Abstract
Stevia leaves are usually used in dried state and undergo the inevitable effect of drying process that changes the quality characteristics of the final product. The aim of this study was to assess temperature effect on Stevia leaves through analysis of relevant bioactive components, antioxidant capacity and content of natural sweeteners and minerals. The drying process was performed in a convective dryer at constant temperatures ranging from 30 to 80 °C. Vitamin C was determined in the leaves and as expected showed a decrease during drying proportional to temperature. Phenolics and flavonoids were also determined and were found to increase during drying below 50 °C. Antioxidant activity was determined by DPPH and ORAC assays, and the latter showed the highest value at 40 °C, with a better correlation with the phenolics and flavonoids content. The content of eight natural sweeteners found in Stevia leaves was also determined and an increase in the content of seven of the sweeteners, excluding steviol bioside, was found at drying temperature up to 50 °C. At temperatures between 60 and 80 °C the increase in sweeteners content was not significant. Stevia leaves proved to be an excellent source of antioxidants and natural sweeteners.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stevia rebaudiana Bertoni, a plant used for sweetening, medicinal, pharmaceutical and feeding purposes, is one of the raw materials that has been claiming great interest and demand on the global markets during the last few years [1]. Most of the studies on Stevia leaves are focused on the plant growing parameters and its adaptation to different edapho-climatic zones [2]. The medicinal and functional properties, such as antiviral and antibacterial capacities [3, 4] or antioxidant activity [5, 6] have also attracted much attention and interest, so that in published data complete and extensive research works on the extraction methods by all available technologies can be found [4, 6, 7]. The first stage in processing of Stevia leaves is by far the drying operation, commonly performed using solar energy. However, solar drying due to an intrinsic lack of systematic process control, often presents multiple disadvantages related to dried product quality, especially those aspects related to a deficient microbiological safety. In consequence, conducting research on dehydration of Stevia leaves is highly recommended. Drying by hot air is a very common and appropriate postharvest technology to extend shelf life of a product, preserving its quality and stability through reduction of moisture content. Furthermore, drying process also add value to food products and a proper management of the drying process, seen from an operational and capital investment point of view, can lead to a higher yield of a high quality product [8]. The undesirable effects of hot-air drying can be reduced to a minimum through proper monitoring of appropriate control variables, such as air drying temperature, which favors improvement of the nutritional value of the food product.
Several investigations have clearly shown the influence of drying temperature on the relevant physical and biochemical quality aspects of various fruits and vegetables, especially on attributes such as color, total phenolic content, vitamin C, β-carotene or antioxidant capacity [8, 9]. In other investigations, the effect of drying methods on some quality characteristics of Stevia leaves has been reported [10, 11]. Knowledge on these properties are necessary to establish quality standard of the dried products and also in the development of new products with desired properties or for quality improvement. Periche et al. [12] found high levels of phenolics and flavonoids compounds in Stevia infusions. Muanda et al. [13] identified 18 phenolic compounds in the Stevia leaves, which revealed a high antioxidant capacity that also come from the flavonoids like apigenin-4-O-β-D-glycoside, kaemp ferol-3-O-rhamnoside, quercetin-3-O-β-D-arabinoside, quercetin-3-O-glucoside and quercetin-3-O-rutinoside, identified by Ghanta et al. [5] and Cacciola et al. [14].
Consequently, a proper application of drying temperature that leads to final products with excellent quality characteristics is crucial. The effects of air drying temperature leading to changes in the concentration profiles of the bioactive components and antioxidant capacity in the Stevia leaves have been reported only scarcely. Therefore, the aim of this study was to quantify the effect of drying temperature in a range between 30 and 80 °C on the Stevia leaves with respect to bioactive components, antioxidant capacity and natural sweeteners.
Materials and Methods
Drying Experiments
The Stevia leaves were obtained from a local nursery at La Serena, Chile. The leaves were separated from the stalk by a clear cut, and selected to provide a homogeneous group, based on date of harvest, color and freshness according to visual analysis. Samples were kept refrigerated at 5.2 ± 0.2 °C until drying process that was performed at six experimental drying temperatures (30, 40, 50, 60, 70 and 80 °C) at a constant air velocity of 2.0 ± 0.2 m/s. The weight of Stevia leaves sample, initially at 80 ± 0.5 g (load density = 2.08 kg/m2), was monitored until achievement of equilibrium condition, using an analytical balance (Ohaus SP402, NJ, USA) with a precision of ±0.01 g connected by an interface system (Ohaus RS232, NJ, USA) to a computer, which recorded and stored data at regular time intervals.
Proximate Analysis, Water Activity and Vitamin C Content
The moisture content was determined employing a vacuum oven (Gallenkamp, OVL570, UK) and an analytical balance (CHYO, Jex120, Japan). Crude protein content was determined using the Kjeldahl method with a conversion factor of 6.25. Lipid content was analyzed gravimetrically following Soxhlet extraction. Crude ash was determined by incineration in a muffle furnace (Felisa, 360D) at 550 °C. Crude fiber was estimated by acid/alkaline hydrolysis of insoluble residues. The methods used for proximate analysis followed the recommendations of AOAC [15]. The water activity was determined at 25 °C by a Novasina water activity instrument (model TH-500, Pfaffikon, Switzerland). The determination of vitamin C was performed by certification of NBS (N-Bromosuccinimide) according to Barakat et al. [16] with some modifications. All analyses were performed in triplicate and expressed in g/100 g dry matter (d.m.).
Preparation of Stevia Leaves Extracts
Extraction was performed using 0.5 g of powdered sample of fresh or dried Stevia leaves mixed to 20 mL of aqueous methanol solution (50 %) on a shaker for 24 h [13]. After centrifugation at 5000 rpm for 25 min at room temperature, the supernatant was removed and transferred to a 25 mL volumetric flask and stored at −80 °C, until performance of the analysis for total phenolic content (TPC), total flavonoid content (TFC) and DPPH (2,2,-dyphenyl-2-picryl-hydrazyl) antioxidant activity.
Determination of Total Phenolic and Flavonoid Content
TPC was determined using Folin-Ciocalteu reagent (FC) as described by Chuah et al. [17] , but with some modifications. Absorbance was measured at 725 nm with a spectrophotometer (Spectronic R20 Genesys™, Illinois, USA) and a gallic acid calibration curve was established for quantification of TPC. Results were expressed as mg gallic acid equivalent (GAE) per 100 g dry matter (mg GAE/100 g d.m.). TFC was measured using a colorimetric assay as described by Dini et al. [18]. Absorbance was determined at 415 nm with distilled water as blank. Total flavonoids in the extracts were expressed as mg quercetin equivalent (QE) per 100 g dry matter (mg QE/100 g d.m.).
Determination of DPPH Radical-Scavenging Activity
The DPPH antioxidant capacity was determined as described by Muanda et al. [13]. Absorbance was monitored at 517 nm using a spectrophotometer (Spectronic R20 Genesys™, Illinois, USA). The antioxidant capacity was expressed as μmoles Trolox equivalent per 100 g dry matter (μmoles TE/100 g d.m.).
Determination of Oxygen Radical Absorbance Capacity (ORAC)
ORAC antioxidant activity was determined as described by Zheng and Wang [19] using sodium fluorescein solution. The fluorescence spectrophotometer was set at 485 nm for excitation and at 535 nm for emission. ORAC value was expressed in μmoles TE/100 g d.m.
Analysis of Phenolic Acids
Extraction procedures of phenolic acids followed the method described by López-Martínez et al. [20] with some modifications. The analysis of phenolic acids was performed using an Agilent 1200 HPLC system (Agilent, Palo Alto, CA, USA), equipped with a high-pressure pump, an automatic injector, a UV-visible diode array detector controlled by ChemStation software. The analytical column was a Kromasil 100-5C18; 250 × 4.6 mm (Eka Chemical, Bohus, Sweden). Selected phenolic acids (gallic, protocatechuic, chlorogenic, caffeic, syringic, vanillic, p-coumaric, trans-sinapic, ellagic, salicylic, trans-ferulic and trans-cinnamic acids) and rutin (quercetin-3-O-rutinoside, a glycoside between the flavonol quercetin and the disaccharide rutinose or α-L-rhamnopyranosyl-(1 → 6)-β-D-glucopyranose) in a methanol-formic acid (99:1) solvent were identified by comparison of retention times, spectra and peak area at maximum absorption wavelength. The content of phenolic compounds were expressed in mg/100 g d.m.
Determination of Sweeteners Content
The determination of sweeteners content (steviol glycosides) was performed according to The Compendium of Food Additive Specifications from Joint FAO/WHO Expert Committee on Food Additives [21]. The method determines the percentages of the individual steviol glycosides by HPLC (Agilent, Palo Alto, CA, USA) using Stevioside (>99.0 % purity) and Rebaudioside A (>97 % purity) as standards. The contents of the seven main steviol glycosides (Rebaudioside A, Rubusoside, Dulcoside, Steviolbioside, Stevioside, Rebaudioside C and Rebaudioside B) was determined and the total content of sweeteners was the sum of the individual steviol glycosides [21].
Determination of Minerals
Mineral elements (K, Ca, Mg, Na, Cu, Mn, Zn, and Fe) in the Stevia leaves were determined using an atomic absorption spectrophotometer (AAS, Shimadzu Instruments, Inc., SpectrAA-220, Kyoto, Japan) after digestion in a mixture of H2SO4, HNO3 and HClO4 in equal proportion. All determinations were performed in triplicate and expressed in mg/100 g d.m..
Statistical Analysis
Data were performed through an analysis of variance (ANOVA) using the Statgraphics®5.1. software. Differences among mean values were established by the LSD at 5 %. In addition, a multiple range test (MRT) was used to demonstrate the existence of homogeneous groups within each of the parameters.
Results and Discussion
Drying Process
In this work, the drying assays performed at a constant air velocity and different air drying temperatures maintained constant at 30, 40, 50, 60, 70 or 80 °C during the whole process, achieved a moisture ratio around 0.01 (1 %) after a drying time of 570, 480, 270, 180, 120 and 60 min, respectively. Analysis of the assays showed that both drying temperature and drying time have a significant effect on the bioactive compounds, which means that any report on the results of a drying process should definitely mention the drying conditions.
Physicochemical Characteristics
In Table 1 values of the proximate composition (moisture, lipid, total ash, protein, crude fiber and carbohydrates) for both fresh and dried Stevia leaves are shown. Influence of process temperature on the proximate composition was observed with significant differences (p < 0.05) between the Stevia leaves samples, all dried at different temperatures. In the fresh Stevia leaves, a moisture content of 76.64 ± 0.37 % wet basis (w.b.) was determined, indicating susceptibility of the fresh leaf to oxidation and enzymatic reactions. It should be noted that initial moisture value of the fresh Stevia leaves was almost of the same order as moisture values reported by Kaya and Aydin [22] for herbal leaves (80 % w.b.). Moisture content of dried Stevia leaves has been reported to lie in a range between 4.5 and 7.7 g/100 d.m. [1, 23, 24], but without stating drying conditions and methods. In our study, as it can be observed in Table 1, this range of moisture content was achieved during convective drying of Stevia leaves at temperatures between 30 and 40 °C. Moisture content may be reduced to a value near to 1 g/100 g d.m. at a drying temperature of 80 °C, which brought water activity down to 0.04 ± 0.01. The drying operation also led to a relative increase in the content of lipid, ash and protein and a relative decrease in the content of carbohydrate and fibre. The increase in fat, ash and protein content as drying temperature increased may be significant but is quite small and is probably due to intrinsic variation in composition of natural food products. However, the decrease in carbohydrate and fiber content as process temperature increased may be the result of enhanced thermal degradation of both compounds especially during the initial drying period. The values of the proximate analysis in Table 1 are similar to values reported in other investigations on dehydrated Stevia leaves that showed lipid, protein, carbohydrate, fiber and ash values to lie between 3.0 and 6.0, 9.8–12, 35.2–61.9, 15.0–18.5 and 6.3–13.1 g/100 d.m., respectively [1, 23, 24].
In Table 1 the contents of vitamin C in the fresh and dried Stevia leaves are also shown, with a significant (p < 0.05) decrease during the drying process at all temperatures. The content of vitamin C in dehydrated Stevia leaves decreased from 130.3 ± 3.4 mg/100 g d.m. in the fresh leaves to 74.1 ± 0.4, 60.9 ± 0.7 and 50.1 ± 0.7 mg/100 g d.m. in the leaves dried at temperatures of 40, 60 and 80 °C, respectively, which meant a reduction to 57, 47 and 38 % of the initial content for the respective drying temperatures. At 30 °C, the dry matter content of the dried Stevia leaves was about 94 % and the content of vitamin C retained in the leaves was 73.7 ± 1.2 mg/100 g wet basis. At 80 °C the dry matter content is around 99 % and the retained content of vitamin C was 49.5 ± 0.5 mg/100 g wet basis. Therefore, the content of vitamin C found in the dried Stevia leaves decreased as drying temperature increased, although it still remained relatively high, which may be considered a valuable asset for preparation of a naturally sweetened herbal infusion in tea mixtures or as water-based extracts in desserts and milk products or directly by the end-user in jams, stewed fruits, etc. On the other hand, the presence of vitamin C in the dried leaves may be used as a quality index and also as an evaluating criterion for thermal treatment. In comparison, in fresh leaves of peppermint (Mentha piperita) and spearmint (Mentha spicata), contents of vitamin C of 140 ± 10 and 480 ± 30 μg/kg fresh tissue were found, respectively [25].
Total Phenolics and Flavonoids Content
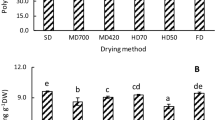
In Fig. 1, contents of total phenolics (TPC) and total flavonoids (TFC) are shown. TPC of 28.76 ± 2.94 mg GAE/100 g d.m. and TFC of 88.65 ± 2.03 mg QE/100 g d.m. were determined in the fresh Stevia leaves. The presence of total phenolics and total flavonoids is a positive indication for the antioxidant properties of Stevia leaves. After air drying (temperature not reported), Tadhani et al. [6] determined a TPC of 25.18 mg GAE/g d.m. and Shukla et al. [26] a TPC of 56.74 mg GAE/g d.m. Stevia leaves. For shade drying, Ghanta et al. [5] reported a value of 0.86 mg GAE/mg d.m. in ethyl acetate extract of Stevia leaves. Similarly, Kim et al. [27] reported TPC of 130.67 mg catechin/g d.m. for air drying of Stevia leaves at 40 °C for 12 h. For TFC, Tadhani et al. [6] reported 21.73 mg GAE/g d.m., Ghanta et al. [5] 0.83 mg QE/mg d.m., Kim et al. [27] 15.64 mg QE/g d.m. and Muanda et al. [13] 20.68 mg catechin/g d.m. for drying at room temperature. In this work, drying process at 30, 40 and 50 °C brought a significant (p < 0.05) increase in TPC and TFC reaching the highest value for both TPC and TFC in leaves dried at 40 °C. TPC almost doubled its initial value with an increase of 91 % (55.05 ± 2.27 mg GAE/100 g d.m.), while TFC increased by 57 % (138.97 ± 3.74 mg QE/100 g d.m.). This increase is related to the formation of phenolic compounds usually associated to availability of precursors of phenolic molecules arising from non-enzymatic inter-conversion between the phenolic molecules [8]. Similarly, TPC increased from 9.6 mg GAE/100 g d.m. in fresh sage to over 30.0 mg GAE/100 g d.m. during air drying at room temperature, while TPC in thyme changed from 20.0 to 112.0 mg GAE/100 g d.m. during similar drying operation. Drying of Stevia leaves at temperatures higher or equal to 60 °C caused on the contrary a slight decrease in TPC and TFC. Drying at 80 °C reduced TPC to 21.11 ± 1.02 mg GAE/100 g d.m. equivalent to 73 % of the original value, while TFC is reduced to 71.87 ± 1.33 mg QE/100 g d.m, equivalent to only 81 % of the initial value, which meant that under the controlled air drying conditions drastic losses of antioxidant compounds did not occur. On the contrary, low temperature drying below 50 °C would increase TPC and TFC in the dried products. According to Mrad et al. [9], a decrease in TPC during drying may be attributed to the binding of polyphenols with other compounds such as proteins, or to alterations in the chemical structure of polyphenols that cannot be extracted nor determined by available methods. In contrast to the results reported in this work, Periche et al. [10] reported an increase in TPC and TFC of dried Stevia leaves. During convective drying at 180 °C for 3 min TPC increased from 44.40 ± 1.04 mg GAE/g d.m. in the fresh Stevia leaves to 76.8 mg GAE/g d.m. in the dried leaves, while TFC increased from 2.52 ± 0.24 to 45.1 mg catechin equivalent/g d.m. Drying at 100 °C for 3 min showed on the other hand a decrease in TPC to 31.5 mg GAE/g d.m., but an increase in TFC to 17.2 mg catechin equivalent/g d.m. During shade drying for 30 days at 20 °C TPC decreased slightly to 39.1 mg GAE/g d.m., while TFC increased to 20.3 mg catechin equivalent/g d.m. A comparison with the results of this study would indicate the concurrent effects of temperature and drying time on the biochemical changes occurring during the drying process.
Effect of drying temperature on total phenolic and flavonoid content of Stevia leaves. Identical lowercase letters above the bars indicate no significant differences (p < 0.05) in TPC values. Identical uppercase letters above the bars indicate no significant differences (p < 0.05) in flavonoids values. TPC total phenolics, TFC total flavonoids
Antioxidant Capacity
Total antioxidant activity of plant and fruit tissues are usually evaluated and expressed as Trolox equivalent (TE), since the occurrence of different antioxidants would require sophisticated measuring techniques to identify and to evaluate individually all the antioxidant compounds. In this study, the antioxidant capacity associated with phenolic compounds in Stevia leaves was evaluated by means of DPPH and ORAC assays. During the drying process compounds having a varying degree of antioxidant activity are generated and accumulated, and antagonistic or synergistic effects with themselves or with the other constituents of samples could also develop. These complex chemical interactions that influence functional properties of food are still under investigation. The DPPH radical scavenging activity as affected by air drying process can be seen in Fig. 2. Fresh Stevia leaves had a value of 3.29 ± 1.33 μmoles TE/100 g d.m. for DPPH and 222.59 ± 7.96 μmoles TE/100 g d.m. for ORAC assays. In any of the dried Stevia leaves samples DPPH radical scavenging activity was not significantly different (p > 0.05), reaching a mean value of 2.79 ± 0.02 μmoles TE/100 g d.m. in the leaves dried at 80 °C. For ORAC assays, the highest value for antioxidant activity was obtained in Stevia leaves dried at 40 °C, which coincided with the highest TPC, also showing a correlation of antioxidant activity with TPC. A similar pattern of behavior was observed for TFC and ORAC values. TFC and ORAC value are highest at 40 °C and lowest at 60 °C. The amount of total phenolic and total flavonoid may be better correlated to ORAC value than to the DPPH radical scavenging activity. In contrast to this study, an antioxidant activity of 52.92 ± 0.84 mg TE/g d.m., measured as DPPH radical scavenging activity of methanolic extract of fresh Stevia leaves, was reported [12]. This antioxidant activity increased to 126 and 64.9 mg TE/g d.m. after drying for 3 min at 180 °C and 100 °C, respectively, which could mean that any synergistic effect that could be developed is stronger at very high drying temperatures than at temperatures below 80 °C. Therefore, drying was found to have a positive effect on the antioxidant activity of Stevia leaves, and dried Stevia leaves could be considered an important source of bioactive components with high antioxidant activity.
Effect of drying temperature on ORAC and DPPH free radical-scavenging activity in Stevia leaves. Identical lowercase letters above the bars indicate no significant differences (p < 0.05) on DPPH values
Identical uppercase letters above the bars indicate no significant differences (p < 0.05) on ORAC values
Phenolic Acid Content
The phenolic acids can be distinguished either as derivatives of benzoic acid or as derivatives of cinnamic acid. The hydroxycinnamic acids are more common than the hydroxybenzoic acids and consist chiefly of p-coumaric, caffeic, ferulic and sinapic acids [28, 29]. The phenolic acids present in Stevia leaves are shown in Table 2. They were mainly the hydroxycinnamic acids: chlorogenic, caffeic and trans-ferulic acids. Gallic, protocatechuic, syringic, vanillic, p-coumaric, trans-sinapic, ellagic, salicylic, and trans-cinnamic acids were not detected [28]. Chlorogenic acid was found as the most abundant phenolic acid in the Stevia leaves. Rutin or quercetin-3-O-rutinoside, reported by Cacciola et al. [14] to be present in Stevia leaves, was also detected. It can be seen in Table 2 that except for caffeic acid, the concentration of the three detected phenolic compounds increased during drying process, although concentration of chlorogenic acid seemed unaffected by drying temperature. However, in the case of chlorogenic acid and the flavonoid rutin some tendency can be observed, with the highest concentration of the two compounds occurring in the product dried at 40 °C, which may indicate a favorable drying condition for the Stevia leaves. The naturally occurring phenolic compounds are antioxidants, which have been shown to have potential health effects [28, 29]. Ferulic acid and rutin are also known for their antibacterial activity. Chlorogenic acid, caffeic and trans-ferulic acids are typical of the family of esters formed between quinic acid and certain trans-hydroxycinnamic acids and are commonly found in coffee and leaves of many plants [28, 30].
Sweeteners Content
The Stevia leaves investigated in this study were characterized by very low amount of steviol glycosides. In terms of percentage of dry matter (g/100 g d.m.), the seven major steviol glycosides detected in fresh Stevia leaves were stevioside with 3.44 %, steviolbioside (2.01 %), rebaudioside A (1.16 %), rubusoside (0.53 %), dulcoside (0.21 %), rebaudioside C (0.16 %), and rebaudioside B (0.11 %). These values were close to those reported by Lemus-Mondaca et al. [1] and Gardana et al. [31]. Total steviol glycosides content in fresh and dried Stevia leaves were found to vary between 7.62 and 21.42 g/100 g d.m. [1]. Moreover, Shafii et al. [32] found in Stevia leaves extracts stevioside (2 to 125 mg/g d.m.), rebaudioside A (2.5 to 164 mg/g d.m.) and rebaudioside C (1.5 to 125 mg/g d.m.). Gardana et al. [31] reported per 100 g d.m. of Stevia leaves 5.8 g of stevioside, 1.8 g of rebaudioside A, 1.3 g of rebaudioside C and 0.7 g of dulcoside A. Woelwer-Rieck et al. [33] reported for dried Stevia leaves obtained from cultivation under different soil conditions, fertile sandy loam and light loamy soil varying concentration of stevioside (79 ± 2.9 and 77.8 ± 6.1 mg/g d.m.) and rebaudioside A (49.3 ± 4.4 and 42.8 ± 2.9 mg/g d.m.). In this work the values obtained for stevioside varied from 34.4 to 123.6 mg/g d.m. and rebaudioside A varied from 11.6 to 40.5 mg/g d.m., which may be considered of the same order of magnitude. However, it may also be a clear evidence of agronomical factors affecting sweeteners content in the leaves, which would definitely need further investigation. In Table 3 the contents of steviol glycosides examined and quantified in this study for each drying temperature can be seen. In all dried Stevia leaves, the predominating steviol glycoside was stevioside. It is worth mentioning that rebaudiosides A and C, very often used to quantify sweeteners potential, were found only in relatively small amounts in the analyzed Stevia leaves. The drying process of Stevia leaves at temperatures from 30 to 80 °C brought about a general increase in content of the detected steviol glycosides, except for steviolbioside that decreased to about 10 % of its original content at drying temperatures over 50 °C, which may be considered as a decomposition due to heat treatment. On the other hand, the increase in sweeteners concentration as a consequence of drying process has also been reported by other authors using freeze drying and shade drying [10]. Therefore, it may be probable that the Stevia leaves contain precursors of the steviol glycosides that chemically react during heating to form the corresponding sweeteners. For most of the sweeteners, a positive increase is observed especially at drying temperatures up to 50 °C, which is an air temperature that usually occurs in the natural habitat of the Stevia plant.
Minerals Content
Minerals, usually required only in very small amounts in human diets, are vital for many metabolic purposes in the human body and are thus called essential trace elements. The main mineral elements sodium, magnesium, phosphorus, sulphur, chlorine, potassium, and calcium are classified as macronutrients, while the minor elements chromium, manganese, iron, cobalt, copper, zinc, selenium, molybdenum and iodine are considered micronutrients. In Table 4 the content of eight essential minerals, i.e., sodium, potassium, calcium, magnesium, copper, iron, manganese, and zinc in Stevia leaves, fresh and dried at different temperatures, are shown. Potassium, calcium, magnesium, and sodium which are nutritionally important, were found in reasonable amount in the analyzed samples of Stevia leaves. The high concentration of these minerals would be very beneficial to health. As reported by many authors potassium predominates, followed by calcium and magnesium or sodium [1]. The high content of potassium determined in all studies is remarkable, and is once again confirmed in this study. Potassium is essential as an activator for enzymes involved in the synthesis of certain peptide bonds [34]. Zinc and iron are found in foods of plant and animal origin and were also present in the Stevia leaves. Zinc is known to be essential for the function and structure of several enzymes, like peptidase, transphosphorylase and others. It is also an essential component of both DNA and RNA polymerase and is well-known for its anti-viral, anti-bacterial, anti-fungal and anti-cancer properties [35]. Iron is important for the biological function of oxygen transport to the body and consequently a lack of this mineral in the diet leads to anaemia. Manganese and copper were also determined in the Stevia leaves. Manganese has an important function in the respiration and nitrogen metabolism, while copper acts as co-enzyme of phenolase, laccase and ascorbic acid oxidase [34]. Apart from sodium that showed a significant decrease in the dried Stevia leaves samples, all the other minerals found in the dried Stevia leaves were of same order of magnitude as in the fresh leaves. The significant differences in minerals content found between the fresh and dried samples were most probably not an effect of the drying process, but may be due rather to a heterogeneous distribution of the minerals in the analyzed sample. It could also mean that the minerals are not evenly distributed in the different plant tissues of Stevia.
Conclusions
Biochemical changes occurred during drying process with significant effect of air drying temperature and drying time, so that the conditions of the drying process should always be mentioned. The content of vitamin C in the dried Stevia leaves decreased as drying temperature increased, although it remained relatively high, which may be considered a valuable asset in preparation for herbal tea mixtures or water-based extracts. Drying process between 30 and 50 °C brought a significant increase in TPC and TFC reaching the highest value in both cases in leaves dried at 40 °C. Antioxidant activity measured as ORAC also showed the highest value at 40 °C, indicating a probable favorable drying condition. Chlorogenic acid, found as the most abundant phenolic acid in Stevia leaves, was unaffected by drying temperatures. Sweeteners content was found to increase especially at drying temperature up to 50 °C, indicating the probable presence of precursors that are transformed during the drying process. Mineral content during drying between 30 and 80 °C did not show a clear tendency to increase or decrease, probably due to uneven distribution in the Stevia plant tissues.
References
Lemus-Mondaca R, Zura-Bravo L, Vega-Gálvez A, Ah-Hen K (2012) Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: a comprehensive review on the biochemical, nutritional and functional aspects. Food Chem 132:1121–1132
Rahmesh K, Singh V, Megeji N (2006) Cultivation of Stevia (Stevia rebaudiana Bertoni): a comprehensive review. Adv Agron 89:137–177
Kedik S, Yartsev E, Stanishevskaya I (2009) Antiviral activity of dried extract of Stevia. Pharm Chem J 43:198–199
Ghosh S, Subudhi E, Nayak S (2008) Antimicrobial assay of Stevia rebaudiana Bertoni leaf extracts against 10 pathogens. Int J Integr Biol 2:27–31
Ghanta S, Banerjee A, Poddar A, Chattopadhyay S (2007) Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener. J Agric Food Chem 55:10962–10967
Tadhani MB, Patel VH, Subhash R (2007) In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J Food Comp Anal 20:323–329
Tadhani MB, Subhash R (2006) Preliminary studies on Stevia rebaudiana leaves: proximal composition, mineral analysis and phytochemical screening. J Med Sci 6:321–326
Kerkhofs N, Lister C, Savage G (2005) Change in colour and antioxidant content of tomato cultivars following forced-air drying. Plant Foods Hum Nutr 60:117–121
Mrad ND, Boudhrioua N, Kechaou N, Courtois F, Bonazzi C (2012) Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod Process 90:433–441
Periche A, Castelló ML, Heredia A, Escriche I (2015) Influence of drying method on steviol glycosides and antioxidants in Stevia rebaudiana leaves. Food Chem 172:1–6
Gasmalla MA, Yang R, Amadou I, Hua X (2014) Nutritional composition of Stevia rebaudiana Bertoni leaf: effect of drying method. Trop J Pharm Res 13:61–65
Periche A, Koutsidis G, Escriche I (2014) Composition of antioxidants and amino acids in Stevia leaf infusions. Plant Foods Hum Nutr 69:1–7
Muanda F, Soulimani R, Diop B, Dicko A (2011) Study on chemical composition and biological activities of essential oil and extracts from Stevia rebaudiana Bertoni leaves. LWT Food Sci Technol 44:1865–1872
Cacciola F, Delmonte P, Jaworska K, Dugo P, Mondello L, Rader J (2011) Employing ultra-high pressure liquid chromatography as the second dimension in a comprehensive two-dimensional system for analysis of Stevia rebaudiana extracts. J Chromatogr A 1218:2012–2018
AOAC (1990). Association of Official Analytical chemists. Official Method of Analysis no. 920.39, 962.09 and 934.06, 15th edn, Arlington, VA, USA
Barakat MZ, El-Wahab MF, El-Sadr MM (1955) Action of N-bromosuccinimide on ascorbic acid. Biochemistry Department, Faculty of Medicine, Abbassia, Cairo, Egypt. Anal Chem 27:536–540
Chuah AM, Lee Y-C, Yamaguchi T, Takamura H, Yin L-J, Matoba T (2008) Effect of cooking on the antioxidant properties of coloured peppers. Food Chem 111:20–28
Dini I, Tenore GC, Dini A (2010) Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT - Food Sci Technol 43:447–451
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170
López-Martínez LX, Oliart-Ros RM, Valerio-Alfaro G, Lee C-H, Parkin KL, Garcia HS (2009) Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT - Food Sci Technol 42:1187–1192
JECFA (2008). Joint FAO/WHO expert committee on food additives. Steviol glycosides. In: Compendium of food additive specifications, 69th meeting, FAO/WHO monographs 5, Rome, Italy
Kaya A, Aydin O (2009) An experimental study on drying kinetics of some herbal leaves. Ener Conv Manage 50:118–124
Abou-Arab A, Abou-Arab A, Abu-Salem M (2010) Physicochemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana Bertoni plant. Afr J Food Sci 4:269–281
Kaushik R, Pradeep N, Vamshi V, Geetha M, Usha A (2010) Nutrient composition of cultivated Stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. J Food Sci Technol 47:27–33
Curutchet A, Dellacassa E, Ringuelet J, Chaves A, Viña S (2014) Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha piperita and Mentha spicata). Food Chem 143:231–238
Shukla S, Mehta A, Menta P, Bajpai V (2012) Antioxidant ability and phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Exp Toxicol Pathol 64:807–811
Kim I, Yang M, Lee O, Kang S (2011) The antioxidant activity and the bioactive compound content of Stevia rebaudiana water extracts. LWT Food Sci Technol 44:1328–1332
Karaköse H, Jaiswal R, Kuhnert N (2011) Characterization and quantification of hydroxycinnamate derivatives in Stevia rebaudiana leaves by LC-MSn. J Agric Food Chem 59:10143–10150
El Gharras H (2009) Polyphenols: food sources, properties and applications – a review. Int J Food Sci Technol 44:2512–2518
Jaiswal R, Patras MA, Eravuchira PJ, Kuhnert N (2010) Profile and characterization of the chlorogenic acid in green Robusta coffee beans by LC-MSn: identification of seven classes of compounds. J Agric Food Chem 58:8722–8737
Gardana C, Scaglianti M, Simonetti P (2010) Evaluation of steviol and its glycosides in Stevia rebaudiana leaves and commercial sweetener by ultra-high performance liquid chromatography-mass spectrometry. J Chromatogr A 1217:1463–1470
Shafii B, Vismeh R, Beaudry R, Warner R, Jones A (2012) Large-scale profiling of diterpenoid glycosides from Stevia rebaudiana using ultrahigh performance liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 403:2683–2690
Woelwer-Rieck U, Lankes C, Wawrzun A, Wüst M (2010) Improved HPLC method for the evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. Euro Food Res Technol 231:581–588
Alabi D, Alausa A (2006) Evaluation of the mineral nutrients and organic food contents of the seeds of Lablab purpureus, Leucaena leucocephala and Mucuna utilis for domestic consumption and industrial utilization. World J Agric Sci 2:115–118
Brisibe E, Umoren U, Brisibe F, Magalhäes P, Ferreira J, Luthria D, Wu X, Prior R (2009) Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem 115:1240–1246
Acknowledgments
The authors gratefully acknowledge the financial support provided by FONDECYT REGULAR PROJECT N°1130558 for publication of this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lemus-Mondaca, R., Ah-Hen, K., Vega-Gálvez, A. et al. Stevia rebaudiana Leaves: Effect of Drying Process Temperature on Bioactive Components, Antioxidant Capacity and Natural Sweeteners. Plant Foods Hum Nutr 71, 49–56 (2016). https://doi.org/10.1007/s11130-015-0524-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0524-3