Abstract
Changes of photosynthetic activity in vivo of individual heterocysts and vegetative cells in the diazotrophic cyanobacterium Anabaena sp. strain PCC 7120 during the course of diazotrophic acclimation were determined using fluorescence kinetic microscopy (FKM). Distinct phases of stress and acclimation following nitrogen step-down were observed. The first was a period of perception, in which the cells used their internally stored nitrogen without detectable loss of PS II activity or pigments. In the second, the stress phase of nitrogen limitation, the cell differentiation occurred and an abrupt decline of fluorescence yield was observed. This decline in fluorescence was not paralleled by a corresponding decline in photosynthetic pigment content and PS II activity. Both maximal quantum yield and sustained electron flow were not altered in vegetative cells, only in the forming heterocysts. The third, acclimation phase started first in the differentiating heterocysts with a recovery of PS II photochemical yields \(F_{\text{v}} /F_{\text{m}} ,\;F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}.\) Afterwards, the onset of nitrogenase activity was observed, followed by the restoration of antenna pigments in the vegetative cells, but not in the heterocysts. Surprisingly, mature heterocysts were found to have an intact PS II as judged by photochemical yields, but a strongly reduced PS II-associated antenna as judged by decreased F 0. The possible importance of the functional PS II in heterocysts is discussed. Also, the FKM approach allowed to follow in vivo and evaluate the heterogeneity in photosynthetic performance among individual vegetative cells as well as heterocysts in the course of diazotrophic acclimation. Some cells along the filament (so-called “superbright cells”) were observed to display transiently increased fluorescence yield, which apparently proceeded by apoptosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several genera of cyanobacteria are able to fix atmospheric nitrogen under conditions of nitrogen limitation. Reduction of dinitrogen to ammonia is catalysed by the nitrogenase, an extremely oxygen-sensitive enzyme that becomes rapidly and irreversibly inhibited by comparatively low concentrations of O2 (reviewed by Postgate 1998). Since cyanobacteria produce oxygen inside their own cells by photosynthesis that provides energy for all cellular processes, including nitrogen fixation, they evolved strategies that protect nitrogenase from oxygen. Various cyanobacteria reconcile nitrogenase activity with photosynthesis in different ways (reviewed e.g., by Bergman et al. 1997; Berman-Frank et al. 2003). (1) Unicellular diazotrophs usually separate both processes temporally, by fixing nitrogen at night while photosynthesising during the day. (2) The marine, filamentous, non-heterocystous genus Trichodesmium carries out photosynthesis and nitrogen fixation during the day inside the same cells, without permanent spatial segregation. Instead, this genus employs a reversible switching of activity of individual cells from oxygenic photosynthesis to nitrogen fixation coupled to a non-oxygenic photosynthesis through the Mehler reaction (Kana 1993; Berman-Frank et al. 2001; Küpper et al. 2004, 2009). (3) Heterocystous diazotrophs are filamentous species, which in the absence of combined nitrogen differentiate 5–10 % of cells in the filament into heterocysts, cells specialised for the functioning of nitrogenase. Heterocysts are supplied with products of photosynthesis formed in the vegetative cells in exchange for organically bound amino nitrogen. Thus, a distinct spatial separation of the two incompatible metabolic activities is achieved (reviewed in Flores and Herrero 2010; Kumar et al. 2010; Maldener and Muro-Pastor 2010; Zhao and Wolk 2008). Although markedly disparate at first glance, the representatives of these three types use many similar processes to provide an environment suitable for the efficient functioning of nitrogenase under aerobic conditions (Berman-Frank et al. 2003).
This paper focuses on the heterocystous cyanobacterium Anabaena sp. strain PCC 7120. Protection of nitrogenase from molecular oxygen in the mature, fully differentiated heterocyst results from concerted cytomorphological and biochemical modifications in the cell (Wolk 1996; Kumar et al. 2010). The changes in cellular structure in the course of differentiation have been extensively studied and an unquestioned consensus picture on their course during the differentiation process is available. Consequently, cytological characteristics are often used as reliable markers for the subsequent stages in heterocyst differentiation (Lang and Fay 1971; Wolk et al. 1994; Fiedler et al. 1998; Hebbar and Curtis 2000).
The picture is less complete and consistent as far as the biochemical characteristics and particularly the activities of the photosynthetic apparatus are concerned, since the methods for biochemical and functional metabolic analysis of individual differentiated cells in situ have been largely lacking. Although mature heterocysts can be separated from vegetative cells (Strohmeier et al. 1994), the isolation procedure may influence the functional components (enzymes) by itself and the very tight and extensive mutual interactions between the cells in the filament become disrupted with unknown consequences on the metabolism. Furthermore, the method of isolation selects heterocysts that passed a certain stage of maturity. With all this in mind, the following characteristics of biochemistry in heterocysts have been reported in literature. Analyses of isolated heterocyst membranes showed that they contain cytochrome c-554, plastocyanin, plastoquinone, P700, cytochrome b-563, Fe–S proteins (Tel-Or et al. 1977), ferredoxin and ferredoxin:NADPH+ oxidoreductase (Tel-Or and Stewart 1976), implying that in the mature heterocysts photosystem I (PS I) activity is predominant. The content of photosynthetic pigments, particularly phycobiliproteins, is considerably reduced (Wolk and Simon 1969). In recent study conducted on membrane proteomes of heterocyst from nitrogen fixing filamentous cyanobacteria Nostoc punctiforme sp. PCC 73102 (Cardona et al. 2009) a significant amount of assembled PS II complexes was found containing the D1, D2, CP43, CP47 and PsbO proteins from these complexes.
Measurements concerning various features and processes related to photosynthesis provided the following results. Ying et al. (2002) studied the fluorescence and absorption spectra of individual vegetative cells and heterocysts in intact filaments in vivo at room temperature and 77 K. Differences were detected between the spectra of vegetative cells and heterocysts, between heterocysts at different stages of differentiation, and even between different parts of the same heterocyst. The latter differences have been attributed to different stages of phycobilisomes (PBS) degradation. The reduced amount of PBS present in mature heterocysts was reported to remain active in light absorption and in transfers of light energy to Chl in PS I (Murry et al. 1981). Recently, by single-cell confocal laser spectroscopy, the differentiation of single-cell pigments in strain Nostoc KU001 has been investigated (Sugiura and Itoh 2012). In this study, typical fluorescence of PS II was detected in heterocysts and the pigment ratios in single cells were determined, suggesting an uncoupling of antennae proteins from PS II in heterocyst. Time-lapse fluorescence microscopy (Kumazaki et al. 2013) revealed a highly synchronous decay in APC and PS II concentration in Anabaena variabilis during heterocyst differentiation with a constant stoichiometric ratio in early stage of nitrogen limitation stress. At the same time the average whole-thylakoid concentration of PSI showed no substantial change during heterocysts differentiation. Photooxidation and photoreduction of P700 showed that functional PS I complexes are present in heterocysts (Donze et al. 1972) and some functional sections of photosynthetic electron transport as well as photophosphorylation have been demonstrated (Wolk and Simon 1969). Earlier studies using isolated mature heterocysts showed strongly reduced fluorescence of Chl a and no light-induced changes in fluorescence yield (FY) (Donze et al. 1972). Heterocysts have also strongly diminished levels of manganese (Tel-Or and Stewart 1975) as well as of cytochrome b-559 (Almon and Böhme 1980), both being essential components of the functional PS II complex. These differences could account for the considerably reduced or inhibited activity of PS II in heterocysts. In addition, the absence of the Hill reaction in isolated heterocysts has been documented (Bradley and Carr 1971; Tel-Or and Stewart 1975). Mature heterocysts have been claimed to lack the ability of assimilating CO2 (Fay and Walshby 1966; Brusca et al. 1989), and it was shown that carboxysomes disintegrate during heterocyst differentiation.
Many publications confirm that high respiratory activity in heterocysts is probably the principal mechanism of active nitrogenase protection. This conclusion is supported by detection of elevated O2 consumption rates in the heterocysts (Fay and Kulasooriya 1972; Stewart et al. 1969; Milligan et al. 2007) as well as increased activities of enzymes of the catabolic pathways (Winkenbach and Wolk 1973; Lex and Carr 1974; Lockau et al. 1978). Alternative cytochrome oxidases (CoxII and CoxIII) are essential for heterocyst function in Anabaena sp. strain PCC 7120 (Jones and Haselkorn 2002; Valladares et al. 2003). Also, the formation of a greatly extended cytoplasmic membranes system (the so called “honeycomb”) is perhaps connected with the enhancement of the respiratory activity (Murry et al. 1981). The occurrence of light-dependent O2 consumption coupled to pseudocyclic electron flow in PS I (Mehler reaction) in heterocysts (Milligan et al. 2007) may be of particular importance under natural conditions (Bradley and Carr 1971; Smith et al. 1986).
The development of heterocysts is mainly regulated on a transcriptional level (reviewed in Adams 2000; Herrero et al. 2004; Meeks and Elhai 2002; Zhang et al. 2006; Zhao and Wolk 2008; Muro-Pastor and Hess 2012). Genome wide expression analysis of the response to nitrogen deprivation in Anabaena revealed three main profiles of gene expression at 8, 13, and 24 h following the elimination (usually called “nitrogen step-down”, NSD) of fixed nitrogen from the growth medium (Ehira et al. 2003; Xu et al. 2008). Analyses of isolated heterocysts showed that many of the upregulated genes were physically clustered on the chromosome and became upregulated in a co-ordinated manner. Eight DNA segments containing 29 ORFs were found to be downregulated; they included the genes encoding cyt550, the large subunit of Rubisco and the D1 protein of PS II. In addition, transcriptional changes, which occur during nitrogen starvation, and transcript boundaries have been investigated by RNA sequencing. These data showed the importance of antisense RNAs in regulation of heterocyst specific genes, hundreds of new genes were identified as differentially expressed and the control of their promoters by two major regulators NtcA and HetR was demonstrated (Flaherty et al. 2011; Mitschke et al. 2011).
Kinetic imaging of Chl variable fluorescence allows investigating in vivo changes in several photosynthetic characteristics of both vegetative cells and heterocysts during the process of heterocyst differentiation. The fluorescence kinetic microscope (FKM) described by Küpper et al. (2000, 2007a) proved helpful in investigating the regulation of photosynthesis in the non-heterocystous cyanobacterium Trichodesmium (Berman-Frank et al. 2001; Küpper et al. 2004, 2008, 2009; Andresen et al. 2010) or in filamentous cyanobacterium Calothrix elenkinii (Adamec et al. 2005). Here, we have used this instrument to follow the response to NSD in individual cells of the filaments of Anabaena sp. PCC 7120. In this way we obtained another important comparison of the physiological processes in vegetative cells and in the differentiating heterocysts during acclimation to NSD.
Materials and methods
Growth conditions
Anabaena sp. strain PCC 7120 was grown in 1:4 diluted A&A medium (Allen and Arnon 1955) with 5 mM NO3 − as source of combined nitrogen. The cultures were grown in glass tubes of 30 mm inner diameter, 200 ml of suspension in each. They were aerated at a rate 0.3 l min−1 by air and illuminated with “cool white” fluorescent tubes (50 μmol m−2 s−1 PAR). The culture tubes were kept at 30 °C in a temperature controlled water bath with glass walls. For the NSD experiment, the cyanobacterial filaments were collected by centrifugation after 3 days of cultivation (i.e., approximately at mid-exponential phase). The sediment was washed twice and resuspended in nitrate-free 1:4 diluted A&A medium (N-free medium). Cells were then kept under the same conditions.
Measurement of nitrogenase activity
Nitrogenase activity was assayed by the acetylene reduction method (Hardy 1973; Maldener et al. 1994). 25 ml of suspension withdrawn from the culture was placed in a 125 ml glass bottle. The bottle was sealed with a rubber stopper and 10 ml of air was replaced with acetylene. After 1 h of incubation, a 10 ml gas sample was taken from the bottle and the ethylene formed was assessed by gas chromatography (Hewlett Packard 5890).
Pigment analysis
Cultures were collected on glass-fiber filters (GF3, Macherey–Nagel, Germany), chlorophyll a and carotenoids were extracted overnight in 100 % acetone at 4 °C. Supernatant was cleared by centrifugation (15 min at 4 °C, 10,000 rpm). Absorption spectra of the pigment extract were recorded (350–800 nm) using the Spectronic Unicam UV-550 spectrometer. Pigment content was determined either using standard equations (Porra et al. 1989) or by spectral deconvolution of the whole spectra into gaussian absorption bands of individual pigments (Küpper et al. 2007b). Spectra of extracted phycobiliproteins were measured following extraction in the phosphate saline buffer (Küpper et al. 2009). The relative content of total phycobiliproteins was then estimated from the area of the 610 nm absorbance band of the phycobiliproteins extract. Exact quantification of individual phycobiliproteins was not possible because not all spectra and extinction coefficients of phycobiliproteins in Anabaena are known.
Dry weight
The GF3 filters were dried in the oven for 3 h at 105 °C and then weighted. 10 ml of the cultures was collected on the filters, dried (for 3 h at 105 °C) and weighted again. Dry weight of the sample (mg/ml) was determined from the difference of both values and the volume filtered.
Elemental analysis
Samples (1 ml) were filtered onto pre-combusted (2 h at 400 °C) 13 mm GF/F filters (Whatman, USA), dried for 2 h at 105 °C and analysed on an elemental soil analyser using a thermo conductivity detector (NC 2110, ThermoQuest, Waltham, MA, USA).
Preparation of samples for Chl fluorescence measurements
Microscopic records of Chl fluorescence kinetics were performed in a measuring chamber (middle version as described by Küpper et al. 2000). For each preparation, few drops of cyanobacterial suspension were put on the inner surface of the chamber’s glass window. To prevent movement of the filaments during the measurements, the surface of the glass window had been coated with BD Cell-Tak adhesive (BD Biosciences, NJ, USA), which fixes cells without influencing their physiological conditions. After few minutes of cell sedimentation, the non-immobilised filaments were washed away. Those sticking to the window were covered with a cellophane sheet soaked in N-free medium and stretched by applying a rubber o-ring. Temperature-controlled (30 °C) medium, saturated with air was passed through the chamber at a rate of about 25 ml min−1, as described previously (Küpper et al. 2004, 2009). While in the above described procedure, new sample for measurements under the microscope was prepared for each time point, for verification of the trends we also followed the changes in the Chl fluorescence of individual filaments that were cultivated in the measuring chamber during the whole NSD transition process (up to 192 h), according to Šetlíková et al. (2005).
Measurements of Chl fluorescence kinetics
Fluorescence kinetics of Anabaena sp. strain PCC 7120 were measured by the FKM constructed in collaboration with the company Photon Systems Instruments (Brno, Czech Republic, www.psi.cz) as described by Küpper et al. (2000), in later parts of this study with the modifications described by Küpper et al. (2007a). Most of the fluorescence measurements were performed in tandem with both blue light (410–490 nm, subsequently called ‘blue excitation’) absorbed mainly by PS II and PS I Chl–protein complexes, and with orange light (575–615 nm, ‘orange excitation’), absorbed mainly by PBSs [for information on used spectral filters, see Küpper et al. (2000, 2007a)]. The irradiance intensities of the two lights were adjusted so as to get an approximately equal average fluorescence response with the given material—the intensity of the blue actinic light was approx. 300 μmol quanta m−2 s−1, the orange actinic was approx 90 μmol quanta m−2 s−1, the saturating lights were 10,000 and 4,000 μmol quanta m−2 s−1 for the blue and orange, respectively. The FKM was operated by the FluorCam software from Photon Systems Instruments.
For all measurements reported here, we designed a measuring protocol to record the main dark-light transients in FY and to follow the initial phase of relaxation in the dark. A schematic representation of a typical record obtained with this protocol is illustrated in Fig. S1. The legend to this figure describes the timing of the events during measurement and specifies the symbols used for labelling important basic fluorescence parameters (such as F 0, F m, F t). These basic parameters were read by the software at predefined time check points for calculation of derived fluorescence parameters (e.g., F v/F m, ΦPSII, NPQ, see below).
The measurements were performed with an automatic subtraction of background signals and a maximum time resolution of 80 ms. In the slower parts of the kinetics, lower time resolution was applied. The duration of the record was limited by the hardware and by the size of the resulting data file.
Analysis of fluorescence kinetic records
The original two-dimensional data from the FKM, i.e., films of Chl fluorescence kinetics, were analysed using the FluorCam software from Photon Systems Instruments as described before (Küpper et al. 2000, 2004, 2007a, 2009). Wherever the calculation of the parameter value involved the value of \(F^{\prime}_{0} \), the latter was calculated by the formula proposed by Oxborough and Baker (1997). Fluorescence parameters were calculated from measured values according to the conventional formulas as reviewed by Roháček (2002). We also mostly adhere to the symbols preferred in his review.
Statistical treatment
The data are compiled from at least three independent experiments. For analysis of pigment composition, dry weight, nitrogenase activity and elemental analysis triplicate samples were collected and analysed. These data are presented as averages with standard deviation error bars.
Results and discussion
The course of events during acclimation to nitrogen deprivation
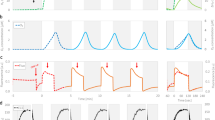
The NSD experiment started with transfer of cells into a medium devoid of bound nitrogen. This initiates the period of nitrogen deprivation of the cells and induces changes both in the macroscopic parameters (Figs. 1A, B, 2) and in the photosynthetic apparatus of individual Anabaena cells (Figs. 3, 4). On a base of revealed consistent character and similarity of the changes between macro- and micro-scopic parameters we have conceptually divided the transition process during which cells readjust to the diazotrophy regime into distinct periods.
(A) Variation of elemental composition of cells and detection of nitrogenase activity measured by acetylene reduction during the NSD. (B) Variation of nitrogen and carbon content of cells related to dry weight. The vertical lines in the graphs show the separation in the physiological phases of the NSD experiment as explained in more detail in the text. The labels of these phases are: P perception period, S stress period, A acclimation period
Variation of basic variable fluorescence parameters with progress of the diazotrophic adaptation. All values are plotted in percent of the value at the start of the experiment. The parameters plotted in the individual panels are indicated by the symbols labelling the Y axes. The left part of each figure represents data obtained with cells under blue excitation, the right part refers to cells under orange excitation
Variation of derived variable fluorescence parameters with progress of the diazotrophic acclimation. The parameters plotted in the individual panels are indicated by the symbols labelling the Y axes. The left part of the figure represents data obtained with cells under blue excitation, the right part refers to cells under orange excitation (see methods for irradiance spectra and intensities)
Perception period
Following the NSD, first a period required to perceive the lack of nitrogen was observed (labelled “P” in Figs. 1, 2, 3, 4). This period lasted less than 20 h. The elemental composition of the biomass has already started to change. When expressed per unit of biomass, both C and N content declined during the first 24 h, however, the decline of N was more pronounced (25 %) than of C (6 %), (Fig. 1B). Therefore the molar N/C ratio (Fig. 1A) significantly declined during the first 24 h, from the initial value of 0.22–0.18. The pigment content of the biomass (Fig. 2) also declined. The initial degradation of phycobilins was less severe than the loss of chlorophylls.
The initial changes of the FYs (intrinsic yield F 0 and maximal yield F m) were different when measured by blue or by orange excitations (Fig. 3). When excited by the blue measuring light that is absorbed mainly by chlorophyll pigments, both yields only slightly declined (left panels in Fig. 3). The amplitude of this decline corresponds to the decline of chlorophyll content (see Fig. 2). However, the decline of the FYs when excited by the orange measuring light (see the right panels of Fig. 3) was much more pronounced, occurred even during the first 12 h after the NSD. Interestingly, the phycobilin content remained almost unchanged. Since the FKM detects fluorescence above approx. 650 nm, this uncoupling between the phycobilin content and FYs can be explained either by decrease in the intrinsic FYs of PBS or by decreased energy transfer from PBS to chlorophyll emitters. In the first case, FY of PBS themselves could decrease by aggregation (or other structural impairment) induced by the onset of nitrogen limitation. This aggregation would precede the breakdown and decrease of PBS that is observed in the later stages of NSD. We can rule out that the FYs would decrease because the composition of PBS would change (for example, if allophycocyanin concentration would decrease relative to phycocyanin), because the shapes of absorption spectra of extracted phycobilins were identical. In the second case, one can speculate that initial stages of nitrogen limitation activate thermal dissipation of energy that is transferred from phycobilisome to the chlorophyll containing reaction centres. However, the PS II photochemical activity remained during the perception period almost constant (Fig. 4). The perception period during acclimation to nitrogen deprivation is most likely caused by the fact that the cells still use up bound nitrogen that was taken up before the NSD that enabled the culture to maintain the original photosynthetic activity. To optimise photosynthesis efficiency and to protect themselves from excessive light, cyanobacteria possess several known molecular mechanisms: state transitions [see e.g., Biggins and Bruce (1989) or Allen and Mullineaux (2004)], blue-green light-induced non-photochemical fluorescence quenching at phycobilisome level (for recent review, see e.g., Kirilovsky and Kerfeld 2012) and phycobilisome decoupling (Kaňa et al. 2009, 2012). Our data suggest that the different characters of FYs changes may result from different regulation mechanisms that took place in cyanobacterial photosynthesis machinery when excited by the blue and the orange light.
Stress period
After about 20 h, the period of nitrogen deficiency stress (labelled “S” in Figs. 1, 2, 3, 4) started. In this period, the macroscopic parameters (elemental and pigment contents, Figs. 1B, 2) continued to decline with similar rates like in the perception period. In addition, in this period we also observed significant drop of FYs of individual cells induced by blue excitation (left panel of Fig. 3). Still, the decline of FYs was distinctly larger under excitation with orange light (right panel in Fig. 3). The latter difference is apparent in changes of F m, F 0 and F v values expressed in percent of the initial value (Fig. 3). During the whole course of experiments, however, in vegetative cells the F m and F 0 values (Fig. 3) showed only small deflections from strict proportionality of their changes and this provides an explanation for only slight variation in the values of F v/F m (Fig. 4). This indicates that despite the loss of pigments, the remaining photosynthetic apparatus is being continuously adjusted to a reasonably functional structure.
The beginning of the decline in pigments coincides with the appearance of first proheterocysts, so it seems to be the time point when the intracellular nitrogen deficiency is developed and the cells start to counteract it by heterocyst differentiation. During this period, a noticeable transient increase in values of the parameters reflecting the maximal dark-acclimated quantum yield of PS II photochemistry (F v/F m) in vegetative cells and the light-acclimated PS II electron transport (ΦPSII = (\((F^{\prime}_{\text{m}} -F^{\prime}_{\text{t}})/F^{\prime}_{\text{m}}\), Genty et al. 1989) in both vegetative and (pro)heterocystous cells was observed (Fig. 4). This rise lasted somewhat longer than the steep drop of F 0 and F m and then parameters started to decline. The decline of ΦPSII was limited only to approximately the 20th hour of experiment. We consider ΦPSII to provide the most reliable estimate of the real efficiency of the thylakoid electron transport, particularly in the specific conditions met in Anabaena records. Therefore we assume that at ~20 h of the experiment the early segment of the nitrogen deficiency stress period, in which only the pigment content (light harvesting) was affected, was terminated and the period of the most severe nitrogen deficiency, in which also the photochemistry was affected, was started. In this period ΦPSII further declined and the most significant changes in the shape of FY transients were observed.
Despite the decrease in all the parameters discussed above, no pronounced changes in the general shape of the FY transients were apparent, i.e., only the amplitudes of the features measured by those fluorescence parameters were changed. Quite frequently transients with very similar shapes were recorded with cells from subsequent samplings even if in the meantime the F v value decreased to about one half.
This indicates that the number of photosynthetic units becomes reduced, but the structure and efficiency of the remaining ones was stable.
Acclimation period
While the previous events displayed similar timing for the vegetative cells and the cells differentiating into heterocysts, the transition to nitrogen deficiency acclimation (marked “A” in Figs. 1, 2, 3, 4) was markedly different for both types of cells and also for the various parameters analysed. In general, the recovery of photochemistry occurred earlier in the heterocysts than in the vegetative cells. A very clear turning point in the quantum yield of PS II as measured by F v/F m (dark-acclimated) and \(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\) (light-acclimated) was observed in the heterocysts already at about 36 h. Judged by F 0, F m and \(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\) of the vegetative cells, the period of nitrogen deficiency stress ended at about 48 h. At this time, the period of acclimation to the nitrogen deficiency began. From its beginning, the acclimation period was characterised by the restoration of the PS II antenna pigments as measured by F 0, which started at 48 h. Also the recovery of the PS II quantum yield measured by \(F^{\prime}_{v} /F^{\prime}_{m}\) started at the same time.
Another important marker in this period that can be determined quantitatively and with a good time resolution is the activity of nitrogenase. First significant quantities of reduced acetylene were detected in samples at the 72nd hour following the NSD. The onset of nitrogenase activity was followed by the recovery of light-acclimated (sustained) electron flow through PS II (as measured by ΦPSII) in the vegetative cells. This clearly shows that the recovery of photosynthesis could only take place when already substantial amounts of fixed nitrogen became available again. Also, the earlier recovery of photochemistry in heterocysts compared to vegetative cells (see above) could result from the earliest onset of nitrogenase, which activity is not yet detectable on macroscopic scale but sufficient to cause the recovery of metabolism on a single-cell levels (host heterocystous cells). We assume that in this time interval the final steady state in the diazotrophic metabolic pattern segregation and efficient metabolic communication between the heterocysts and the vegetative cells got gradually established.
Acclimated state
The final acclimated state was approached at the end of the experiments (150 h). In the acclimated state, the vegetative cells reached about the same PS II-associated antenna size (as judged by F 0) compared to the initial unstressed state before NSD. Also PS II activity as measured by F v, F v/F m and \(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\), reached again the levels (or was even higher) before NSD. In the heterocysts, which were the first cells to start the recovery of PS II activity, this recovery was incomplete, as it is seen best in the curves of light-acclimated PS II quantum yield (\(F^{\prime}_{\text{v}} /F^{\prime}_{\text{m}}\); Fig. 4). At the same time, non-photochemical quenching in mature heterocysts was much higher than in the recovered vegetative cells (Fig. 4). The mechanism of activated non-photochemical quenching in heterocysts of Anabaena deserves further investigation. It has been recently found that Anabaena genome possesses an analogue to the Orange-Carotenoid-Protein (D. Jallot and D. Kirilovsky, personal comm.) that forms basis for NPQ quenching in Synechocystis PCC6803 (Kirilovsky and Kerfeld 2012).
These features indicate that, in contrast to earlier assumptions based on data obtained with isolated heterocysts, heterocysts have active PS II reaction centres, but nevertheless do not carry out much PS II-mediated electron flow and instead thermally dissipate most of the captured excitons. Recent finding by Cardona et al. (2009) that light driven in vitro electron transfer from PS II in heterocyst thylakoid membranes could also be measured and the fact that the authors could not detect any partially disassembled PS II complexes lacking CP43 are being in a good agreement with our experiments conducted in vivo on an individual cell scale. This situation raises the question of the purpose of maintaining an active PS II in these cells. First, it could serve in a specifically PS II-dependent process of thermal exciton quenching and thus be necessary for avoiding excess excitation of PS I. Second, at present it cannot be excluded that under certain environmental circumstances Anabaena heterocysts may carry out the Mehler reaction, as it has been shown in the non-heterocystous marine filamentous cyanobacterium Trichodesmium (see “Introduction” section; Kana 1993; Berman-Frank et al. 2001). To ensure we monitor the earliest generation of heterocysts during NSD, for each measurement the heterocysts with the most developed envelope were chosen along the investigated filament. However, after the onset of nitrogenase activity in the heterocysts of the first generation, the flow of metabolites with bound nitrogen along the filament is established. This could lead to the situation when, in contrast to the heterocysts from the first generation, the second generation heterocysts that differentiate already in recovering filament are able to complete the synthesis of their envelopes before PS II inactivation. Then a relatively high PS II activity in morphologically fully developed heterocysts accompanied by a strong non-photochemical quenching could be a regulatory mechanism required specifically in the second and later generations of heterocysts during their transition to maturity. If so, future measurements following the fate of individual cells in a single filament under long-term nitrogen-limited growth should not show the recovery of PS II at the end of heterocyst differentiation.
Interaction of vegetative cells with heterocysts: heterogeneity along the filament
The differentiation of the heterocysts is controlled by a mechanism which results in their formation at a defined spacing along the filament. We were also interested whether the interaction of the vegetative cells with the differentiating heterocysts is reflected in the heterogeneity of photosynthetic characteristics of individual cells along the filament. This heterogeneity was neglected in the routine measurements shown and discussed above, in which always data from three heterocysts and their neighbouring vegetative cells were processed to characterise the sample. We have, therefore, analysed a string of cells including two (pro)heterocysts 72 h after the NSD and results of the analysis of all cells along the filament are presented in Fig. 5. In this particular experiment, nitrogen fixation was already significant at 72 h after NSD. That means the filament in Fig. 5 was in the middle of the acclimation period.
Heterogeneity of FY along the Anabaena filament. Top left panel image of F m values per pixel expressed in a false colour scale (from black = 0 via blue to red = maximum). Other panels values of the FY parameters (indicated as label of the Y axis) calculated from average FY values for the individual cells of the filament plotted against the serial number of the cell in the filament. The orientation of the string follows from the serial numbers for some of the cells indicated the FY image. Arrows show heterocysts (cells 7, 17) of the filaments
In Fig. 5A the image of F m values in individual pixels of the records is displayed in a false colour scale (from black = 0 via blue to red = maximum). Average values of F 0 and F v for individual cells are graphically plotted against the serial cell number in the other panels of the figure. Cells No. 7 and No. 17 were mature heterocysts as confirmed by a snapshot of the filament taken after the measurement with the microscope operating in the transmitted light. A well-developed characteristic heterocyst cell envelope and polar nodules within these cells are apparent (picture not shown).
The variation of fluorescence parameters along the filament in principle corresponds to what can be expected from the generalised results presented above. The F 0 and F v values of the heterocysts were lower than of the neighbouring vegetative cells. The F 0 values of the heterocysts amount between 55 and 65 % of the value for vegetative cells, which is roughly in line with the ratio of the corresponding points at 72 h in the F 0 panel of Fig. 3. With F v values the ratio of signals in the heterocyst Nos. 7 and 17 also agrees with the points in the corresponding graph. The PS II photochemical yields of the heterocysts are comparable with the vegetative cells.
Superbright cells
Beside the lower FY of heterocysts, we often observed cells with significantly (up to 300 %) higher FYs F 0 than the neighbouring vegetative cells. These cells often still showed variable fluorescence comparable with the vegetative cells, indicating that part of the antenna pigments is uncoupled from the still active reaction centres of PS II. These bright cells were quite dynamic, they formed within 30 min (Fig. 6) and soon almost completely lost their fluorescence.
Dynamics of bright cells. Images of F 0 values per pixel expressed on grey scale (black = 0 to white = maximum). Serial images of the same filament were taken every 30 min. Numbers indicate cell(s) that suddenly increased their fluorescence yield. Usually, the high intensity state disappeared within 30 min. Subsequently, the cells showed significantly less fluorescence
Conclusions
Application of single-cell fluorescence kinetic microscopy enabled the investigation of the in vivo regulation of photosynthesis in Anabaena sp. strain PCC 7120 during the adaptation to nitrogen deprivation. Changes in the character of fluorescence kinetics as well as in fluorescence parameters were investigated in Anabaena vegetative cells as well as in differentiating heterocyst. Our results demonstrate the presence of active PS II reaction centres in pro- and heterocysts. This might be connected to the occurrence of light-dependent O2 consumption coupled to pseudocyclic electron flow (Mehler reaction) in heterocysts (Milligan et al. 2007). Measurements of photochemical performance on single-cell level allowed us to show the dynamics and heterogeneity of PS II activity during the formation of heterocysts.
Abbreviations
- Chl:
-
Chlorophyll
- FKM:
-
Fluorescence kinetic microscope as defined by Küpper et al. (2000)
- F 0 :
-
Minimal fluorescence yield of a dark adapted sample in non-actinic measuring light
- F m :
-
Maximum fluorescence yield of a dark adapted sample
- F s :
-
Steady state fluorescence under actinic irradiance, after the end of the induction transient
- F v :
-
F m − F 0 = Variable fluorescence
- F p :
-
Fluorescence yield at the peak of the induction curve after the onset of actinic light
- FY:
-
Fluorescence yield
- NSD:
-
Nitrogen step-down
- NPQ:
-
(F m − \(F^{\prime}_{\text{m}})\)/F m = Non-photochemical quenching
- PBS:
-
Phycobilisomes
- PS:
-
Photosystem
- ΔF :
-
\(F^{\prime}_{\text{m}} -F^{\prime}_{\text{t}}\), i.e., the response of fluorescence yield to a saturating irradiation pulse in light acclimated state
- ΦPSII :
-
\(F^{\prime}_{\text{m}} -F^{\prime}_{\text{t}}/F^{\prime}_{\text{m}}\) = Light-acclimated electron flow through PS II (Genty et al. 1989)
References
Adamec F, Kaftan D, Nedbal L (2005) Stress-induced filament fragmentation of Calothrix elenkinii (Cyanobacteria) is facilitated by death of high-fluorescence cells. J Phycol 41:835–839
Adams DG (2000) Heterocyst formation in cyanobacteria. Curr Opin Microbiol 3:518–624
Allen MB, Arnon DI (1955) Studies on nitrogen-fixing blue-green algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol 30:366–372
Allen JF, Mullineaux CW (2004) Probing the mechanism of state transitions in oxygenic photosynthesis by chlorophyll fluorescence spectroscopy, kinetics and imaging. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis advances in photosynthesis and respiration. Springer, Dordrecht, pp 663–678
Almon H, Böhme H (1980) Components of activity of the photosynthetic electron transport system of intact heterocysts isolated from the blue-green algae Nostoc muscorum. Biochim Biophys Acta 592:113–120
Andresen E, Lohscheider J, Setlikova E, Adamska I, Simek M, Küpper H (2010) Acclimation of Trichodesmium erythraeum ISM101 to high and low irradiance analysed on the physiological, biophysical and biochemical level. New Phytol 185:173–188
Bergman B, Gallon JR, Rai AN, Stal LJ (1997) N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev 19:139–185
Berman-Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, Falkowski P (2001) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294:1534–1537
Berman-Frank I, Lundgren P, Falkowski P (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res Microbiol 154:157–164
Biggins J, Bruce D (1989) Regulation of excitation-energy transfer in organisms containing phycobilins. Photosynth Res 20:1–34
Bradley S, Carr NG (1971) The absence of functional photosystem II in heterocysts of Anabaena cylindrica. J Gen Microbiol 68:13–14
Brusca JS, Hale MS, Carrasco CD, Golden JW (1989) Excision of an 11-kilobase-pair DNA element from within the nifD gene in Anabaena variabilis heterocysts. J Bacteriol 171:4138–4145
Cardona T, Battchikova N, Zhang PP, Stensjo K, Aro EM, Lindblad P, Magnuson A (2009) Electron transfer protein complexes in the thylakoid membranes of heterocysts from the cyanobacterium Nostoc punctiforme. Biochim Biophys Acta 1787:252–263
Donze M, Haveman J, Schiereck P (1972) Absence of photosystem II in heterocysts of the blue-green algae Anabaena. Biochim Biophys Acta 256:157–161
Ehira S, Ohmori M, Sato N (2003) Genome-wide expression analysis of the responses to nitrogen deprivation in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. DNA Res 10:97–113
Fay P, Kulasooriya SA (1972) Tetrazolium reduction and nitrogenase activity in heterocystous blue-green algae. Arch Microbiol 87:341–352
Fay P, Walshby AE (1966) Metabolic activities of isolated heterocysts of the blue-green alga Anabaena cylindrica. Nature 209:94–95
Fiedler G, Arnold M, Hannus S, Maldener I (1998) The DevBCA exporter is essential for envelope formation in heterocysts of the cyanobacterium Anabaena sp. strain PCC 7120. Mol Microbiol 27:1193–1202
Flaherty BL, Van Nieuwerburgh F, Head SR, Golden JW (2011) Directional RNA deep sequencing sheds new light on the transcriptional response of Anabaena sp. strain PCC 7120 to combined-nitrogen deprivation. BMC Genomics 12:332
Flores E, Herrero A (2010) Compartmentalized function through cell differentiation in filamentous cyanobacteria. Nat Rev Microbiol 8:39–50
Genty B, Briantais J, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochem Biophys Acta 990:87–92
Hardy RWF (1973) Applications of the acetylene reduction assay for nitrogenase. Soil Biol Biochem 5:47–81
Hebbar PB, Curtis SE (2000) Characterization of devH, a gene encoding a putative DNA binding protein required for heterocyst function in Anabaena sp. strain PCC 7120. J Bacteriol 182:3572–3581
Herrero A, Muro-Pastor AM, Valladares A, Flores E (2004) Cellular differentiation and the NtcA transcription factor in filamentous bacteria. FEMS Microbiol Rev 28:469–487
Jones KM, Haselkorn R (2002) Newly identified cytochrome c oxidase operon in the nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120 specifically induced in heterocysts. J Bacteriol 184:2491–2499
Kana TM (1993) Rapid oxygen cycling in Trichodesmium thiebautii. Limnol Oceanogr 38:18–24
Kaňa R, Prášil O, Komárek O, Papageorgiou GC, Govindjee (2009) Spectral characteristic of fluorescence induction in a model cyanobacterium, Synechococcus sp (PCC 7942). Biochim Biophys Acta 1787:1170–1178
Kaňa R, Kotabová E, Komárek O, Šedivá B, Papageorgiou GC, Govindjee, Prášil O (2012) The slow S to M fluorescence rise in cyanobacteria is due to a state 2 to state 1 transition. Biochim Biophys Acta 1817:1237–1247
Kirilovsky D, Kerfeld CA (2012) The orange carotenoid protein in photoprotection of photosystem II in cyanobacteria. Biochim Biophys Acta 1817:158–166
Kumar K, Mella-Herrera RA, Golden JW (2010) Cyanobacterial heterocysts. Cold Spring Harb Perspect Biol 2:a000315
Kumazaki S, Akari M, Hasegawa M (2013) Transformation of thylakoid membranes during differentiation from vegetative cell into heterocyst visualized by microscopic spectral imaging. Plant Physiol 161:1321–1333
Küpper H, Šetlík I, Trtílek M, Nedbal L (2000) A microscope for two-dimensional measurements of in vivo chlorophyll fluorescence kinetics using pulsed measuring radiation, continuous actinic radiation, and saturating flashes. Photosynthetica 38:553–570
Küpper H, Ferimazova N, Šetlík I, Berman-Frank I (2004) Traffic lights in Trichodesmium. Regulation of photosynthesis for nitrogen fixation studied by chlorophyll fluorescence kinetic microscopy. Plant Physiol 135:2120–2133
Küpper H, Aravind P, Leitenmaier B, Trtílek M, Šetlík I (2007a) Cadmium-induced inhibition of photosynthesis and long-term acclimation to Cd-stress in the Cd hyperaccumulator Thlaspi caerulescens. New Phytol 175:655–674
Küpper H, Seibert S, Parameswaran A (2007b) Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: quantification of chlorophylls and carotenoids in crude extracts by fitting with gauss peak spectra. Anal Chem 79:7611–7627
Küpper H, Šetlík I, Seibert S, Prášil O, Šetlíková E, Strittmatter M, Levitan O, Lohscheider J, Adamska I, Berman-Frank I (2008) Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. New Phytol 179:784–798
Küpper H, Andresen E, Wiegert S, Šimek M, Leitenmaier B, Šetlík I (2009) Reversible coupling of individual phycobiliprotein isoforms during state transitions in the cyanobacterium Trichodesmium analysed by single-cell fluorescence kinetic measurements. Biochim Biophys Acta 1787:155–167
Lang N, Fay P (1971) The heterocysts of blue-green algae. II. Details of ultrastructure. Proc R Soc Lond B 178:193–203
Lex M, Carr NG (1974) The metabolism of glucose by heterocysts and vegetative cells of Anabaena cylindrica. Arch Microbiol 101:161–167
Lockau W, Peterson RB, Wolk CP, Burris RH (1978) Modes of reduction of nitrogenase in heterocysts isolated from Anabaena species. Biochim Biophys Acta 502:298–308
Maldener I, Muro-Pastor AM (2010) Cyanobacterial heterocysts. In: Encyclopedia of life sciences (ELS). Wiley, New York
Maldener I, Fiedler G, Ernst A, Fernandez-Pinas F, Wolk CP (1994) Characterization of devA, a gene required for the maturation of proheterocysts in the cyanobacterium Anabaena sp. strain PCC 7120. J Bacteriol 176:7543–7549
Meeks JC, Elhai J (2002) Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol Mol Biol Rev 66:94–121
Milligan AJ, Berman-Frank I, Gerchman Y, Dismukes GC, Falkowski PG (2007) Light-dependent oxygen consumption in nitrogen-fixing cyanobacteria plays a key role in nitrogenase protection. J Phycol 43:845–852
Mitschke J, Vioque A, Haas F, Hess WR, Muro-Pastor AM (2011) Dynamics of transcriptional start site selection during nitrogen stress-induced cell differentiation in Anabaena sp. PCC7120. Proc Natl Acad Sci USA 108:20130–20135
Muro-Pastor AM, Hess WR (2012) Heterocyst differentiation: from single mutants to global approaches. Trends Microbiol 20:548–557
Murry MA, Olafsen AG, Benemann JR (1981) Oxidation of diaminobenzidine in the heterocyst of Anabaena cylindrica. Curr Microbiol 6:201–206
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and \(F^{\prime}_{v} /F^{\prime}_{m}\) without measuring F 0′. Photosynth Res 54(2):135–142
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975:384–394
Postgate JR (1998) Nitrogen fixation, 3rd edn. Cambridge University Press, Cambridge 112
Roháček K (2002) Chlorophyll fluorescence parameters: the definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 40:13–29
Šetlíková E, Šetlík I, Küpper H, Kasalický V, Prášil O (2005) The photosynthesis of individual algal cells during the cell cycle of Scenedesmus quadricauda studied by chlorophyll fluorescence kinetic microscopy. Photosynth Res 84:113–120
Smith DL, Partiquin DG, Dijak M, Curry GM (1986) The effect of light-dependent oxygen consumption on nitrogenase activity in Anabaena cylindrica. Can J Bot 64:1843–1848
Stewart WD, Haystead A, Pearson HW (1969) Nitrogenase activity in heterocysts of blue-green algae. Nature 224:226–228
Strohmeier U, Gerdes C, Lockau W (1994) Proteolysis in heterocyst-forming cyanobacteria: characterization of a further enzyme with trypsine-like specifity, and of a prolyl endopeptidase from Anabaena variabilis. Z Naturforsch C 49:70–78
Sugiura K, Itoh S (2012) Single-Cell confocal spectrometry of a filamentous cyanobacterium Nostoc at room and cryogenic temperature. Diversity and differentiation of pigment systems in 311 cells. Plant Cell Physiol 53:1492–1506
Tel-Or E, Stewart WDP (1975) Manganese and photosynthetic oxygen evolution by algae. Nature 258:715–716
Tel-Or E, Stewart WDP (1976) Photosynthetic electron transport. ATP synthesis and nitrogenase activity in isolated heterocysts of Anabaena cylindrica. Biochim Biophys Acta 423:189–195
Tel-Or E, Lujik W, Parker L (1977) An inducible hydrogenase in cyanobacteria enchances N2 fixation. FEBS Lett 78:49–52
Valladares A, Herrero A, Pils D, Schmetterer G, Flores E (2003) Cytochrome c oxidase genes required for nitrogenase activity and diazotrophic growth in Anabaena sp. PCC 7120. Mol Microbiol 47(5):1239–1249
Winkenbach F, Wolk CP (1973) Activities of enzymes of the oxidative and reductive pentose phosphate pathways in heterocysts of a blue-green alga. Plant Physiol 52:480–483
Wolk CP (1996) Heterocyst formation. Annu Rev Genet 30:59–78
Wolk CP, Simon RD (1969) Pigments and lipids of heterocysts. Planta 97:126–134
Wolk CP, Ernst A, Elhai J (1994) Heterocyst metabolism and development. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht
Xu X, Elhai J, Wolk CP (2008) Transcriptional and developmental responses by Anabaena to deprivation of fixed nitrogen. In: Herrero A, Flores E (eds) The cyanobacteria molecular biology, genomics and evolution. Caister Academic Press, Norfolk, pp 382–422
Ying L, Huang X, Huang B, Xie J, Zhao J, Zhao XS (2002) Fluorescence emission and absorption spectra of single Anabaena sp. strain PCC7120 cells. Photochem Photobiol 76(3):310–313
Zhang C-C, Laurent S, Sakr S, Peng L, Bedú S (2006) Heterocyst differentiation and pattern formation in cyanobacteria: a chorus of signals. Mol Microbiol 59:367–375
Zhao J, Wolk CP (2008) Developmental biology of heterocysts, 2006. In: DE Whitworth (ed) Myxobacteria: multicellularity and differentiation. ASM Press, Washington, D.C., pp 397–418
Acknowledgments
The authors would like to thank Vit’a Lukes for help with data analysis. Financial support was provided by the GACR grant 206/08/1683 and by the project Algatech (CZ.1.05/2.1.00/03.0110). HK would like to thank the “Studienstiftung des Deutschen Volkes” for a fellowship during the initial part of this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper is dedicated to Dr. Ivan Šetlík (1928–2009), our friend and mentor, who initiated this and several other studies on single-cell physiology of algae and diazotrophic cyanobacteria and was involved in the construction of the fluorescence kinetic microscope.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ferimazova, N., Felcmanová, K., Šetlíková, E. et al. Regulation of photosynthesis during heterocyst differentiation in Anabaena sp. strain PCC 7120 investigated in vivo at single-cell level by chlorophyll fluorescence kinetic microscopy. Photosynth Res 116, 79–91 (2013). https://doi.org/10.1007/s11120-013-9897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-013-9897-z