Abstract
Moderately high temperature reduces photosynthetic capacities of leaves with large effects on thylakoid reactions of photosynthesis, including xanthophyll conversion in the lipid phase of the thylakoid membrane. In previous studies, we have found that leaf temperature of 40°C increased zeaxanthin accumulation in dark-adapted, intact tobacco leaves following a brief illumination, but did not change the amount of zeaxanthin in light-adatped leaves. To investigate heat effects on zeaxanthin accumulation and decay, zeaxanthin level was monitored optically in dark-adapted, intact tobacco and Arabidopsis thaliana leaves at either 23 or 40°C under 45-min illumination. Heated leaves had more zeaxanthin following 3-min light but had less or comparable amounts of zeaxanthin by the end of 45 min of illumination. Zeaxanthin accumulated faster at light initiation and decayed faster upon darkening in leaves at 40°C than leaves at 23°C, indicating that heat increased the activities of both violaxanthin de-epoxidase (VDE) and zeaxanthin epoxidase (ZE). In addition, our optical measurement demonstrated in vivo that weak light enhances zeaxanthin decay relative to darkness in intact leaves of tobacco and Arabidopsis, confirming previous observations in isolated spinach chloroplasts. However, the maximum rate of decay is similar for weak light and darkness, and we used the maximum rate of decay following darkness as a measure of the rate of ZE during steady-state light. A simulation indicated that high temperature should cause a large shift in the pH dependence of the amount of zeaxanthin in leaves because of differential effects on VDE and ZE. This allows for the reduction in ΔpH caused by heat to be offset by increased VDE activity relative to ZE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photosynthetic capacities of leaves are reduced by moderately high temperature (30–40°C). In addition to deactivation of Rubisco (Salvucci and Crafts-Brandner 2004), moderately high temperature can also have large effects on thylakoid membrane structure and thylakoid reactions of photosynthesis. Moderately high temperature increases the fluidity (Raison et al. 1982) and leakiness (Weis 1981; Bukhov et al. 1999; Havaux and Niyogi 1999; Schrader et al. 2004) of thylakoid membranes, causes thylakoid membrane de-stacking (Armond et al. 1980; Gounaris et al. 1983; Gounaris et al. 1984; Xu et al. 2006), and induces state 1 to state 2 transitions (Ovaska et al. 1990; Mohanty et al. 2002; Schrader et al. 2004; Haldimann et al. 2008; Zhang and Sharkey 2009). Under steady-state light, the acceptor side of photosystem II (PSII) tends to become less reduced, while the donor side and reaction centre chlorophyll (P700) of PSI tends to become more reduced under moderately high temperature (Zhang and Sharkey 2009). Transthylakoid proton conductance and counterion movements are significantly increased by heat while the pH component (ΔpH) of the transthylakoid proton motive force (pmf) was much reduced under moderately high temperature (Zhang et al. 2009). The thylakoid lumen pH is a key regulator of thylakoid reactions of photosynthesis (Kramer et al. 2004).
The conversion of violaxanthin to zeaxanthin via the intermediate antheraxanthin in the xanthophyll cycle plays an important role in photoprotection of plants and algae (Gilmore 1997; Demmig-Adams 1998; Niyogi 1999; Ort 2001). Under excess light conditions, low lumen pH induces the conversion of violaxanthin to zeaxanthin, the protonation of the PsbS (a polypeptide of PSII), and/or a conformational change of LHCII, which work together to dissipate the excess light energy from chlorophyll in the form of heat to prevent overexcited PSII centers (Müller et al. 2001; Li et al. 2004; Horton et al. 2005). This is termed energy-dependent quenching (q E), one of the major and most rapid components of non-photochemical quenching (NPQ) (Niyogi 2000). Zeaxanthin has two other important functions besides its role in photoprotection. First, it has been reported to stabilize thylakoid membranes by reducing their fluidity (Sarry et al. 1994; Havaux et al. 1996; Havaux 1998). Second, it can act as an antioxidant and prevent lipid peroxidation (Lim et al. 1992; Sarry et al. 1994; Havaux et al. 2007).
Zeaxanthin is produced through the de-epoxidation of violaxanthin via the intermediate antheraxanthin by violaxanthin de-epoxidase (VDE), and it is converted back to antheraxanthin and finally to violaxanthin through epoxidation by the zeaxanthin epoxidase (ZE) (Siefermann-Harms 1985; Jahns et al. 2009). VDE activity is pH dependent (Pfündel and Dilley 1993; Yamamoto and Bassi 1996). It binds to the thylakoid membrane at pH < 6.5 and is activated at pH < 6.2, with its activity saturated at pH < 5.8 (Pfündel and Dilley 1993; Hager and Holocher 1994). The optimum pH for ZE is 7.8 in the light and 7.4 in the dark, and it is inactive when pH is below 5.5 (Siefermann and Yamamoto 1975), indicating its possible location on the stromal side of the thylakoid membrane. A transmembrane model has been proposed for the xanthophyll cycle, in which de-epoxidation of violaxanthin occurs in the lipid matrix on the luminal side and epoxidation of zeaxanthin occurs on the stromal side (Siefermann and Yamamoto 1975). Therefore, the lumenal pH will have a large effect on the activity of VDE. The accumulation of zeaxanthin is controlled by the competition between zeaxanthin formation through VDE and zeaxanthin consumption by ZE (Takizawa et al. 2007). In this way, zeaxanthin accumulation will be governed not only by the lumenal pH but also by the activity of ZE.
Heat has been found to increase zeaxanthin accumulation in some experiments, and zeaxanthin can help leaves tolerate heat (Havaux 1998). However, heat also reduces the ΔpH across the thylakoid membrane (Zhang et al. 2009), which should reduce the amount of zeaxanthin in leaves at high temperature. A slight heat-induced reduction in zeaxanthin content was found in light-adapted tobacco leaves, but the reduction was not as great as predicted by the reduced ΔpH (Zhang et al. 2009). The increase in zeaxanthin at high temperature could be replicated by using dark-adapted leaves illuminated for just 5 min.
In this study, new experiments were conducted to investigate the time course of heat effects on zeaxanthin accumulation and decay during light induction. In addition to tobacco, in which observations of decreased zeaxanthin at high temperature in light were first made, we used Arabidopsis thaliana leaves. In order to follow the time course of changes in zeaxanthin content, we monitored the absorbance change at 505 nm (ZΔA505), with corrections for the electrochromic shift (ECS) and light scattering effects previously demonstrated, to follow changes in Z content (Zhang et al. 2009) at either 23 or 40°C. The npq1 mutant, deficient in zeaxanthin accumulation, was utilized to test the suitability of the optical method for zeaxanthin measurement. Estimates of the effect of heat on VDE and ZE activities were used in a model of zeaxanthin accumulation. The effects of moderately high temperature on zeaxanthin accumulation and decay were investigated and discussed.
Materials and methods
Plant material
Nicotiana tabacum WU38 (tobacco) plants were grown in a greenhouse with supplemental light of 200–300 μmol photons m−2 s−1 provided by 1,000-W high-pressure sodium lights, 16-h photoperiod, and day/night temperature around 23°C. Young, fully expanded, and intact leaves from 6-week-old plants were used for measurements.
Arabidopsis thaliana (Arabidopsis) ecotype Columbia 0 (Col-0) and npq1 (mutant deficient in zeaxanthin accumulation in the Col-0 background) (Niyogi et al. 1998) were grown in soil under 100 μmol photons m−2 s−1 light intensity in a growth chamber, with 8/16 h day/night, 23/19°C day/night. Plants were fertilized weekly with half strength Hoagland’s nutrient solution. Young, fully expanded, and intact leaves from 6-week-old plants were used for measurements.
Spectroscopic measurement
Optical measurements of zeaxanthin accumulation and decay, the ECS, and chlorophyll fluorescence were conducted in a newly constructed spectrophotometer instrument, which was modified from the previous non-focusing optics flash spectrophotometer (NoFOSpec) (Avenson et al. 2004; Takizawa et al. 2008; Zhang et al. 2009). Zeaxanthin accumulation has a signature absorbance peak at 505 nm (Yamamoto and Kamite 1972); however, because it overlaps with other components, such as ECS (peak at 518 nm) and q E-related absorbance changes (peak at 535 nm), multi-wavelength deconvolution is necessary to get a more precise measurement of zeaxanthin level. The deconvolution for zeaxanthin was similar to that described previously (Zhang et al. 2009), except for the additional 488 nm signal (peak of the reversed ECS absorbance change), which was used for further refining the measurements. The relative extinction coefficients for each component (zeaxanthin, ECS, and q E) at three wavelengths (505, 520, and 535 nm) were determined as described (Kramer and Sacksteder 1998; Zhang et al. 2009). For this purpose, 488 nm light was used to finely tune the optical measurement. The deconvolution formulas were solved by linear algebra giving the following equation for calculating relative zeaxanthin changes:
The ECS signal in tobacco was obtained by the formula
Dark-adapted, intact tobacco leaves, which should have little zeaxanthin, were used for measurements. A dark baseline was first recorded for 30 min and then actinic light (347, 665, or 1,000 μmol photons m−2 s−1) provided by red-light-emitting diodes (LEDs) was on for 45 min to induce zeaxanthin formation, followed by 30 min dark or weak light to dissipate zeaxanthin. During the experiment, four measuring wavelengths (488, 505, 520, and 535 nm) were recorded simultaneously. The amount of zeaxanthin accumulated was estimated by the deconvoluted 505 signal (ZΔA505). The rate of zeaxanthin accumulation at light initiation was estimated by the linear slope of the corresponding ZΔA505 curve. During the post-illumination darkness, the rate of zeaxanthin decay was estimated by linear fitting of the corresponding ZΔA505 curve, with the absolute value of the linear slope taken as the decay rate. For weak light experiments, leaves were first illuminated with 1,000 μmol photons m−2 s−1 light for 45 min, followed by weak light (25 μmol photons m−2 s−1) for 30 min, instead of darkness.
The long-period ECS (25-s dark interval) with four wavelengths was performed at the end of 3 or 45 min light to estimate the pH component (∆pH) of the transthylakoid pmf in leaves as described (Cruz et al. 2001; Zhang et al. 2009). The ECS signal was deconvoluted from 488-, 505- and 535-nm signals using Eq. 2. The inverted phase of the ECS is proportional to the ∆pH component of the transthylakoid pmf. ∆pH reported is denoted as “relative” because it was measured by absorbance change, and one unit is equivalent to a change of 0.001 absorbance units.
Energy-dependent quenching (q E) was estimated from the saturation-pulse-induced chlorophyll a fluorescence yields in light-adapted tobacco leaves by equations below, where F ′m is the maximum fluorescence yield caused by a saturating flash pulse in leaves illuminated for 45 min, and F ″m is its recovery in the dark after the light was off for 20 min. This simplified method not requiring long periods of dark adaptation depends on there being very little photo inhibition during the experiments, as was found for heat-treated Arabidopsis (Zhang and Sharkey 2009).
Zeaxanthin accumulation and decay in Arabidopsis leaves was monitored by the spectrophotometer using the same method as in tobacco, except that only 1,000 μmol photons m−2 s−1 was used for 45-min illumination. The zeaxanthin contribution in Arabidopsis was obtained with the formula
The ECS signal in Arabidopsis was obtained by the following formula
Heat treatment and gas exchange
The experiments described above were performed under either 23 or 40°C, each with leaves from different plants. The high-temperature treatment in tobacco leaves was same as before (Zhang et al. 2009). Intact tobacco leaves were clamped into the leaf chamber in the spectrophotometer under flowing humidified ambient air (79% N2, 21% O2, 372 parts per million by volume, a mole fraction, and ppmv CO2). Heat treatment was imposed by flowing water from either of two thermostated water baths, one set at 23°C and the other at 40°C, through a metal block that was attached to the actinic and measuring light pipe of the spectrophotometer, just above the leaf. The temperature of the leaf (monitored by thermocouple attached to the leaf) was switched between 23 and 40°C in 2 min.
The net CO2 assimilation rate in tobacco was measured by gas exchange as described (Zhang and Sharkey 2009). Mixed gas (79% N2, 21% O2, and 372 ppmv CO2) with controlled humidity (dew point at 16.5°C) flowed into the leaf chamber and passed over the abaxial leaf surface. The H2O and CO2 before and after the leaf chamber were measured using a CO2/H2O analyzer (Model LI-6262, LI-COR, Lincoln, Neb. USA). The net CO2 assimilation rate and stomatal conductance under 45-min illumination were calculated using equations of Farquhar and von Caemmerer (1982).
For Arabidopsis experiments, the spectrophotometer was connected with a LI-COR 6400 (LI-COR, Inc., Lincoln, NE) which provided controlled gas concentration (79% N2, 21% O2, and 400 ppmv CO2) and humidity (dew point at 16.5°C), and allowed measurement of net CO2 assimilation rates and stomatal conductance simultaneous with the optical measurements. Intact Arabidopsis leaves were clamped into the LI-COR leaf chamber that was anchored in the light path of the spectrophotometer. Two thermostated water baths, one set at 23°C and the other at 40°C, were connected to the leaf chamber for heat treatment.
Results
Optical measurement of effect of temperature on zeaxanthin
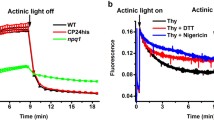
Moderately high temperature increased the zeaxanthin accumulation rates at light initiation and zeaxanthin decay rates during the initial darkness after illumination (Fig. 1). The amount of zeaxanthin (ZΔA505) in tobacco leaves increased much faster upon illumination at 40°C than at 23°C (Fig. 1A). Higher levels of zeaxanthin were found at 40°C until 10–15 min after turning on the light. By the end of 45-min light, ZΔA505 at 40 and 23°C reached a similar level. Following illumination, ZΔA505 at 40°C decayed much faster than that at 23°C. Similar effects of heat on zeaxanthin accumulation and decay were found in intact leaves of Arabidopsis, except that the ZΔA505 rose much more rapidly under high temperature and ZΔA505 decay in darkness at 23°C was much slower than that in tobacco (Fig. 1B). The Arabidopsis mutant deficient in zeaxanthin accumulation, npq1, exhibited little change of ZΔA505 in response to illumination or darkness at either 23 or 40°C (Fig. 1B), verifying our optical method employed to monitor zeaxanthin accumulation and decay in vivo.
Monitoring zeaxanthin accumulation and decay by ZΔA505. Dark-adapted, intact tobacco and Arabidopsis leaves were given 45 min illumination, followed by a 30-min post-illumination dark period. The 505-nm signal (ZΔA505), representing the amount of zeaxanthin, was deconvoluted by 488-, 520-, and 535-nm signals. Zeaxanthin accumulation and decay were monitored by ZΔA505 before, during, and after the light, with the signal before illumination as the dark baseline. Measurements were performed at either 23 or 40°C with separate leaves. Three light intensities (347, 665, and 1,000 μmol photons m−2 s−1) were used individually for tobacco, and 1,000 μmol photons m−2 s−1 light was used for Arabidopsis. The curves from 1,000 μmol photons m−2 s−1 light are presented here as demonstrations. The npq1 mutant is deficient in zeaxanthin accumulation, and it is on the background of Columbia-0 (Col)
Effect of temperature on zeaxanthin and photosynthesis
Three-minute illumination induced zeaxanthin formation in tobacco leaves, but there was more zeaxanthin accumulation at 40°C than at 23°C at three light intensities (Student’s t-test, P < 0.05) (Fig. 2A). At the end of 45-min light, zeaxanthin levels increased at both temperatures as compared to that at the end of 3-min light (Fig. 2B). However, the difference in zeaxanthin content between leaves at 23°C and 40°C disappeared after 45-min light. Within the noise level, both the heated and the control leaves had a similar amount of zeaxanthin at the end of 45-min light (P > 0.05), and high light intensity induced relatively more zeaxanthin than low light. Net CO2 assimilation rates in tobacco leaves with 3-min light were about 25% of those in leaves with 45-min light (Fig. 2C, D), indicating that by the end of 3-min light, photosynthesis was not fully activated.
Zeaxanthin accumulation and CO2 assimilation in tobacco leaves with 3- or 45-min light. Dark-adapted, intact tobacco leaves were illuminated for 45 min with one of the three light intensities (347, 665, 1,000 μmol photons m−2 s−1) at either 23 or 40°C, each with a different leaf. A, B, The amounts of zeaxanthin accumulated with 3- or 45-min light was estimated by ZΔA505. C, D Net CO2 assimilation rates were measured by gas exchange (mean ± SE, n = 3–5)
Heat effects on zeaxanthin accumulation and net CO2 assimilation rates in Arabidopsis leaves at 1,000 μmol photons m−2 s−1 (Fig. 3) were similar to those in tobacco leaves (Fig. 2). With 3-min light, Arabidopsis leaves at 40°C had more zeaxanthin and higher net CO2 assimilation rates than those at 23°C (P < 0.01). By the end of 45-min light, leaves at 40°C had slightly less zeaxanthin than leaves at control temperature, but the difference was not significant (P > 0.05); the net CO2 assimilation rates at 40°C were significantly higher than those at 23°C (P < 0.01).
Zeaxanthin accumulation and CO2 assimilation in leaves of Arabidopsis with 3- or 45-min light. Dark-adapted, intact Arabidopsis (Col-0) leaves were illuminated for 45 min with 1,000 μmol photons m−2 s−1 light intensity at either 23 or 40°C, each with different leaves. A The amount of zeaxanthin accumulated with 3 or 45 min light was estimated by ZΔA505. B Net CO2 assimilation rates were measured by gas exchange (mean ± SE, n = 6–8)
High temperature increased the stomatal conductance in tobacco leaves at all the three light intensities (Fig. 4A). At the same temperature, leaves illuminated with higher light had higher stomatal conductance than those with lower light, although the difference between 347 and 665 μmol photons m−2 s−1 was small. In tobacco leaves at 40°C, the rate of increase of stomatal conductance between 15 and 20 min of illumination was greater than at 23°C. However, after 45 min of illumination, the stomatal conductance in tobacco leaves at 40°C reached a plateau but continuously increased in leaves at 23°C. In Arabidopsis, the stomatal conductance was comparable in leaves at 23 and 40°C until 20 min of illumination (Fig. 4B). Afterward, stomatal conductance at 23°C continued to increase while stomatal conductance at 40°C remained constant (Fig. 4B).
Stomatal conductance in leaves of tobacco and Arabidopsis during 45-min illumination. Stomatal conductance was measured by gas exchange. A Dark-adapted, intact tobacco leaves were illuminated for 45 min with one of the three light intensities (347, 665, and 1,000 μmol photons m−2 s−1) at either 23 or 40°C, each with a different leaf. B Dark-adapted, intact Arabidopsis (Col-0) leaves were illuminated for 45 min with 1,000 μmol photons m−2 s−1 light intensity at either 23 or 40°C, each with a different leaf (mean ± SE, n = 3–6 for tobacco, n = 4–6 for Arabidopsis)
Temperature and ΔpH
In tobacco leaves at 23°C, the ∆pH component of the transthylakoid pmf was reduced by the end of 45 min of illumination with 1,000 μmol photons m−2 s−1 light relative to the value at the end of 3-min light (Fig. 5). The ∆pH in tobacco leaves heated to 40°C was lower at 3 min than after 45 min at 23°C, but no further reduction after 45 min was seen. In Arabidopsis, the ΔpH at 23°C with 45-min light was reduced to 66% of that with 3-min light, and 70% for heated leaves with 3 min light, and 40% for heated leaves with 45-min light.
Relative ∆pH in leaves of tobacco and Arabidopsis during 45-min illumination. Dark-adapted, intact tobacco and Arabidopsis (Col-0) leaves were illuminated for 45 min with 1,000 μmol photons m−2 s−1 at either 23 or 40°C. ∆pH, relative, estimates the pH component of the transthylakoid pmf, and was measured by the inverted phase of the extended ECS (25 s dark) deconvoluted by subtracting 488-, 505-, and 535-nm signals from 520-nm signal. ∆pH here is denoted as “relative” because it was measured by absorbance change and one unit is equivalent to a change of 0.001 absorbance units (mean ± SE, n = 4 for tobacco, n = 3–5 for Arabidopsis)
At the end of 45 min light, the ∆pH was reduced by high temperature in all the three light intensities in tobacco leaves (Fig. 6), consistent with our previous report in light-adapted leaves (Zhang et al. 2009). Heat caused little change of zeaxanthin accumulation (ZΔA505) when compared at the same light intensity (P > 0.05). The relationship between zeaxanthin accumulation and ∆pH was shifted to lower pH at 40°C compared with 23°C (Fig. 6A). q E decreased at 40°C as compared with 23°C, but with the same light intensity (Fig. 6B). Nonetheless, the relationship between q E and light-induced ∆pH was shifted to lower pH at 40°C compared with 23°C except possibly at the lowest light intensity. The relationship between q E and zeaxanthin accumulation is consistent with a lower luminal pH at high temperature (specifically, lower q E per zeaxanthin), and the amount of zeaxanthin was nearly the same at high temperature as at low temperature (Fig. 6C).
Zeaxanthin and qE in response to ΔpH in tobacco leaves with 45-min light. Dark-adapted, intact tobacco leaves were illuminated for 45 min with one of the three light intensities (347, 665, and 1,000 μmol photons m−2 s−1) at either 23 or 40°C. Two data points with the same light intensity but one at 23°C and the other at 40°C were connected by dashed lines. The amount of zeaxanthin accumulated with 45-min light was estimated by ZΔA505. The measurement of ∆pH is the same as in Fig. 5. q E (the energy-dependent quenching, activated by lumen acidification) was measured by chlorophyll a fluorescence and calculated as (F ″m /F ′m −1), F ′m is the maximal fluorescence level from leaves illuminated for 45 min, and F ″m is its recovery in the dark after the light. (mean ± SE, n = 3)
Activities of VDE and ZE
Heat increased the activities of both VDE and ZE in intact leaves of tobacco and Arabidopsis (Fig. 7). The experiments were started with dark-adapted leaves which should have little zeaxanthin initially and thus little substrate for ZE; therefore the initial rate of accumulation of zeaxanthin after the light was turned on should mainly reflect the activity of VDE. At light initiation, the VDE activity estimated by zeaxanthin accumulation rates in tobacco leaves at 40°C was 3–4 times faster than that at 23°C but exhibited little dependence on light intensity (Fig. 7A).
Estimating the activities of VDE and ZE based on zeaxanthin accumulation and decay rates. A, B Dark-adapted, intact tobacco leaves were illuminated for 45 min with one of three light intensities (347, 665, and 1,000 μmol photons m−2 s−1) at either 23 or 40°C, each with a different leaf. The activity of VDE at light initiation (the first few seconds after light was on) was estimated by the initial accumulation rate of zeaxanthin (υΔA505), which is the linear slope of the corresponding ZΔA505 curve. The activity of ZE by the end of light was estimated by the initial decay rate of zeaxanthin (υΔA505−Decay) for the first few seconds after light was off which is the absolute value of the linear slope of the corresponding ZΔA505 curve (mean ± SE, n = 3). C, D, Dark-adapted, intact Arabidopsis (Col-0) leaves were illuminated for 45 min with 1,000 μmol photons m−2 s−1 light at either 23 or 40°C. The estimations of VDE and ZE activities were same as in tobacco (mean ± SE, n = 7–8)
Upon the cessation of light, photosynthetic electron transport and proton flux will stop rapidly. As a result, the transthylakoid ∆pH will be dissipated. Owing to its dependence on ∆pH, the activity of VDE should cease in a few seconds as ΔpH decreases in the dark, but the activity of ZE during the initial dark period should continue at a rate close to the one before the light was turned off. If this holds true, then the initial decay rate of zeaxanthin (υΔA505−Decay), just after the light was turned off, should reflect the activity of ZE during illumination. During the first few seconds of darkness the ZE activity in tobacco leaves was about twice as fast at 40°C as at 23°C, and it was slightly faster following lower light than higher light (Fig. 7B). In Arabidopsis leaves, both VDE and ZE activities were doubled at 40°C as compared with those at 23°C (Fig. 7C, D).
The zeaxanthin decay rate was significantly reduced after 15-min darkness in both tobacco and Arabidopsis (Fig. 8). However, switching from high light to 25 μmol photons m−2 s−1, rather than to darkness resulted in the high initial rate of decay extending for much longer time. Fifteen minutes after the turning off the actinic light, the decay rates of zeaxanthin in weak light illumination (25 μmol photons m−2 s−1) were much faster in both tobacco and Arabidopsis leaves than those in darkness, but the maximum rate was similar regardless of darkness or weak light.
Zeaxanthin decay in darkness or weak light. Dark-adapted, intact tobacco (A) or Arabidopsis (Col-0) (B) leaves were illuminated for 45 min with 1,000 μmol photons m−2 s−1 light at 23°C, then the 1,000 μmol photons m−2 s−1 light was off, either darkness or 25 μmol photons m−2 s−1 weak light was on for 30 min. The upward and downward arrows indicate the onset and cessation of 1,000 μmol photons m−2 s−1 light
Discussion
Moderately high temperature accelerated the activities of both VDE and ZE in intact tobacco and Arabidopsis leaves, but to different degrees (Figs. 1, 7). As proposed previously (Takizawa et al. 2007), zeaxanthin accumulation in the steady state is affected by the balance between VDE, which is highly dependent on lumen pH, and ZE, which is independent of lumen pH. Furthermore, Takizawa et al. (2007) proposed that changing the ratio of activities of VDE and ZE will shift the lumen pH dependence of zeaxanthin accumulation. We adapted the analysis in Takizawa et al. (2007) for the data reported here (Appendix), and the results indicate that there could be a large shift in the pH dependence of zeaxanthin accumulation because of the differential effects of heat on VDE and ZE (Fig. 9). This provides one potential explanation for the small change in zeaxanthin in heated leaves, even though the ΔpH is reduced and thus thylakoid lumen pH is increased. However, given the complexity of violaxanthin/zeaxanthin interconversion, other explanations could also account for this phenomenon. For example, it is possible that the conversion reached a maximum because of violaxanthin availability to VDE, and therefore the conversion rate became insensitive to pH.
High temperature increased zeaxanthin accumulation in dark-adapted leaves with 3-min illumination, but not in leaves with 45-min illumination (Figs. 2, 3), confirming our previous results (Zhang et al. 2009). There are several possibilities to explain this phenomenon. In dark-adapted leaves with a short illumination time, there was less zeaxanthin accumulated initially; therefore there was less substrate for ZE activity; however, with illumination of longer duration (45 min), zeaxanthin accumulation induced by light may inhibit the enzyme activity of VDE (Havir et al. 1997) and stimulate ZE activity. As a result, the heat-accelerated VDE activity was dominant when dark-adapted leaves had just 3-min light, resulting in more zeaxanthin accumulation at 40°C than at 23°C. However, after 45-min illumination, the inhibition of VDE by zeaxanthin and the stimulation of ZE by both zeaxanthin and heat resulted in a balance between VDE and ZE, producing comparable or sometimes less zeaxanthin accumulation in heat-treated, light-adapted leaves.
Second, photosynthesis was not fully activated by the end of 3-min light but was so after 45 min of illumination (Figs. 2, 3). In tobacco, the increase in photosynthetic rate between 3 and 45 min may have been caused in part by stomatal opening, but in Arabidopsis, the increase in photosynthesis with time at 40°C was not the result of stomatal effects to any great degree (Fig. 4). By the end of 45-min light when photosynthesis is fully induced, the increased rate of use of ATP in photosynthesis could make the ΔpH smaller than that at 3 min (Fig. 5).
VDE activity during initial illumination was increased at 40°C in intact leaves of tobacco and Arabidopsis, which is consistent with the results from in vitro experiments showing that the conversion from violaxanthin to zeaxanthin is highly temperature dependent (Latowski et al. 2002). What caused the increased activity of VDE under moderately high temperature? Latowski et al. (2002) found that from 4°C to 25°C, the maximal time required for the conversion of violaxanthin to antheraxanthin was dramatically shortened from 217 min to 5.6 min, but there was little effect on the time required for the conversion from antheraxanthin to zeaxanthin which is much faster (around 3 min), indicating that the conversion from violaxanthin to antheraxanthin is rate limiting and much more sensitive to temperature. Jahns et al. (2009) proposed that the release of violaxanthin from antenna proteins and its subsequent diffusion from the antenna-binding site to the VDE-binding site are the rate-limiting steps of de-epoxidation. It could be because the release of violaxanthin or its diffusion to VDE is faster under high temperature. The properties of the thylakoid membrane lipid have a strong impact on xanthophyll conversion (Latowski et al. 2000; Latowski et al. 2002; Goss et al. 2005), and the activity of VDE is proposed to be limited by xanthophyll diffusion within the membrane (Macko et al. 2002). Heat stress can increase the fluidity of the thylakoid membrane (Raison et al. 1982), which may accelerate the diffusion of violaxanthin toward the VDE-binding site and make violaxanthin more accessible to VDE.
The increased ZE activity at high temperature may result from the activation energy of the enzyme. In addition, the increased fluidity of the thylakoid membrane at high temperature may increase the diffusion of zeaxanthin to the site where ZE is located. In contrast, ZE activity was slowed at 4°C compared with 20°C (Reinhold et al. 2008). Moreover, ZE activity in leaves illuminated by lower light was faster than in those with higher light (Fig. 7), consistent with the hypothesis that ZE activity is reduced by high light, which may involve a direct modification of the enzyme (Reinhold et al. 2008).
The rapid decay of zeaxanthin immediately after turning off the light slowed considerably in darkness but not under weak light (Fig. 8), as reported by Hager (1966). Zeaxanthin formation requires NADPH. Arabidopsis mutants with reduced NADPH concentration accumulated high levels of zeaxanthin, suggesting a possible role for NADPH as an important regulator of ZE activity (Takahashi et al. 2006). The maintenance of ZE activity by weak light could result from maintenance of reduced NADPH by 25 μmol photons m−2 s−1 light, but other explanations, such as phosphorylation, are also possible.
In summary, zeaxanthin accumulation and decay were monitored in vivo by the deconvoluted 505-nm absorbance in dark-adapted, intact tobacco and Arabidopsis leaves under 45-min light induction at either 23 or 40°C. Moderately high temperature increased the activities of both VDE and ZE, which may result from heat-increased fluidity of the thylakoid membrane. More zeaxanthin was accumulated in heated leaves initially when photosynthesis was not activated and ZE may be limited by the amount of zeaxanthin; however, by the end of 45-min light, heated leaves did not have more zeaxanthin than those at 23°C. Nonetheless, relatively more zeaxanthin and q E were formed than expected, given the reduced ΔpH found at high temperature. A large shift in the pH dependence of zeaxanthin accumulation allowed for the effect of heat in reducing the ΔpH to be offset by increased VDE activity. ZE activity was accelerated by weak light at 23°C, confirming previous in vitro findings.
References
Armond PA, Björkman O, Staehelin LA (1980) Dissociation of supramolecular complexes in chloroplast membranes—a manifestation of heat damage to the photosynthetic apparatus. Biochim Biophys Acta 601:433–442
Avenson TJ, Cruz JA, Kramer DM (2004) Modulation of energy dependent quenching of excitons in antenna of higher plants. Proc Natl Acad Sci USA 101:5530–5535
Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59:81–93
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001) Contribution of electric field Δφ to steady-state transthylakoid proton motive force in vitro and in vivo. Control of pmf parsing into Δφ and ΔpH by counterion fluxes. Biochemistry 40:1226–1237
Demmig-Adams B (1998) Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol 39:474–482
Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology I. Encyclopedia of plant physiology new series. Springer, Berlin, pp 549–587
Gilmore AM (1997) Mechanistic aspects of xanthophyll cycle-dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant 99:197–209
Goss R, Lohr M, Latowski D, Grzyb J, Vieler A, Wilhelm C, Strzalka K (2005) Role of hexagonal structure-forming lipids in diadinoxanthin and violaxanthin solubilization and de-epoxidation. Biochemistry 44:4028–4036
Gounaris K, Brain APR, Quinn PJ, Williams WP (1983) Structural and functional-changes associated with heat-induced phase-separations of non-bilayer lipids in chloroplast thylakoid membranes. FEBS Lett 153:47–52
Gounaris K, Brain ARR, Quinn PJ, Williams WP (1984) Structural reorganization of chloroplast thylakoid membranes in response to heat-stress. Biochim Biophys Acta 766:198–208
Hager A (1966) Die Zusammenhänge zwischen lichtinduzierten Xanthophyll-Umwandlungen und Hill-Reaktion. Berichte Deutsche Botanische Gesellschaft 79:94–107
Hager A, Holocher K (1994) Localization of the xanthophyll-cycle enzyme violaxanthin de-epoxidase within the thylakoid lumen and abolition of its mobility by a (light-dependent) pH decrease. Planta 192:581–589
Haldimann P, Gallé A, Feller U (2008) Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol 28:785–795
Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3:147–151
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96:8762–8767
Havaux M, Tardy F, Ravenel J, Chanu D, Parot P (1996) Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. Plant Cell Environ 19:1359–1368
Havaux M, Dall’Osto L, Bassi R (2007) Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol 145:1506–1520
Havir EA, Tausta SL, Peterson RB (1997) Purification and properties of violaxanthin de-epoxidase from spinach. Plant Sci 123:57–66
Horton P, Wentworth M, Ruban A (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579:4201–4206
Jahns P, Latowski D, Strzalka K (2009) Mechanism and regulation of the violaxanthin cycle: the role of antenna proteins and membrane lipids. Biochim Biophys Acta 1787:3–14
Kramer DM, Sacksteder CA (1998) A diffused-optics flash kinetic spectrophotometer (DOFS) for measurements of absorbance changes in intact plants in the steady-state. Photosynth Res 56:103–112
Kramer DM, Kanazawa A, Cruz JA, Ivanov B, Edwards GE (2004) The relationship between photosynthetic electron transfer and its regulation. In: Govindjee X, Papageorgiou GC (eds) Chlorophyll fluorescence: the signature of green plant photosynthesis. Kluwer, Norwell, pp 251–278
Latowski D, Kostecka A, Strzalka K (2000) Effect of monogalactosyldiacylglycerol and other thylakoid lipids on violaxanthin de-epoxidation in liposomes. Biochem Soc Trans 28:810–812
Latowski D, Kruk J, Burda K, Skrzynecka-Jaskier M, Kostecka-Gugala A, Strzalka K (2002) Kinetics of violaxanthin de-epoxidation by violaxanthin de-epoxidase, a xanthophyll cycle enzyme, is regulated by membrane fluidity in model lipid bilayers. Eur J Biochem 269:4656–4665
Li XP, Gilmore AM, Caffarri S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Lim BP, Nagao A, Terao J, Tanaka K, Suzuki T, Takama K (1992) Antioxidant activity of xanthophylls on peroxyl radical-mediated phospholipid peroxidation. Biochim Biophys Acta 1126:178–184
Macko S, Wehner A, Jahns P (2002) Comparison of violaxanthin de-epoxidation from the stroma and lumen sides of isolated thylakoid membranes from Arabidopsis: implications for the mechanism of de-epoxidation. Planta 216:309–314
Mohanty P, Vani B, Prakash JS (2002) Elevated temperature treatment induced alteration in thylakoid membrane organization and energy distribution between the two photosystems in Pisum sativum. Z Naturforsch 57c:836–842
Müller P, Li XP, Niyogi KK (2001) Non-photochemichal quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Niyogi KK (1999) Photoprotection revisited: Genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 6:455–460
Niyogi KK, Grossman AR, Bjorkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
Ort DR (2001) When there is too much light. Plant Physiol 125:29–32
Ovaska J, Mäenpää P, Nurmi A, Aro EM (1990) Distribution of chlorophyll-protein complexes during chilling in the light compared with heat-induced modifications. Plant Physiol 93:48–54
Pfündel EE, Dilley RA (1993) The pH-dependence of violaxanthin deepoxidation in isolated pea chloroplasts. Plant Physiol 101:65–71
Raison JK, Roberts JKM, Berry JA (1982) Correlations between the thermal-stability of chloroplast (thylakoid) membranes and the composition and fluidity of their polar lipids upon acclimation of the higher-plant, Nerium oleander, to growth temperature. Biochim Biophys Acta 688:218–228
Reinhold C, Niczyporuk S, Beran KC, Jahns P (2008) Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions. Biochim Biophys Acta 1777:462–469
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186
Sarry JE, Montillet JL, Sauvaire Y, Havaux M (1994) The protective function of the xanthophyll cycle in photosynthesis. FEBS Lett 353:147–150
Schrader SM, Wise RR, Wacholtz WF, Ort DR, Sharkey TD (2004) Thylakoid membrane responses to moderately high leaf temperature in Pima cotton. Plant Cell Environ 27:725–735
Siefermann D, Yamamoto HY (1975) Properties of NADPH and oxygen-dependent zeaxanthin epoxidation in isolated-chloroplasts—a transmembrane model for the violaxanthin cycle. Arch Biochem Biophys 171:70–77
Siefermann-Harms D (1985) Carotenoids in photosynthesis 1. Location in photosynthetic membranes and light-harvesting function. Biochim Biophys Acta 811:325–355
Takahashi H, Watanabe A, Tanaka A, Hashida SN, Kawai-Yamada M, Sonoike K, Uchimiya H (2006) Chloroplast NAD kinase is essential for energy transduction through the xanthophyll cycle in photosynthesis. Plant Cell Physiol 47:1678–1682
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767:1233–1244
Takizawa K, Kanazawa A, Kramer DM (2008) Depletion of stromal Pi induces high ‘energy-dependent’ antenna excition quenching (qE) by decreasing proton conductivity at CFo-CF1 ATP synthase. Plant Cell Environ 31:235–243
Weis E (1981) Reversible effects of high, sublethal temperatures on light-induced light scattering changes and electrochromic pigment absorption shift in spinach leaves. Z Pflanzenphysiol 101:169–178
Xu S, Li JL, Zhang XQ, Wei H, Cui LJ (2006) Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidant metabolites, and ultrastructure of chloroplasts in two cool-season turfgrass species under heat stress. Environ Exp Bot 56:274–285
Yamamoto HY, Bassi R (1996) Carotenoids: localization and function. In: Ort DR, Yocum CF (eds) Oxygenic photosynthesis: the light reactions. Springer, Netherlands, pp 539–563
Yamamoto HY, Kamite L (1972) Effects of dithiothreitol on violaxanthin deepoxidation and absorbance changes in 500 nm region. Biochim Biophys Acta 267:538
Zhang R, Sharkey TD (2009) Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth Res 100:29–43
Zhang R, Cruz JA, Kramer DM, Magallanes-Lundback ME, DellaPenna D, Sharkey TD (2009) Moderate heat stress reduces the pH component of the transthylakoid proton motive force in light-adapted intact tobacco leaves. Plant Cell Environ 32:1538–1547
Acknowledgments
This research was supported by the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, Grant number 2004-35100-14860 and Project number MICL03483 (TDS) and the US Department of Energy (DE-FG02-08ER15955, (DMK).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Model of zeaxanthin dependence on pH
The relative amount of zeaxanthin was modeled following Takizawa et al. (2007) and presented briefly here. The synthesis of zeaxanthin was modeled as
where Z is the relative concentration of zeaxanthin (strictly Z + A), k 1 is the rate constant of VDE, and 1−Z is the relative concentration of violaxanthin. The disappearance of zeaxanthin by ZE was modeled as
where the value for k 2, the ZE rate constant, was taken from the initial decay of zeaxanthin upon darkening the leaf. Although the factors controlling ZE and VDE are complex (see Discussion), the only requirement for this analysis is that the concentration of V affects the rate of VDE and that the concentration of Z affects the rate of ZE. The concentration of Z at equilibrium will be that concentration where formation equals disappearance:
Solving for Z.
Because of the pH dependence of k 1, Z will depend on the ΔpH. The pH of the lumen was estimated as
where pHL is the pH of the lumen; pHS is the pH of the stroma, taken to be 7.8 and constant (Takizawa et al. 2007). The activity of VDE (k 1) was modeled as.
where k 0 is a theoretical maximal rate of VDE at optimal pH, nH is an effective Hill coefficient, and pK is the effective pK of protonation. The values developed by Takizawa et al. (2007) were used here (nH = 4, pK = 6). The scale for ΔpH was estimated by assuming that the optical signal reported in Fig. 5 after 3 min represented a maximum ΔpH and was equal to 2.5 pH units. The ΔpH after 45 min were estimated as 1.9 and 1.2 for tobacco at 23 and 40°C, respectively, and 2.2 and 0.9 for Arabidopsis at 23 and 40°C, respectively. k 0 was adjusted until k 1 was equal to the observed values at the indicated ΔpH. The results shown in Fig. 9 illustrate that the relationship between ΔpH and zeaxanthin concentrations can vary because of changes in the ratios of rates of VDE and ZE activity, as first shown by Takizawa et al. (2007). The results of this modeling and data reported here show that the effects of heat, in causing a loss of ΔpH but increasing VDE activity, can be offsetting resulting in little change in zeaxanthin concentration in moderately heat-stressed leaves.
Rights and permissions
About this article
Cite this article
Zhang, R., Kramer, D.M., Cruz, J.A. et al. The effects of moderately high temperature on zeaxanthin accumulation and decay. Photosynth Res 108, 171–181 (2011). https://doi.org/10.1007/s11120-011-9672-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-011-9672-y