Abstract
In plants, green non-foliar organs are able to perform photosynthesis just as leaves do, and the seed-enclosing pod acts as an essential photosynthetic organ in legume and Brassica species. To date, the contribution of pod photosynthesis to seed yield and related components still remains largely unexplored, and in Arabidopsis thaliana, the photosynthetic activity of the silique (pod) is unknown. In this study, an Arabidopsis glk1/glk2 mutant defective in both leaf and silique photosynthesis was used to create tissue-specific functional complementation lines. These lines were used to analyze the contribution of silique wall photosynthesis to seed yield and related traits, and to permit the comparison of this contribution with that of leaf photosynthesis. Our results showed that, together with leaves, the photosynthetic assimilation of the silique wall greatly contributed to total seed yield per plant. As for individual components of yield traits, leaf photosynthesis alone contributed to the seed number per silique and silique length, while silique wall photosynthesis alone contributed to thousand-seed weight. In addition, enhancing the photosynthetic capacity of the silique wall by overexpressing the photosynthesis-related RCA gene in this tissue resulted in significantly increased seed weight and oil content in the wild-type (WT) background. These results reveal that silique wall photosynthesis plays an important role in seed-related traits, and that enhancing silique photosynthesis in WT plants can further improve seed yield-related traits and oil production. This finding may have significant implications for improving the seed yield and oil production of oilseed crops and other species with pod-like organs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meeting the increasing food demands of the growing world population requires increasing the yield of staple crops. Traditional agriculture coupled with selective breeding for improved morphological traits as well as the increased availability of high-quality irrigation and fertilizer has made great contributions to sustained increases in yield, but these increases are neither sufficient nor sustainable in the future (Murchie et al. 2009). While 90–95% of crop yield is produced by photosynthesis, improving photosynthetic efficiency has played only a minor role in the remarkable increases in crop production over the past 50 years, and further increases in potential yield will rely largely on improving photosynthesis (Zhu et al. 2010). Therefore, it is important to employ our extensive knowledge of this fundamental process to improve crop productivity (Singh et al. 2014).
In addition to green leaves, which are the primary sources of photosynthate production, higher plants can use nearly all vegetative and reproductive structures to perform photosynthetic CO2 assimilation. Green leaves, stems, and green sterile flower organs optimized for light harvesting and photosynthetic performance are characterized by net photosynthetic assimilation using atmospheric carbon dioxide (Aschan and Pfanz 2003; Raven and Griffiths 2015). Evidence of photosynthetic activity in non-leaf organs has been found in, siliques (pods) of Brassica species (Singal et al. 1987; Sharma and Ghildiyal 1992; Mogensen et al. 1997; Hua et al. 2012), pods of legume plants (Crookston et al. 1974; Atkins et al. 1977; Quebedeaux and Chollet 1975; Zhang et al. 2017), rice spikelets (Imaizumi et al. 1997), wheat ears and awns (Tambussi et al. 2005; Li et al. 2006; Zhou et al. 2016), peach and tomato fruit (Pavel and DeJong 1993; Xu et al. 1997), cotton bracts and capsules (Hu et al. 2012), and green seeds of rapeseed and Arabidopsis thaliana (Ruuska et al. 2004; Allorent et al. 2015).
Among non-foliar organs, pods (also termed “siliques” in the Brassicaceae) are optimized for active photosynthesis. Pod photosynthetic assimilation is dominated by the activity of the pod wall, with limited photosynthesis also occurring in the seed embryos within the pod (Allorent et al. 2015). Research on pod photosynthesis has been performed mostly in legume and Brassica species. It has demonstrated that pod wall photoassimilation plays an essential role in provisioning a carbon supply for seed development and yield production (Bennett et al. 2011). Studies on soybean revealed that pod wall photosynthesis contributed 7.34–15.06% of all seed weight (Yang et al. 2008). Shading pods in alfalfa plants significantly reduced both total pod weight and seed weight per pod, and it was suggested that the contribution to the both total pod weight and seed weight per pod of pod wall photosynthesis was similar to that of the leaves in the early and last stages of seed filling (Wang et al. 2016). In Brassica species, the silique wall serves as a major photosynthetic organ during seed filling and oil synthesis. Its photosynthetic contribution to seed yield is much more substantial, and can reach as high as 70–100% (Singal et al. 1995). In Brassica juncea plants, shading treatments applied to siliques caused 95% seed yield loss by decreasing seed number per silique (SNS) and total seed size (Sharma and Ghildiyal 1992). Results from rapeseed indicated that silique photoassimilation was a major contributor to seed weight, and that the photosynthetic activity of the silique wall was highly associated with seed oil content (Hua et al. 2012). The results of defoliation experiments on four Brassica species suggested that silique wall and leaf photosynthesis were complementary in determining seed yield, although the relative contribution of silique wall photosynthesis varies considerably among species (Ramana and Ghildiyal 1997). However, more research on the contribution of pod photosynthesis to seed yield and its related components is necessary. Moreover, it will also be important to ask how the knowledge of silique wall photosynthesis can be used to improve crop seed yield (Bennett et al. 2011). Even in A. thaliana, the importance of the silique as a site of photosynthetic activity remains unclear.
In this study, we used a glk1/glk2 double mutant in A. thaliana which is deficient in both leaf and silique photosynthesis (Fitter et al. 2002; Waters et al. 2008, 2009) to study functional complementation. We were able to restore both silique wall and leaf photosynthesis. Furthermore, we performed analyses of the relative contribution of silique wall and leaf photosynthesis to yield-related traits. Last, to test whether enhancing silique photosynthesis can further improve yield-related traits in the wild-type (WT) accession, we overexpressed the photosynthesis-related gene RCA in silique wall tissue, and compared its performance to that of leaf tissue.
Materials and methods
Constructs and plant transformation
For the construction of tissue-specific expression vector, leaf-specific promoter PL (PAt4G14400) and the silique wall-specific promoter PS (PAt1G56100) (Guo et al. 2015) were cloned into pBI vector to produce pBIPL and pBIPS vectors.
For making the functional complementation constructs, full-length GLK1 (AT2G20570) gene was digested with KpnI and cloned into pBIPL and pBIPS vectors in front of the tissue-specific promoter, thus produced the PLG1 and PSG1 constructs. The complementation constructs were introduced into glk1/glk2 double mutant with Agrobacterium tumefaciens strain GV3101 using the floral dip method (Clough and Bent 1998).
For making the RCA overexpression constructs, full-length RCA (AT2G39730) gene was digested with KpnI and cloned into pBIPL and pBIPS vectors in front of the tissue-specific promoter, thus produced the PLRCA and PSRCA constructs. The RCA overexpression constructs were transformed into Arabidopsis with Agrobacterium tumefaciens strain GV3101 using the floral dip method (Clough and Bent 1998).
Plant materials and growth conditions
WT, glk1/glk2 mutant, PLG1/PSG1, and PLRCA/PSRCA transgenic Arabidopsis plants were grown in pots with compost soil. The glk1/glk2 mutant is a homozygous dSpm insertional double mutant kindly provided by the group of Professor Jane A. Langdale (Fitter et al. 2002; Waters et al. 2008, 2009). Seeds of T5 generation homozygous transgenic lines (screened by herbicide Basta) were pre-incubated in the dark for 3 days at 4 C before transferring to a growth room with a continuous artificial light period of 16 h (22 °C) and a dark period of 8 h (20 °C) at a photon flux density of 100 µmol m−2 s−1 and a humidity of 50% ± 5%.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis
The RNA was isolated from leaf tissues using an RNA extraction kit (Takara, Dalian), according to the manufacturer’s instructions. The first-strand cDNA was synthesized by the Prime Script RT reagent Kit (Takara, Dalian). Real-time quantitative PCR was performed using 2 µl of cDNA in a 20 µl reaction volume with SYBR Premix Ex Taq (Takara) on a 7500-Fast real-time PCR System (Applied Biosystems). Gene-specific primers were designed (Supplemental Table S1). The Arabidopsis Actin1 (At2g37620) gene was used as an internal control to normalize the expression level of the target gene (Liu et al. 2012). Each treatment was repeated three times independently. The thermal cycler was set as follows: an initial incubation at 50 °C for 2 min and 95 °C for 5 min, followed by 40 cycle at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s. The relative quantification of transcription levels was determined by the methods described previously (Livak and Schmittgen 2001).
Chlorophyll content and photosynthesis detection
Tissue samples (1.0 g) were obtained from fully expanded leaves and 10-day siliques after flowering. After the encapsulated ovules were removed, the silique wall tissue was used for extraction. At least three replicates were taken from different plants of each line. After freezing in liquid nitrogen, the samples were ground to a power in 50 ml of extraction solution (2:1 acetone:95% alcohol) and incubated in the dark at 4 °C overnight to ensure complete extraction of chlorophyll. The cell debris was pelleted by centrifugation for 1 min at 15,000×g, and absorbance of the supernatant was measured at 646.6, 663.6, and 750 nm, as described previously (Liu et al. 2012). Chlorophyll content was expressed in micrograms.
The leaf photosynthetic parameters were measured with a portable photosynthesis system LI-6400XT (LI-COR, USA) adapted with a needle chamber in the growth room. The measurement conditions were as follows: 22 °C, a photon flux density of 100 µmol m−2 s−1, and a humidity of 50% ± 5%. All data were determined as the means of eight plants.
Seed oil content determination
Seed oil content was determined using a Foss NIRSystems 5000 near-infrared reflectance spectroscope according to the WinISI III manual instructions for routine analysis (Foss NIRSystems Inc., http://www.foss-nirsystems.com). The oil content calibration equation was determined using a modified partial least squares regression method (Wang et al. 2010).
Yield analysis
Mature plants were harvested and immediately used for analysis on the traits of silique number per plant (SNP), silique length, and SNS. SNP was expressed as effective SNP in this study. Sixteen plants of each line and six siliques per plant were used for analysis of the above traits. Then seeds were dried and processed for analysis on the traits of seed yield per plant (SYP), thousand-seed weight (TSW), and seed oil content. For RCA overexpressing plants, four independent lines were used in the analysis of yield-related traits for both PLRCA and PSRCA overexpressors.
Contribution analysis of leaf and silique photosynthesis
To conduct a simplified analysis, we presumed that photoassimilates for seed yield-related traits were contributed only via leaf and silique photosynthesis. The relative photosynthetic contributions of silique and leave tissues to seed-related traits were assessed by determining the degree of recovery of each individual trait by comparing complemented lines with mutant and WT plants. The contribution and relative contribution were calculated as follows:
Results
Tissue-specific restoration of silique wall and leaf photosynthesis in a photosynthesis-defective mutant
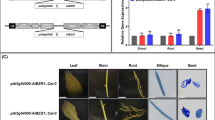
To investigate the effect of silique wall photosynthesis on seed yield and related traits, we used a special mutant defective in both leaf and silique photosynthesis, which has mutations at both the AtGLK1 and AtGLK2 loci. These loci are responsible for coordinating photosynthesis in A. thaliana (Fitter et al. 2002; Waters et al. 2008, 2009). The resulting glk1/glk2 mutant plants exhibit a pale green phenotype in both leaf and silique tissues throughout development (Fig. 1a, b and Supplemental Fig. S1). Compared to WT plants, the net photosynthetic rate (NPR) of mutant leaves was decreased by about 40% (Fig. 1c), suggesting a similar decrease of NPR in mutant siliques, although the precise value is difficult to measure. To restore photosynthetic function in mutant silique and leaf tissues, we performed functional complementation by introducing a full-length GLK1 gene into the glk1/glk2 mutant. This introduced GLK1 gene was driven by two tissue-specific promoters, PL and PS. The PL promoter is leaf-specific, while PS is active only in the silique wall (Supplemental Fig. S2; Guo et al. 2015). The two complemented lines were named PLG1 and PSG1, respectively.
Phenotype, chlorophyll content, NPR and gene expression in glk1/glk2 mutant and complemented plants comparing with WT. glk1/2, glk1/glk2 double mutant; G1, GLK1; SW, silique wall. Error bars indicate ± SE of three biological repeats. The different letters indicate significant differences (P < 0.05) among treatments as determined by the Duncan’s test. a Difference in leaf color of glk1/glk2 mutant and complemented plants comparing with WT. b Chlorophyll content in glk1/glk2 mutant, complemented plants and WT. c Leaf net photosynthesis rate (NPR) of glk1/glk2 mutant and complemented plants comparing with WT. d GLK1 expression in leaf and silique wall tissues of glk1/glk2 mutant, complemented plants and WT. e GLK2 expression in leaf and silique wall tissues of glk1/glk2 mutant, and WT
The complemented lines were produced using genetic transformation. PLG1 plants exhibited leaves with a normal green color throughout, except for the siliques, which were pale green, while the PSG1 plants exhibited siliques with a normal green color but pale green leaves throughout the rest of the plant (Fig. 1a, b and Supplemental Fig. S1). Transcriptional analysis revealed that GLK1 expression was restored in both PLG1 and PSG1 plants, and expression ranged up to ten times more than that found in the glk1/glk2 mutant (Fig. 1d, e). Assays of the chlorophyll content of the lines showed that the chlorophyll levels of PLG1 leaves and PSG1 silique wall were 2–3 times as high as those found in the glk1/glk2 mutant, and were nearly similar to those found in WT plants. The net leaf photosynthetic rate of PLG1 plants increased up to 1.5 times the rate found in the mutant, which was also nearly the same as that found in WT plants (Fig. 1c). These results indicated that photosynthesis was successfully restored in the leaf and silique wall of PLG1 and PSG1 plants, respectively.
The effect of silique wall and leaf photosynthesis on seed yield in Arabidopsis thaliana
To study the relative contributions to net photosynthesis of photosynthesis in silique wall and leaf tissues of A. thaliana, we conducted a systematic analysis of seed yield-related traits in the two complemented lines, along with mutant and WT plants. Traits were assessed as part of this analysis included, effective SNP, SNS, TSW, and silique length (Fig. 2). Our results showed that mutant SYP was only about 1/3 of that of WT, despite the fact that SYP was significantly (i.e., up to two times) increased in PLG1 plants with restored leaf photosynthesis relative to the mutant. In PSG1 plants with restored silique wall photosynthesis, SYP recovered somewhat, reaching 64.04% of that found in the WT. With respect to the effective SNP, the mutant realized only about 1/3 of the mean number of siliques found in the WT, while in PLG1- and PSG1-complemented plants, SNP was restored somewhat, to approximately 80% of the mean SNP found in the WT. PSG1-complemented plants showed higher SNP values than PLG1-complemented plants. Compared to the WT, the TSW was fully recovered after PSG1 complementation, which had only silique wall photosynthesis restored, although we found no difference in this trait between mutant and PLG1 plants. Besides, the traits of SNS and the silique length were only restored in PLG1 plants with leaf photosynthesis recovered, nearly the same as those found in WT.
Analysis of seed yield-related traits in glk1/glk2 mutant, WT and complemented plants. glk1/2, glk1/glk2 double mutant; G1 GLK1, TSW thousand-seed weight. Data are expressed as the mean ± SE (n = 16). The different letters indicate significant differences (P < 0.05) among treatments as determined by the Duncan’s test
The seed oil content, the core economic yield trait for oilseeds, was also analyzed (Fig. 2). In glk1/glk2 mutant plants, seed oil content was reduced to 88% of that found in WT plants. PLG1 plants showed a 2.88% increase in seed oil content relative to the mutant, and PSG1 plants showed a 10.75% increase. Compared to WT plants, seed oil content was nearly fully restored in PSG1 plants.
The relative contributions of silique and leaf photosynthesis to yield components
Photosynthetically active leaves and siliques provide the majority of the nutrients and assimilates required for seed development and filling. To obtain a simplified analysis, we presumed that photoassimilates for seed yield-related traits were contributed only via leaf and silique photosynthesis. The relative photosynthetic contributions of siliques and leaves to seed-related traits were assessed by determining the degree of recovery of each individual trait by comparing complemented lines with mutant and WT plants. For the total SYP, we found that leaves contributed 61.3% of photosynthetic demand, and siliques contributed 38.7% (Fig. 3). For the individual yield components, silique and leaf photosynthesis showed nearly the same relative contributions to the effective SNP. While leaf photosynthesis contributed nearly all the photosynthetic demand to SNS and silique length, silique wall photosynthesis contributed nearly all the photosynthetic demand to the TSW. As for oil content—the key trait for oil containing seeds—the silique contributed nearly four times as much as did the leaves.
Yield-related traits in plants with enhanced silique wall photosynthesis in the wild-type background
To test whether enhancing silique photosynthesis could further improve yield-related traits in plants of the WT background with normal photosynthetic capacity, the photosynthesis-related gene RCA was overexpressed in A. thaliana to enhance photosynthesis specifically in silique wall tissue (driven by the PS promoter) and in leaf tissue (driven by the PL promoter) (Supplemental Fig. S3). These two RCA-overexpressing lines were also examined to assess whether seed yield-related traits differed relative to the WT. This analysis showed that SYP increased by about 25% in PLRCA plants relative to the WT, but not in PSRCA lines (Fig. 4). TSW showed an increase of only 11.17% in PSRCA plants relative to the WT, although this was still significant. Silique length and SNS showed a similar pattern; both traits were only increased in PLRCA plants, to about 7% to that of WT plants. As for seed oil content, this trait was enhanced by about 5% in PLRCA plants compared to the WT, and PSRCA plants showed an increase twice as large as that of the PLRCA plants. Finally, we found no significant differences in SNP between WT plants and those of the overexpressed lines.
Discussion
As in leaves, green non-foliar organs also perform light harvesting and photosynthetic assimilation. Among them, pods, or siliques, as they are called in the Brassicaceae, are active photosynthetic organs during development, and perform functions beyond simply enclosing and protecting developing seeds. Photosynthesis in pods occurs mainly in the pod wall, which is itself a modified type of leaf (Bennett et al. 2011). Previous research has reported that assessed on the basis of the quantity of assimilate produced per unit of chlorophyll, pod walls have greater photosynthetic potential than leaves (King et al. 1998; Bennett et al. 2011). Thus, studying pod wall photosynthetic activity may help increase seed yields.
Considerable research on pod photosynthesis in legume and Brassica species has been performed, which demonstrates the essential role played by pod wall photosynthesis in seed development and yield. In Brassica species, the silique wall acts as the major photosynthetic organ during seed filling and oil synthesis, because Brassica leaves senescence shortly after the onset of flowering. However, quantifying the contribution of pod photosynthesis to seed yield still remains largely unexplored. In A. thaliana, the contribution of the silique to total photosynthetic activity is unclear. To the best of our knowledge, this study is the first to analyze the photosynthetic contribution of the silique wall in A. thaliana. Here, the relative contribution of silique wall photosynthesis to traits linked to seed yield was analyzed and compared to those of leaves. Our results showed that both silique wall and leaf photosynthesis contributed to total SYP. As for the individual components of yield traits, only leaf photosynthesis was found to contribute to the SNS and silique length, while only silique wall photosynthesis was found to contribute to TSW. In oilseed rape, the SNP was identified as the decisive trait for seed yield (Diepenbrock 2000). According to the results of present study, both silique wall and leaf photosynthesis contributed to this trait, and in almost equal amount. These findings suggest that the photosynthetic capacity of the silique wall is particularly important not only for seed development and filling, but also for the early development and survival of young siliques. Overall, this study reveals that the photosynthetic assimilation of the silique wall plays a complementary role with that of the leaf in A. thaliana seed production.
Little attention has been paid to the role played by silique photosynthesis in seed oil production. According to the results of our study, seed oil content was strongly determined by silique wall photoassimilation, as leaf photosynthesis had a minimal impact on this trait (Fig. 3). Given that a similar result was also found in rapeseed (Hua et al. 2012), more attention should be paid to silique (pod) wall photosynthetic capacity as a determinant of seed oil production in oilseed crops.
Silique (pod) photosynthesis makes an important contribution to seed development and filling, as well as to yield-related traits, although it is surprising that little research has been carried out on how this might be manipulated to enhance crop yield (Bennett et al. 2011). In the present study, overexpression of the photosynthetic gene RCA driven by a silique wall-specific promoter PS in WT background enhanced silique photosynthesis. The PSRCA plants showed significantly increased TSW and seed oil content (Fig. 4). However, this line did not exhibit enhanced SYP, which may be due to the slight reduction in the SNP. This reduction may be a negative effect of the overexpression of RCA, as this trait is decisive for yield. Nevertheless, enhancing silique wall photosynthesis in A. thaliana resulted in a significant increase of seed weight and oil production. This finding may have promising implications for improving seed yield and oil production in oilseed crops and other species with pod-like structures.
In the previous researches, there are two commonly used strategies to study the non-foliar photosynthesis. One involves using stable C-isotope tracers to label photoassimilated metabolites and to characterize their partitioning into different organs (Tcherkez et al. 2011; Ma et al. 2014). The other strategy involves shading target photosynthetic organs by wrapping with aluminum foil (Hua et al. 2012; Wang et al. 2016). In this study, we used an alternative approach to analyze the photosynthetic contributions of silique walls with leaves in A. thaliana. Here the Atglk1/glk2 double mutant, which is defective in photosynthesis as it possesses mutations in the photosynthesis-regulatory GLK gene pairs, was used for photosynthetic analyses of both leaf and non-foliar organs. GLK gene pairs optimize photosynthesis by regulating chloroplast development (Waters et al. 2008, 2009). Overexpressing GLK specifically in A. thaliana root tissue could induce chloroplast biogenesis in the root, which shows the photosynthetic potential of the root tissue (Kobayashi et al. 2013). These findings indicate that modulating the expression of GLK gene pairs may be a valuable method to further study photoassimilation in various photosynthetic organs.
Conclusion
In the present study, we demonstrated the important contribution of silique wall photosynthesis to seed yield and oil production in the model plant A. thaliana. Enhancing photosynthetic capacity of the silique wall in the WT background further increased seed weight and oil production. Our findings suggest that the photosynthetic capacity of the silique (pod) wall is important for breeding efforts focused on improving seed and oil production in oilseed crops and related species.
References
Allorent G, Osorio S, Ly VuJ, Falconet D, Jouhet J, Kuntz M et al (2015) Adjustments of embryonic photosynthetic activity modulate seed fitness in Arabidopsis thaliana. New Phytol 205(2):707–719
Aschan G, Pfanz H (2003) Non-foliar photosynthesis—a strategy of additional carbon acquisition. Flora 198(2):81–97
Atkins CA, Kuo J, Pate JS, Flinn AM, Steele TW (1977) Photosynthetic pod wall of pea (Pisum sativum L.): distribution of carbon dioxide-fixing enzymes in relation to pod structure. Plant Physiol 60(5):779–786
Bennett EJ, Roberts JA, Wagstaff C (2011) The role of the pod in seed development: strategies for manipulating yield. New Phytol 190(4):838–853
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Crookston RK, O’Toole J, Ozbun JL (1974) Characterization of the bean pod as a photosynthetic organ. Crop Sci 14(5):708–712
Diepenbrock W (2000) Yield analysis of winter oilseed rape (Brassica napus L.): a review. Field Crops Res 67(1):35–49
Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA (2002) GLK gene pairs regulate chloroplast development in diverse plant species. Plant J 31(6):713–727
Guo Y, Zhan GM, Yang XY, Hua W, Lv YT (2015) Leaf and silique specific promoters of Arabidopsis thaliana. Chin J Oil Crop Sci 37(1):1–8
Hu YY, Zhang YL, Luo HH, Li W, Oguchi R, Fan DY et al (2012) Important photosynthetic contribution from the non-foliar green organs in cotton at the late growth stage. Planta 235(2):325–336
Hua W, Li RJ, Zhan GM, Liu J, Li J, Wang XF et al (2012) Maternal control of seed oil content in Brassica napus: the role of silique wall photosynthesis. Plant J 69(3):432–444
Imaizumi N, Samejima M, Ishihara K (1997) Characteristics of photosynthetic carbon metabolism of spikelets in rice. Photosynth Res 52(2):75–82
King SP, Badger MR, Furbank RT (1998) CO2 refixation characteristics of developing canola seeds and silique wall. Funct Plant Biol 25(3):377–386
Kobayashi K, Sasaki D, Noguchi K, Fujinuma D, Komatsu H, Kobayashi M et al (2013) Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors. Plant Cell Physiol 54(8):1365–1377
Li X, Wang H, Li H, Zhang L, Teng N, Lin Q et al (2006) Awns play a dominant role in carbohydrate production during the grain-filling stages in wheat (Triticum aestivum). Physiol Plant 127(4):701–709
Liu J, Hua W, Yang HL, Zhan GM, Li RJ, Deng LB et al (2012) The BnGRF2 gene (GRF2-like gene from Brassica napus) enhances seed oil production through regulating cell number and plant photosynthesis. J Exp Bot 63(10):3727–3740
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–∆∆CT method. Methods 25(4):402–408
Ma F, Jazmin LJ, Young JD, Allen DK (2014) Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proc Natl Acad Sci USA 111(47):16967–16972
Mogensen VO, Jensen CR, Mortensen G, Andersen MN, Schjoerring JK, Thage JH et al (1997) Pod photosynthesis and drought adaptation of field grown rape (Brassica napus L.). Eur J Agron 6(3–4):295–307
Murchie EH, Pinto M, Horton P (2009) Agriculture and the new challenges for photosynthesis research. New Phytol 181(3):532–552
Pavel EW, DeJong TM (1993) Estimating the photosynthetic contribution of developing peach (Prunus persica) fruits to their growth and maintenance carbohydrate requirements. Physiol Plant 88(2):331–338
Quebedeaux B, Chollet R (1975) Growth and development of soybean (Glycine max [L.] Merr.) pods: CO2 exchange and enzyme studies. Plant Physiol 55(4):745–748
Ramana S, Ghildiyal MC (1997) Contribution of leaf photosynthesis towards seed yield in Brassica species. J Agron Crop Sci 178(3):185–187
Raven JA, Griffiths H (2015) Photosynthesis in reproductive structures: costs and benefits. J Exp Bot 66(7):1699–1705
Ruuska SA, Schwender J, Ohlrogge JB (2004) The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol 136(1):2700–2709
Sharma P, Ghildiyal MC (1992) Contribution of leaf and pod photosynthesis to seed yield in mustard. Photosynthetica 26(1):91–94
Singal HR, Sheoran IS, Singh R (1987) Photosynthetic carbon fixation characteristics of fruiting structures of Brassica campestris L. Plant Physiol 83(4):1043–1047
Singal HR, Talwar G, Dua A, Singh R (1995) Pod photosynthesis and seed dark CO2 fixation support oil synthesis in developing Brassica seeds. J Biosciences 20(1):49–58
Singh J, Pandey P, James D, Chandrasekhar K, Achary V, Kaul T et al (2014) Enhancing C3 photosynthesis: an outlook on feasible interventions for crop improvement. Plant Biotechnol J 12(9):1217–1230
Tambussi EA, Nogués S, Araus JL (2005) Ear of durum wheat under water stress: water relations and photosynthetic metabolism. Planta 221(3):446–458
Tcherkez G, Mahé A, Hodges M (2011) 12C/13C fractionations in plant primary metabolism. Trends Plant Sci 16(9):499–506
Wang X, Liu G, Yang Q, Hua W, Liu J, Wang H (2010) Genetic analysis on oil content in rapeseed (Brassica napus L.). Euphytica 173(1):17–24
Wang H, Hou L, Wang M, Mao P (2016) Contribution of the pod wall to seed grain filling in alfalfa. Sci Rep 6:26586
Waters MT, Moylan EC, Langdale JA (2008) GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J 56(3):432–444
Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA (2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21(4):1109–1128
Xu HL, Gauthier L, Desjardins Y, Gosselin A (1997) Photosynthesis in leaves, fruits, stem and petioles of greenhouse-grown tomato plants. Photosynthetica 33(1):113–123
Yang Y, Cang J, Wang XD, Cui L, Wang X, Zhou Z (2008) Photosynthetic characteristics of soybean pod and its contribution to yield. J Northeast Agric Univ 39(12):51–56
Zhang W, Mao P, Li Y, Wang M, Xia F, Wang H (2017) Assessing of the contributions of pod photosynthesis to carbon acquisition of seed in alfalfa (Medicago sativa L.). Sci Rep 7:42026
Zhou B, Serret MD, Elazab A, Pie JB, Araus JL, Aranjuelo I, Sanz-Sáez Á (2016) Wheat ear carbon assimilation and nitrogen remobilization contribute significantly to grain yield. J Integr Plant Biol 58(11):914–926
Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261
Acknowledgements
This work was supported by the National Program for Basic Research of China (2015CB150200) and the Natural Science Foundation of Hubei province (2015CFB348).
Author information
Authors and Affiliations
Contributions
WH, HW and XZ conceived and designed the research; XZ, LZ, CK, YG, CH, LD, XS, GZ, and ZH performed the experiments and bioinformatics; XZ and LZ analyzed the data; and XZ wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.



Rights and permissions
About this article
Cite this article
Zhu, X., Zhang, L., Kuang, C. et al. Important photosynthetic contribution of silique wall to seed yield-related traits in Arabidopsis thaliana. Photosynth Res 137, 493–501 (2018). https://doi.org/10.1007/s11120-018-0532-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-018-0532-x