphere was reported by people who had undergone CNS radiotherapy. Patients with CNS tumours who had not been irradiated had average HRQL results similar to the assessments given in the group of patients after leukaemia treatment. Similar observations are reported in the research paper by Bhat et al. [7], while Speechley et al. [6] in addition to radiotherapy points out a lower assessment of the quality of life in the group of older children and youth, who perceive their imperfections in a critical way and more frequently face problems with acceptance in peer groups. In our analysis, a parameter with a significant influence on the quality of life was the time from the completion of treatment. The longer the time was, the better the patients assessed their quality of life, nevertheless, the evaluations still remained lower than in the general population. Not all authors confirm such an observation [7], although an analysis carried out in Canada in a group of young adults, 10 years after completion of oncological treatment showed their generally good adaptation and only an insignificantly poor quality of life, especially if irradiated [25].
Our study has several limitations imposed on the analyses of variables putatively associated with HRQOL outcomes. One is the relatively small size of the study sample that comes from a single centre, which suggests that there is a need to perform a prospective study recruiting patients from many centres and including more homogeneous groups of patients with brain tumour. Also, we used only generic HRQOL instrument permitting comparisons across healthy samples and survivors, but we did not evaluate the quality of life of survivors using PedsQL™ Cancer Module. The main reason was the lack of linguistic validation of that tool. However, we think the cancer module is a better instrument for children undergoing treatment than for off-treatment patients because it includes items that seems not to be relevant for survivors (e.g. questions about children concerns about nausea, needles and other treatment-related procedures). That missing data could probably better differentiate the HRQOL between brain tumour and leukaemia groups. Another issue is that because of the lack of similar analyses concerning Polish survivors of paediatric cancers, we compared our findings mainly with American researchers’ results. It is obvious that Polish and American populations differ in many aspects of life. However, most of results presented in our study performed on Polish children after cessation of anticancer therapy was comparable to reference data of American studies [5–8, 15, 18]. Thus, the background of these observations seems to be beyond cultural differences between Poland and USA. The similarities in quality of life of Polish paediatric cancer survivors, when compared to American ones, can possibly be explained by several factors. Firstly—although Poland is a transitional society, it is progressing in transformation towards capitalist countries, adopting western models of social live and behaviour. Secondly—taking into account relatively good system of social care for children in Poland, this age group, when compared to the elderly, is possibly the most advantaged social group [26], what might influence their quality of life. Thirdly—since the health declines with an increasing age, especially in older adults, it might be the subject to different level of social life in general population in each country [27], however, this possibly does not affect paediatric patients. Fourthly—socioeconomic variables may operate differently in Poland than in the United States. As is expected, family income and employment are associated with better health; however, the relationship between income and health does not reach significance for Poland, contrary to US [26].
To sum up, it can be stated that both the results of own examination and reports from other researchers confirm the validity of monitoring the quality of life of children and youth after completion of oncological treatment. Since at the present time we are not able to prevent cancer, we can only treat it more effectively. Therefore, it is advisable when developing new therapeutic strategies to take into consideration the knowledge about factors that influence the quality of life. To make this possible, an assessment of quality of life should be given the status of an independent parameter in an evaluation of treatment results, complimentary to the time criteria that have been applied up to now.
From oncological point of view, a contemporary major challenge is to reduce treatment-related long-term side effects. As radiotherapy is currently thought to have more neurocognitive sequel than chemotherapy [28], we would argue for the feasibility of reduction or omitting CNS irradiation and for testing the new therapeutic strategies such as the use of neuroprotectors and chemo- or radiosensitizers. Finally, in future with better understanding the pathobiology of neopalstic cells, it should be possible to individualize oncological treatment towards molecularly targeted therapy with few or no toxic effects [29, 30].
Abbreviations
- ALL:
-
Acute lymphoblastic leukaemia
- ANLL:
-
Acute non lymphoblastic leukaemia
- BT:
-
Brain tumour
- CNS:
-
Central nervous system
- Gy:
-
Gray
- HRQL:
-
Health-related quality of life
- PNET:
-
Primitive neuroectodermal tumour
References
Gurney, J. G., Smith, M. A., Bunin, G. R. (1999). CNS and miscellaneous intracranial and intraspinal neoplasms. In L. A. G. Ries, M. A. Smith, J. G. Gurney, et al. (Eds.), Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995 (pp. 51–64). Bethesda, MD: National Cancer Institute, SEER Program. NIH Pub. No. 99-4649.
Ries, L. A. G., Eisner, M. P., Kosary, C. L., Hankey, B. F., Miller, B. A., Clegg, L., et al. (2004). SEER cancer statistics review, 1975–2002. http://seer.cancer.gov/csr/1975_2002/. Posted to the SEER web site 2005.
Mertens, A. C., Yasui, Y., Neglia, J. P., Potter, J. D., Nesbit, M. E., Jr., Ruccione, K., et al. (2001). Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. Journal of Clinical Oncology, 19(13), 3163–3172.
Moller, T. R., Garwicz, S., Barlow, L., Falck Winther, J., Glattre, E., Olafsdottir, G., et al. (2001). Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. Journal of Clinical Oncology, 19(13), 3173–3181.
Meeske, K., Katz, E. R., Palmer, S. N., Burwinkle, T., & Varni, J. W. (2004). Parent proxy-reported health-related quality of life and fatigue in pediatric patients diagnosed with brain tumors and acute lymphoblastic leukemia. Cancer, 101, 2116–2125.
Speechley, K. N., Barrera, M., Shaw, A. K., Morrison, H. I., & Maunsell, E. (2006). Health-related quality of life among child and adolescent survivors of childhood cancer. Journal of Clinical Oncology, 24, 2536–2543.
Bhat, S. R., Goodwin, T. L., Burwinkle, T. M., Lansdale, M. F., Dahl, G. V., Huhn, S. L., et al. (2005). Profile of daily life in children with brain tumors: An assessment of health-related quality of life. Journal of Clinical Oncology, 23, 5493–5500.
Russell, K. M., Hudson, M., Long, A., & Phipps, S. (2006). Assessment of health-related quality of life in children with cancer: Consistency and agreement between parent and child reports. Cancer, 106, 2267–2274.
Eiser, C., Vance, Y. H., Horne, B., Glaser, A., & Galvin, H. (2003). The value of the PedsQLTM in assessing quality of life in survivors of childhood cancer. Child: Care, Health and Development, 29(2), 95–102.
Shankar, S., Robison, L., Jenney, M. E., Rockwood, T. H., Wu, E., Feusner, J., et al. (2005). Health-related quality of life in young survivors of childhood cancer using the Minneapolis-Manchester Quality of Life-Youth Form. Pediatrics, 115, 435–442.
Cardarelli, C., Cereda, C., Masiero, L., Viscardi, E., Faggin, R., Laverda, A., et al. (2006). Evaluation of health status and health-related quality of life in a cohort of Italian children following treatment for a primary brain tumor. Pediatric Blood & Cancer, 46, 637–644.
Pickard, A. S., Topfer, L. A., & Feeny, D. H. (2004). A structured review of studies on health related quality of life and economic evaluation in pediatric acute lymphoblastic leukemia. Journal of the National Cancer Institute Monographs, 33, 102–125.
Foreman, N. K., Faestel, P. M., Pearson, J., Disabato, J., Poole, M., Wilkening, G., et al. (1999). Health status in 52 long-term survivors of pediatric brain tumors. Journal of Neurooncology, 41, 47–53.
Meeske, K. A., Patel, S. K., Palmer, S. N., Nelson, M. B., & Parow, A. M. (2007). Factors associated with health-related quality of life in pediatric cancer survivors. Pediatric Blood & Cancer, 49, 298–305.
Varni, J. W., Burwinkle, T. M., Katz, E. R., Meeske, K., & Dickinson, P. (2002). The PedsQL in pediatric cancer: Reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer, 94, 2090–2106.
Varni, J. W., Seid, M., & Kurtin, P. S. (2001). PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care, 39, 800–812.
Varni, J. W., Burwinkle, T. M., & Seid, M. (2006). The PedsQL 4.0 as a school population health measure: Feasibility, reliability, and validity. Quality of Life Research, 15, 203–215.
Palmer, S. N., Meeske, K. A., Katz, E. R., Burwinkle, T. M., & Varni, J. W. (2007). The PedsQL brain tumor module: Initial reliability and validity. Pediatric Blood & Cancer, 49(3), 287–293.
Varni, J. W., Katz, E. R., Colegrove, R., Jr, & Dolgin, M. (1993). The impact of social skills training on the adjustment of children with newly diagnosed cancer. Journal of Pediatric Psychology, 18(6), 751–767.
Kavale, K. A., & Forness, S. R. (1996). Social skills deficits and learning disabilities: A metaanalysis. Journal of Learning Disabilities, 29(3), 226–237.
Upton, P., Lawford, J., & Eiser, C. (2008). Parent–child agreement across child health-related quality of life instruments: A review of the literature. Quality of Life Research, 17(6), 895–913.
Varni, J. W., Seid, M., & Rode, C. A. (1999). The PedsQL: Measurement model for the pediatric quality of life inventory. Medical Care, 37(2), 126–139.
Bastiaansen, D., Koot, H. M., Bongers, I. L., Varni, J. W., & Verhulst, F. C. (2004). Measuring quality of life in children referred for psychiatric problems: Psychometric properties of the PedsQL 4.0 generic core scales. Quality of Life Research, 13(2), 489–495.
Aarsen, F. K., Paquier, P. F., Reddingius, R. E., Streng, I. C., Arts, W. F., Evera-Preesman, M., et al. (2006). Functional outcome after low-grade astrocytoma treatment in childhood. Cancer, 106, 396–402.
Maunsell, E., Pogany, L., Barrera, M., Shaw, A. K., & Speechley, K. N. (2006). Quality of life among long-term adolescent and adult survivors of childhood cancer. Journal of Clinical Oncology, 24, 2527–2535.
Szaflarski, M., & Cubbins, L. A. (2004). Self-reported health in Poland and the United States: A comparative analysis of demographic, family and socioeconomic influences. Health (London), 8(1), 5–31.
Ross, C. E., & Wu, C. L. (1996). Education, age, and the cumulative advantage in health. Journal of Health and Social Behavior, 37(1), 104–120.
Mulhern, R. K., & Butler R. W. (2004). Neurocognitive sequelae of childhood cancers and their treatment. Pediatric Rehabilitation, 7(1), 1–14 (discussion 15–16).
Luttjeboer, M., & Kaspers, G. J. (2006). Medulloblastoma: Need for targeted treatment. Expert Reviews of Anticancer Therapy, 6(5), 649–652.
Pui, C. H., Robison, L. L., & Look, A. T. (2008). Acute lymphoblastic leukaemia. Lancet, 371(9617), 1030–1043.
Acknowledgments
The Quality of Life study described in this paper was carried out using the PedsQL™, developed by Dr. James W. Varni. The Authors thank also anonymous Reviewers for their effort to improve the quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pogorzala, M., Styczynski, J., Kurylak, A. et al. Health-related quality of life among paediatric survivors of primary brain tumours and acute leukaemia. Qual Life Res 19, 191–198 (2010). https://doi.org/10.1007/s11136-009-9580-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-009-9580-1