Abstract
Photosynthesis is a very efficient photochemical process. Nevertheless, plants emit some of the absorbed energy as light quanta. This luminescence is emitted, predominantly, by excited chlorophyll a molecules in the light-harvesting antenna, associated with Photosystem II (PS II) reaction centers. The emission that occurs before the utilization of the excitation energy in the primary photochemical reaction is called prompt fluorescence. Light emission can also be observed from repopulated excited chlorophylls as a result of recombination of the charge pairs. In this case, some time-dependent redox reactions occur before the excitation of the chlorophyll. This delays the light emission and provides the name for this phenomenon—delayed fluorescence (DF), or delayed light emission (DLE). The DF intensity is a decreasing polyphasic function of the time after illumination, which reflects the kinetics of electron transport reactions both on the (electron) donor and the (electron) acceptor sides of PS II. Two main experimental approaches are used for DF measurements: (a) recording of the DF decay in the dark after a single turnover flash or after continuous light excitation and (b) recording of the DF intensity during light adaptation of the photosynthesizing samples (induction curves), following a period of darkness. In this paper we review historical data on DF research and recent advances in the understanding of the relation between the delayed fluorescence and specific reactions in PS II. An experimental method for simultaneous recording of the induction transients of prompt and delayed chlorophyll fluorescence and decay curves of DF in the millisecond time domain is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delayed (chlorophyll) fluorescence (DF) is light that is emitted from green plants, algae and photosynthesizing bacteria in the red-infra-red region of the spectrum for a short time after they have been exposed to light, but after the prompt fluorescence has decayed. The source of this emission is either chlorophyll a, or bacteriochlorphyll molecule, depending upon the organism used. In the specialized literature, this phenomenon is described by several terms, each reflecting its specific characteristics—e.g., delayed fluorescence, delayed luminescence, delayed light emission, and afterglow. This phenomenon was discovered by Strehler and Arnold (1951) when they were attempting to use the firefly luminescence for the measurement of the light-induced accumulation of ATP in the green alga Chlorella; the authors discovered that even without the addition of luciferase and luciferin, there was a long-lived glow from algal cells and chloroplasts in darkness following illumination. The observed DF was characteristic of different photosynthesizing samples used—leaves (Strehler and Arnold 1951), chloroplasts and photosynthesizing bacteria (Arnold and Thompson 1956).
For more than half a century this amazing property of photosynthetic systems has attracted the interest of many researchers (see e.g., Shuvalov and Litvin 1969; Barber and Kraan 1970; Wraight and Crofts 1971; Itoh and Murata 1973; Hipkins and Barber 1974; Jursinic and Govindjee 1977, 1982; Malkin and Barber 1978; Grabolle and Dau 2005). Several outstanding reviews have appeared that discuss the internal relationship between DF and the storage of light energy in photosynthesis (see, e.g., Lavorel 1975; Amesz and Van Gorkom 1978; Malkin 1979; Lavorel et al. 1982; Jursinic 1986; Veselovskii and Veselova 1990; Gaevsky and Morgun 1993; Radenovic et al. 1994; Tyystjarvi and Vass 2004). But even now, the full picture of the phenomenon remains unclear. There are two main reasons for this: (a) photosynthesis is a very complicated process that includes a multitude of simultaneous reactions, and DF is correlated with many of these reactions in a specific way; (b) it is technically difficult to measure DF because its spectrum is the same as that of prompt fluorescence (PF), but its intensity is lower by at least two orders of magnitude. As DF is emitted for a long time after the disappearance of PF, measurement of the two types of light emission is based only on their temporal separation. For measurement of DF, each laboratory usually constructs its own unique equipment, which as a rule has considerably different characteristics from the equipment of other laboratories. For this reason, even when the same sample is used, researchers often find differences in their results. Comparison of such results is difficult and sometimes leads to incorrect interpretations. In this review, we summarize contemporary ideas about the mechanism(s) of DF and its relationship with different photosynthetic reactions and present basic ideas for the understanding of this phenomenon for beginning researchers. The methodical approaches for the measurement of DF are also discussed. Our aim is to give the basic know-how about delayed fluorescence and to initiate attempts for integrating this potentially highly informative method with other methods in use (see Amesz and Hoff 1996; Aartsma and Matysik 2008). We hope that simultaneous measurements with several biophysical techniques would provide a powerful means for a complete understanding of the photosynthetic process in vivo.
Origin of delayed fluorescence
Delayed fluorescence is emitted predominantly from Photosystem (PS) II (Jursinic 1986). In mutant algae in which PS II is missing, DF is very weak or not observed at all. Light emission from PS II particle suspensions is 60–90 times more intense compared to that from PS I suspensions (Amesz and Van Gorkom 1978). This is why in this review delayed fluorescence will be discussed in terms of PS II.
The similarity between the emission spectra of delayed and prompt chlorophyll fluorescence (PF) (Arnold and Thompson 1956; Clayton 1969; Sonneveld et al. 1980a; Grabolle and Dau 2005) shows that in both cases the photon is a result of the radiative deactivation of the singlet excited state of the PS II antenna chlorophyll (Chl) a (Krause and Weis 1991; Lang and Lichtenthaler 1991).
The PF emission is practically extinguished about 5 ns after the light is switched off (Jursinic 1986; Krause and Weis 1991); its intensity decays polyphasically with characteristic lifetimes that range from several ps to 2 ns (Miloslavina et al. 2006). On the contrary, DF has components that decay in very different time domains: in the nanoseconds (Christen et al. 2000), microseconds (Jursinic and Govindjee 1977; Jursinic et al. 1978; Wong et al. 1978; Christen et al. 1998; Mimuro et al. 2007; Buchta et al. 2008), milliseconds (Hipkins and Barber 1974; Barber and Neumann 1974; Zaharieva and Goltsev 2003; Goltsev et al. 2005; Buchta et al. 2007), seconds (Rutherford et al. 1984; Hideg et al. 1991; Katsumata et al. 2008), and even in the hour time range (Hideg et al. 1990).
The different lifetimes of PF and DF show that the mechanism of the formation of the excited singlet state of the PS II chlorophyll antenna pigment that produces the emission is different for PF and DF. In the case of PF, chlorophyll is excited either via direct absorption of light, or by fast energy transfer from other chlorophyll molecules. In DF the excited antenna chlorophyll is formed as a result of back electron transfer and charge recombination in the reaction center of PS II, followed by repopulation of the excited chlorophyll (Chl*) state of the PS II antenna system by fast energy transfer (Arthur and Strehler 1957). Delayed fluorescence exists because each of the redox reactions of the photosynthetic electron transport is reversible. The absorption of a light quantum leads to the formation of Chl* state, energy transfer to P680 and charge separation between P680 and Pheo. The separated charges in the couple P680 +Pheo− however can recombine, which will lead to P680 * formation. The rapid exciton equilibration (Dau and Sauer 1996) delocalizes the excitation among about 200 PS II antennae chlorophylls, including the light-harvesting complexes (denoted as \( Chl_{200}^{*} \)by Grabolle and Dau (2005)), which can emit fluorescence. This emission decays with a lifetime of 2–4 ns and in theory already can be considered as the fastest-decaying component of delayed fluorescence. In practice this component is considered a variable fluorescence, which is a part of PF (Schatz et al. 1988), because its short life-time makes its separation from PF impossible.
In fact, all electron transfer reactions in the reaction center of PS II—in its donor as well as in its acceptor side, are reversible. Depending on the “location” in electron transport chain from which the electron returns, the DF emission is delayed with different times after the absorption of the photon that had originally induced the primary charge separation. The charge recombination which is a result of back electron transport from different PS II electron carriers can lead to the formation of “secondary excited” Chl* and DF emission with much longer lifetimes (micro-, milliseconds and even seconds). Because of the decreasing probability of this “long distance” reversal of the electron transport, the intensity of the emitted DF will decrease with the distance between the separated charges. With time, as the charge couples that can recombine start to disappear, the DF intensity decreases. This is the reason why in dark, after illumination by a single light pulse or by continuous light, the DF decay can be observed. The time course of the DF dark decay is described by a generally decreasing multi-component function in which all the different DF decay components overlap. This decay is first monotonous, but, under certain conditions, may form a transient maximum in the seconds time range (Desai et al. 1983; Hideg et al. 1991).
Each DF decay component is a result of reversal of electron transfer reactions in a specific state of PS II, for example S3Z+QA −QB, or S3Z+QA −QB =. The lifetime of different DF components is determined by the lifetime of the corresponding PS II state. The possibility to use DF for the investigation of photosynthesis depends on the correct identification of the different DF components and their connection to the specific reactions that occur in the photosynthetic machinery (Jursinic 1986; Tyystjarvi and Vass 2004; Goltsev et al. 2005).
The initial parts of the DF dark decay curve (the region of the fastest decay) can be represented by a sum of exponential functions (Lavorel 1975). The second and tens-of-second regions of the DF dark decay curve, however, cannot be described with sufficient accuracy as a sum of exponential functions. In these regions the curve is better described by a hyperbolic function, which can be explained by the simultaneous participation of many electron-transfer reactions, each having a different rate (Lavorel 1975), or with the heterogeneity of the system with respect to the depth of the energy traps, which is characterized by a normal distribution of the \( \Updelta G^{*} \) values (Lavorel and Dennery 1982; Tyystjarvi and Vass 2004).
Three types of processes can determine the kinetics of the DF dark decay:
-
(1)
Redox reactions in which one of the separated charges takes part; these reactions result in the decrease of the concentration of charge couples; in this case the luminescence is referred to as “leakage” type. This mechanism of DF decay explains mainly the fastest DF components (micro- and sub-millisecond time range).
-
(2)
Charge recombination reactions, resulting in the formation of secondarily excited states and DF quanta emission; this is “deactivation” type luminescence (Lavorel 1975). This process contributes to the slower DF components (milliseconds and longer). In both (1) and (2) cases, the DF intensity decreases because of a decrease in the concentration of the separated charge couples.
-
(3)
A third type of processes affect the kinetics of DF dark decay by changing the rate constant of recombination of charge couples, and correspondingly the DF quantum yield. These processes are related to the dark deactivation of the energized state of the thylakoid membrane (ΔμH+ or ΔΨ).
When the reactions that determine the dark decay are of the first order (as it is for the leakage type DF, where the disappearance of only one of the separated charges is responsible for the decrease of the concentration of the DF emitting state), then the DF relaxation curve can be described as a sum of exponential functions, where the kinetic components differ in lifetime (τ) and amplitude (L):
where, L(t) is DF emitted at time t after the light is switched off; L i is the amplitude of the i-th component, and τ i is its characteristic lifetime. With this model, the deactivation type of DF can also be described, when the separated charges remain in the same protein complex, as is for example for the PS II state Z+P680QA −.
The PS II redox states that mainly generate DF are \( P_{680}^{ + } Pheo^{ - } \), \( P_{680}^{ + } Q_{A}{}^{ - } \), \( Z^{ + } Q_{A}{}^{ - } \), \( Z^{ + } Q_{B}{}^{ - } \) and \( S_{i} ZQ_{B}{}^{ - } \). Delayed fluorescence, emitted in the micro- and millisecond time domain, is mostly related to \( Z^{ + } Q_{A}{}^{ - } \) state of PS II. For an ensemble of PS II, the actual population of the Chl*-state (fraction of PS II in the Chl*-state denoted as [Chl *]) is assumed to be determined by the free-energy difference between the excited-antenna state and the radical-pair state of PS II reaction center, \( Z^{ + } Q_{A}{}^{ - } \), reached at this time according to:
where, \( \Updelta G_{{Z^{ + } Q_{A}{}^{ - } }}^{*} = \Updelta G_{3}^{*} \) (see Fig. 1) represents the difference in the Gibbs free energy (\( \Updelta G_{3}^{*}\,<\,0 \)) between the excited-antenna state (Chl*) and the PS II radical-pair state (\( Z^{ + } Q_{A}{}^{ - } \)), k B is the Boltzmann constant, and T is the absolute temperature in Kelvin.
Energy level diagram for the Photosystem (PS) II-states participating in Delayed Fluorescence (DF) generation. ΔG values indicate differences in Gibbs free energy between the states participating in ns to ms DF generation. k 1 * is the rate constant of excited antenna state decay by primary charge separation in PS II; k 1 is the rate constant of primary charge separation in excited PS II reaction center chlorophyll; k 2 is electron transfer (ET) from Pheo to QA; k 3 is ET from Z to P680 +; P680 +Pheo− is the singlet and [P680 +Pheo−]3 is the triplet state of PS II radical pair, respectively. The rate constants k 3 and k 5 represent ET in the donor and k 4—in the acceptor side of PS II. For selected rate constants, the approximate values of the corresponding time constants, i.e., the reciprocal rate constants are given. The G value of the Chl 200 * state is chosen to be zero. Modified after Grabolle and Dau (2005)
Consequently the amount of DF quanta emitted from this state (L) is given by:
where, c is a proportionality coefficient that depends on the amount of active reaction centers of PS II in the sample and on the equipment constant that is used to measure the DF (this coefficient connects the value that is monitored by the equipment, in volts, with the actual number of emitted DF quanta); φ f is the quantum yield for fluorescence emission of the antenna chlorophylls.
Delayed fluorescence of PS II, measured few milliseconds in dark after a flash-excitation, is shown to decrease rapidly. A decrease in the fluorescence intensity could be explained by 1) reoxidation of the reduced acceptor (QA −) and the transition from one radical-pair state \( Z^{ + } Q_{A}{}^{ - } \), to another radical-pair state \( Z^{ + } Q_{B}{}^{ - } \) (with rate constant k 4) or by 2) the reduction of Z+ and the transition (with rate constant k 5) from state \( S_{i - 1} Z^{ + } Q_{B}{}^{ - } \) to state \( S_{i} ZQ_{B}{}^{ - } \). A third reaction that decreases the concentration of the separated charge couples is charge recombination between Z+ and QA −. The kinetics of the DF decay depends on the rates of the three reactions. If the direct redox reactions are broken by physical or chemical treatments, the concentration of charge-separated states will decrease with time because of recombination and emission. In this case, the DF decay can be described by first-order kinetics (Tyystjarvi and Vass 2004):
where, \( [Z^{ + } Q_{A}{}^{ - }] \left( t \right) \) is the temporary concentration of reaction centers (RC) in the \( Z^{ + } Q_{A}{}^{ - } \) state during the measurement period; \( [Z^{ + } Q_{A}{}^{ - }] \left( 0 \right) \) is its concentration at the beginning of measurement; \( k_{r}^{*} \)is the rate constant of repopulation of Chl * after recombination of radical-pair \( Z^{ + } Q_{A}{}^{ - } \); \( k_{r0}^{*} \) is the temperature-independent part of the recombination rate constant (frequency factor) and can be determined experimentally.
In biological systems, where the separated charges are stabilized by the redox reactions on the acceptor and donor sides, the kinetics of the DF dark relaxation is significantly more complex and includes many kinetic components.
Measurement of delayed fluorescence
There are two principal approaches to the measurement of DF. One of these is to measure the curve of the DF dark relaxation after excitation with a saturating flash of light (of ns duration), after which different kinetic components of the curve and their relationship with the processes of charge transfer in the electron-transfer chain of the chloroplasts are evaluated. In the second approach, the induction curve (IC) of the DF is recorded during the transition of dark-adapted samples to light-adapted state.
Delayed fluorescence dark relaxation curves
There are two approaches to the analysis of the dark relaxation of DF—measurement after illumination of a dark-adapted sample by a single pulse (usually by a laser), or measurement after continuous illumination. The two approaches give different results. In both cases, the low intensity of the DF requires that several curves be averaged in order to improve the signal-to-noise ratio (Lavorel et al. 1986). However, with advancement in instrumentation (gated photomultipliers, better amplifiers, etc.) used for DF measurement, it is now possible to get excellent signal-to-noise ratio even from a single DF decay. To cover wider time range, Holgar Dau and co-workers (Grabolle and Dau 2005; Buchta et al. 2007) split the signal from the photomultiplier and then applied different amplifications to the two channels. Then, the signals of the two channels were analyzed at different time intervals.
In general, it is a good practice to start measuring DF as soon as possible after the excitation light is switched off; further, it is important to avoid both the scattered light and the prompt fluorescence, especially during the first nanoseconds. The scattered light is easily removed by placing complementary filters in front of the detector, since the wavelength of fluorescence is longer than the one of the scattered light (the exciting light). To avoid the detector form measuring prompt fluorescence, the detection system is turned off during the first nanoseconds after the illumination of the sample. This is achieved either electronically (by using gated photomultipliers) or mechanically by placing a shutter in front of the multiplier. Both methods have problems: the electronic method often gives artefact signals (Grabolle and Dau 2005), and the mechanical shutters are, in most cases, too slow.
Even in the relatively short period of time in which the DF relaxation curve is recorded, several kinetic components have been resolved (Lavorel et al. 1986).
Delayed fluorescence induction curves
When a dark-adapted sample is exposed to continuous light, we can measure DF induction curve, just as we measure PF induction curve. Both measurements reflect changes in the photosynthesis machinery during dark-to-light adaptation. For DF induction, however, it is necessary to use alternating light and dark cycles (Fig. 2). During the light period, prompt fluorescence can be measured, but during the dark period, DF is measured with one caveat: right after light period, the DF detector is kept off for a short period to avoid measuring PF decay. For millisecond DF measurements, the light and dark periods vary from several hundred μs to several ms. Usually, a phosphoroscope is used, the main part of which is a mechanical modulator providing the light-dark cycles; continuous light is usually chopped by a rotating disk. The duration of the light and dark periods (when DF is measured) is controlled by the rate of rotation of the disk and the size and position of the openings (Radenovic et al. 1994).
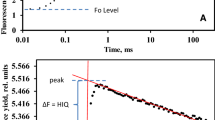
Schematic representation of the timing protocol of an electronic-based non-phosphoroscopic M-PEA (Hansatech Instruments, King’s Lynn, UK) for simultaneous recording of prompt (blue points) and delayed chlorophyll fluorescence (red lines). Black line represents the prompt fluorescence (PF) transient drawn through PF points recorded before the dark periods (PF smoothed). Bottom bar: Time scheme of alteration of actinic light and dark periods. During the periods marked with blue color the actinic light is on and chlorophyll fluorescence is measured. With red color, we have marked the time intervals when the light is off and delayed fluorescence is recorded. The sensor begins DF measurement 5 μs after the actinic illumination is switched off (marked with thin black line between blue and red regions). One measuring cycle includes light and dark periods in the ratio 3:1. The cycle duration increases during the induction in sequence: 400 μs, 4, 10, 100 ms, 1 and 10 s. The DF measurement period increases from 100 μs to maximal value of 240 ms
The DF signal, measured in each dark interval, is a poly-phasic decrease of the DF intensity. Frequently, a distinct time period is chosen, and its averaged DF intensity is then analyzed (Zaharieva and Goltsev 2003). This averaging results in a single point of the IC. Using several time points, a complete IC can be built. Alternatively, the full DF decay is recorded after each illumination, and each curve is analyzed by multi-exponential simulations (Fig. 2).
An obvious advantage of the type of measurement, described above, is that during the illumination periods, PF can also be recorded, and thus one can simultaneously obtain and compare both PF and DF induction curves. The variable part of PF intensity decreases slightly (a few percent change) after every dark period as a result of QA − dark reoxidation. During the same dark period, DF value drops drastically (~10 times or more). This suggests that the QA − dark reoxidation does not determine DF μs dark decay. We speculate that DF μs dark relaxation is predominantly the result of redox reactions on the donor side of PS II.
One of the problems of the phosphoroscopic method is that the measured light intensity is a complex function of the history of sample illumination in the previous measurement cycles. This means that in each cycle there will be very slow components that have not yet decayed but that were excited during previous illumination cycles. These components may be low in amplitude, but they add up and at later stages of the induction period, they might significantly modify the recorded DF (Lavorel et al. 1986). A way to avoid this pile-up of the slow components was proposed by Schreiber and Schliwa (1987), who subtracted the signal at the end of a measuring cycle from the DF intensity in the next cycle.
Review of delayed fluorescence decay
The DF decay up to ms time domain is a result of the equilibrium reactions in PS II (Radenovic et al. 1994; Goltsev et al. 2005) discussed later in this paper.
Photosystem II has a shallow trap in P680 since excited energy states of the antenna chlorophyll a molecules are at similar energy levels; further, there is a fast equilibrium between the excited state P680 * and the ion-radical pair P680 •+Pheo•− (reactions 3 and −3 in Fig. 3) (Schatz et al. 1988). Delayed fluorescence that originates from the recombination of P680 +Pheo− with a lifetime of 2–4 ns (Shuvalov and Klimov 1976; Jursinic 1986), can not be distinguished from PF (Schatz et al. 1988; Christen et al. 2000). This DF component can be measured (Shuvalov and Klimov 1976) only when the rest of the states that are in equilibrium with P680 +Pheo− have been eliminated through a suitable chemical treatment. At 77 K, the excited state of P680 * is, however, not shared with antenna chlorophyll a and DF originates directly from the PS II reaction center (Mimuro et al. 2007) and decays with lifetime in the range of 15–25 ns.
The decay of DF in the ns time range reflects the kinetics of reduction of P680 •+ by Z (reaction 5 in Fig. 3) (Christen et al. 2000). For thylakoid membranes, this decay is described by three exponentials with lifetimes of about 50 ns, 300 ns and 1.5 μs. The last two DF kinetic components oscillate, with a period of 4, with the number of the saturating pulses; these results show that they are related to the processes on the PS II electron donor side. In isolated preparations of light-harvesting complex of PS II, there is DF, and it decays with lifetimes of 65 and 800 ns, which suggests that these DF components are related to the formation of small quantities of radical pairs Chl•+Chl•− even in the antenna complexes and to their radiative recombination (Christen et al. 2000).
When QA is reduced, three light emission components are observed, with lifetimes of 1 μs (Van Best and Duysens 1977), 5–10 μs, and 35–40 μs (Jursinic and Govindjee 1982); these are suggested to arise as a result of charge recombination in the ZP680 +PheoQA − state (Sonneveld et al. 1980b). It is possible that this type of light emission is a result of the formation of triplet states [P680 +Pheo−]T, which is accelerated by the presence of QA −, and of their reversal to the [P680 +Pheo−]S states (Jursinic and Govindjee 1982; Jursinic 1986). However, triplet exciton model for DF has not been supported (Stacy et al. 1971). It has been suggested that the lifetime of the 5–10 μs component also depends on the rate of electron transfer from Z to P680 + (Lavorel 1973).
Ten μs after photon absorption, about 80% of all PS II are in Z+QA − state (Jeans et al. 2002). At this time, an equilibrium between the populations of the excited antenna state, Chl 200 *, and of the Z+QA − state is reached (Grabolle and Dau 2005). From this moment up to the ms time range, most of DF is a result of charge recombination between Z+ and QA − (Van Gorkom and Donze 1973; Jursinic 1986). In the μs time domain, the DF decays are dominated by processes on the (electron) donor side of PS II. In dark-adapted PS II membrane particles, the DF dark relaxations show strong dependence on the number of the saturating laser pulses (Grabolle and Dau 2005). The DF decays induced by the third flash are kinetically resolved in four components: three are in the μs time range with time constants of 14 μs, 65 μs, 203 μs, which reflect steps in the S3 → S4 transition and one slow, ms component related to dioxygen formation during S4 → S0 transition (Buchta et al. 2007).
The sub-ms (120–200 μs) component (Lavorel et al. 1982; Gekhman 1988) in the DF decay curve is associated with the normal functioning of the PS II reaction center (Gaevsky and Morgun 1993). Goltsev (2005) suggested that it is due to leakage type luminescence generated from the reaction centers in the Z+P680QA −QB state, and the deactivation of these states is a result of the forward reaction of re-oxidation of QA − by QB, which occurs with a rate constant of 2500–5000 s−1 (Lazar 1999).
The first real ms component of the DF decay curve in stationary “light” conditions (after long light-dark cycles) has a lifetime of about 1 ms (Gaevsky and Morgun 1993) to 2–3.5 ms (Zaharieva and Goltsev 2003; Goltsev et al. 2005). Its amplitude is an order of magnitude lower than that of the μs component in isolated pea chloroplasts, but is higher in whole leaves (Gekhman 1988). With laser pulse illumination this DF component has a time constant of 1.2–2 ms and is clearly S-state dependent (Goltsev et al. 1980; Buchta et al. 2007). When DF is measured with the phosphoroscope method, after a few light cycles, the S-state distribution is changed. The DF quantum yield of S3-state exceeds by an order of magnitude of that of the S2-state, and by 3 orders of magnitude of that of the S1-state (Buchta et al. 2007). When there is a random distribution of different S-states, the DF is emitted mainly by PS II in the S3-state. DF decay in the ms time domain is a result of “leakage”, both on the donor and the acceptor sides. At the donor side, DF lifetime may reflect mono-exponential dioxygen formation and disappearance of S3-states. At the acceptor side, DF dark relaxation is related to the reoxidation of QA − by the electron transport chain with fully reduced QB (QB =). In a chloroplast suspension with an exogenous acceptor (especially under uncoupling conditions) and in whole leaves, the lifetime of the ms component probably reflects, besides reactions in oxygen evolving complex, the kinetics of oxidation of reduced PS II quinone acceptors by the plastoquinone (PQ) pool (Gaevsky and Morgun 1993; Goltsev et al. 2005). The following equation shows these acceptor side reactions:
The acceleration of the electron transfer is expected to be accompanied by a decrease of the lifetime of the ms component.
The amplitude of the ms component depends on the difference in the potentials on the two sides of the membrane (Fleishman 1971; Venediktov et al. 1980). Interestingly, it decreases under both phosphorylating conditions and when uncouplers of phosphorylation are present (Wraight and Crofts 1971). A major explanation is that transmembrane electric field facilitates the electron transfer from QA − to Pheo, and then to P680 +, reducing the activation free energy of the charge recombination. In thylakoid suspensions, the intensity of ms DF increases exponentially with increasing transmembrane diffusion electric gradient (see Jursinic et al. 1978; Venediktov et al. 1980).
Delayed fluorescence that decays with lifetimes of about several tens of seconds has been investigated by many authors (Rutherford et al. 1984; Rutherford and Inoue 1984; Hideg et al. 1991; Katsumata et al. 2008; Berden-Zrimec et al. 2008). The slow DF components (with lifetimes of several seconds) relate to the recombination of the S2 and S3 states of the oxygen evolving complex with QA − and QB − (Joliot et al. 1971; Lavorel 1975). This component has been identified to originate from recombination occurring in RCs in the S2QB − or S3QB − state. Its lifetime correlates with the time of re-oxidation of QB −, which is about 22 s (Robinson and Crofts 1983).
The slow decay of luminescence in the second time range is well described by a second-order kinetics. The experimental decay curve is fitted well with a first or a second-order hyperbola (Tyystjarvi and Vass 2004 and references therein).
When the photosynthetic sample is illuminated by infrared light, the relaxation curve has a maximum at 30–40 s after the light is switched off (Katsumata et al. 2008 and references therein). The complex kinetics of the dark relaxation and emission spectra of the slow components indicate that both photosystems I and II take part in the generation of excited states of PS II antennae chlorophylls and by this way—in the emission of this type of luminescence (Schmidt and Senger 1987).
Induction curve
During the illumination of dark-adapted leaves, the DF intensity, like the PF intensity, undergoes a series of complex induction transitions that reflect the function of the photosynthetic electron transport chain (Malkin 1979). The PF induction curves have been extensively studied and used as a tool in photosynthesis research and plant physiology (Dau and Hansen 1989; Dau 1994; Lazar 1999; Schreiber et al. 2000; Tsimilli-Michael and Strasser 2008).
The DF induction curve reflects processes that occur in photosynthesizing samples when they are exposed to light after a period of dark adaptation. The best resolution of the IC maxima can be obtained after a dark adaptation period of 5–15 min. After the induction period, which lasts about 2–3 min at room temperature and saturating light intensity, a stationary level of DF is reached (Veselovskii and Veselova 1990; Radenovic et al. 1994).
We know much less about the DF induction curve than about the PF transients. The DF induction curve, unlike that of PF, has several maxima and minima. Their number and amplitude, however, depend on the recording period, or in other words, on the kinetic components of DF being measured (Zaharieva and Goltsev 2003). Moreover, the amplitudes and lifetimes of the ms DF during the induction period vary significantly (Goltsev et al. 2005). There is no consensus nomenclature of the maxima that are observed in the DF induction curve, and no consensus about the number and interpretation of these maxima. Goltsev and co-workers (Goltsev and Yordanov 1997; Zaharieva and Goltsev 2003; Goltsev et al. 2005) suggest that the maxima (denoted by I) and minima (labelled as D) should be numbered in sequence according to their position in the IC (I1, D1, I2, D2 etc.).
The changes in DF intensity during the induction period are correlated with different processes that include the build-up of the electrical and proton transmembrane gradient (Wraight and Crofts 1971), and depend on the availability of electron acceptors (Ruby 1976) and donors (Mar et al. 1975), and on the state of the oxygen-evolving complex (Zankel 1971; Van Gorkom and Donze 1973).
Two phases can be observed in the DF induction curve (Fig. 4): a fast and a slow one, lasting 200 ms and several minutes, respectively (Itoh et al. 1971; Itoh and Murata 1973; Malkin and Barber 1978). The fast phase includes two maxima I1 and I2, after which DF drops to a minimum labelled as D2 (Goltsev and Yordanov 1997; Goltsev et al. 2003). After a small step, labelled as I3, the slow phase begins. During this phase DF rises to a maximum I4 and then, through several transient maxima (I5 and I6), DF intensity decreases to a stationary level S (Itoh and Murata 1973; Goltsev et al. 2003).
Simultaneous measurements of light absorption measured as relative changes of photocurrent at the upper side of the leaf at 820 nm (I(820), brown line, right axis), Prompt Fluorescence (PF, blue line, left axis) and Delayed Fluorescence (DF, left axis), recorded from 10 μs to 100 s. The induction curves were recorded in dark adapted Camellia leaves from 10 μs to 20 s of illumination. Actinic light intensity was 5000 μmol (photons) m−2 s−1. These transients were measured at the Hansatech Instruments Company (UK) during 2008 and 2009 with their new instrument labeled M-PEA
The fast phase of the DF induction curve occurs during the increase of the PF intensity from Fo to Fp (see Papageorgiou and Govindjee 2004).Two maxima can be seen in this phase—I1 and I2. The first one, I1, coincides with the increase of PF from Fo (the “O” level) to Fi (the I level). At this time, the transient decrease of I(820) (Fig. 4) shows that reduced plastocyanin and P700 + must have accumulated, probably due to the lack of donors for PS I (lack of reduced plastoquinone) (Schansker et al. 2003). This results in the accumulation of a transmembrane electrical gradient (Satoh and Katoh 1983). The appearance of I1, like the transition from Fj (J level) to Fi (I level), can be related to two phenomena: (1) photochemical—accumulation of certain light-emitting states of the PS II RC, and (2) non-photochemical—increase of the DF due to the electrical gradient formed by PS I when P700 is oxidized (Pospisil and Dau 2002). The I2 maximum (usually only a shoulder) is probably related to the prolonged reopening of PS II RCs by the electron transfer from the reduced QB to PQ before the full reduction of the PQ pool (I2–D2 transition). The relative size of this maximum increases with the decrease of the size of the PS II antenna and when the measuring temperature is increased (Zaharieva et al. 2001).
After about 0.5 s of illumination, because of the reduction of the plastoquinone pool, there is a decrease of the outflow of electrons from QA −, and the RCs shift to a “closed” state ZP680QA −QB = (phase D2) (Gaevsky and Morgun 1993; Zaharieva and Goltsev 2003; Goltsev et al. 2005). Under these conditions, the amplitude of the 160-μs component decreases, and the lifetime of the ms component increases (Zaharieva and Goltsev 2003). In the presence of ferricyanide, an artificial electron acceptor, and uncouplers of the phosphorylation, this increase of the lifetime is insignificant and no I2–D2 is observed. This indicates that the I1–I2–D phase correlates with the processes of reduction of the PQ pool, and the time when the D2 minimum is reached, can be an indicator of the reducing activity of the PS II complex (Gekhman 1988).
The increase of DF to the next maximum, labelled as I4, occurs in parallel with a slight decrease of the PF intensity and oxidation of P700 (Goltsev et al. 2005). The accumulation of P700 + suggests that at this time the light-induced activation of the ferredoxin:NADP+-oxidoreductase takes place (Harbinson and Hedley 1993; Schansker et al. 2006), i.e., the linear electron transport is activated, and the transmembrane proton gradient starts to accumulate. The increase of the DF intensity in the slow phase (towards the I4 maximum) is associated with the formation of the proton gradient (Wraight and Crofts 1971; Evans and Crofts 1973) that increases the rate constant of radiative recombination in the PS II RCs.
On the other hand, it can be assumed that the increase of luminescence during the D2–I4 phase is not directly connected with the transmembrane proton gradient formation, but instead to the neutralization of the negatively charged buffer groups that are present on the internal surface of the thylakoid membrane. During the DF increase to the I4 maximum, an additional difference in the electric potentials appears that is caused by the accumulation of Mg2+ ions inside the thylakoids as a result of a H+/Mg2+ exchange when the buffering capacity of the thylakoids is saturated (Gaevsky and Morgun 1993). Later, this potential decreases when Mg2+ is exported out of the thylakoids along the gradient of the electric potential. This process describes the decrease of DF in the phase I4–I6 and can be observed in chloroplast suspensions in non-phosphorylating conditions, when ΔpH produces only osmotic work (Grigoryev et al. 1982). During continuous illumination, ΔpH of the thylakoid membrane increases while the electrical component of the membrane potential decreases to a minimal value (Rottenberg 1977). Grigoryev et al. (1982) suggested that the decrease (I4–I6–S) of the induction curve partly reflects the changes in the transmembrane electrical gradient ΔΨ, which occurs as a result of the secondary ion transport (Rottenberg 1977).
The peak M2 that appears late in the PF induction curve is usually associated with the activation of the dark reactions of the Calvin–Benson cycle (Ireland et al. 1984). After this maximum, the acceleration of the electron transport induces a decrease of the PF that coincides with the I6 peak of the DF induction curve. The maximum I6 can only be seen at a temperature 25°C or higher.
The amplitude of the I4–I6–S phase in the DF induction curve in whole leaves is much larger than the one seen in chloroplasts (Gaevsky and Morgun 1993). In chloroplasts suspended in a medium without an electron acceptor, this decrease is rarely observed (Itoh et al. 1971; Grigoryev et al. 1982). In intact leaves the cause of the DF decrease is the start of the CO2 fixation cycle, the consumption of ATP (Pavlova et al. 1978) and the acceleration of the kinetics of millisecond DF dark decay (from 4 down to 2 ms, see Fig. 5).
Time course of dark relaxation parameters of delayed chlorophyll fluorescence in barley leaves (a, b) and leaf disks from Arabidopsis thaliana (c, d) during light to dark adaptation. Several parameters were calculated by fitting of the experimental DF decays using the following equation: \( DF(t) = L_{1} \times \exp \left( { - t/\tau_{1} } \right) + L_{2} \times \exp \left( { - t/\tau_{2} } \right) + L_{3} \), where, L 1, L 2 and L 3 are the amplitudes of the kinetic components (a, c); τ 1 and τ 2 are lifetimes (b, d) (Goltsev et al. 2003)
Delayed fluorescence intensity can be integrated for different DF decay intervals and can be presented as different induction curves. Some experimental setups allow optional selections for one or more decay intervals (Fig. 4). The measurement of induction curves for the DF that decays in different intervals allows the representation of the DF intensity as a function not only of the induction time, but also of the interval of the measurement.
Figure 5 shows the change of the parameters (amplitude, L, and lifetime, τ), obtained after deconvolution of the DF decays, and recorded at different times during the induction period in intact leaves.
The changes in the time constants of the sub-ms and ms DF decays are modulated by photoinduced changes of the oxygen evolving complex (predominantly in S3-state), the state of the PS II acceptors, and the energization of the thylakoid membrane and the temperature of the sample. The comparison of the rate constants of electron transfer reactions in the PS II acceptor side with the lifetimes of measured DF kinetic components shows which transitions between the particular states of the PS II reaction center determine DF decays (Zaharieva and Goltsev 2003).
The emission decaying in the sub-ms time domain is connected with the Z+P680QA −QB −state, and the one decaying in the ms time domain is connected with the Z+P680QA −QB = state. The lifetimes (τ 1 and τ 2) are determined by the probabilities of forward and backward electron transport in the PSII states, described above. These assumptions are in agreement with experiments done with the modifiers of PS II electron transport (Goltsev et al. 1998) or of thylakoid membrane fluidity (Zaharieva et al. 1998), as well as with the interpretation of temperature dependences of DF decay parameters (Zaharieva et al. 2001). The ms time constant increases approximately twofold during the first second of illumination (Fig. 5). This increase, observed at the beginning of the induction period, is presumably a result of the photoinduced PQ pool reduction (Goltsev et al. 1998): when the PQ pool is reduced, one of the ways for disappearance of the Z+P680QA −QB = state (namely, dissociation of the PQ molecule from the QB binding site) becomes blocked. After the first few seconds of illumination, the lifetime of this component only slightly decreases. Therefore we can conclude that after PS I activation (photoactivation of the ferredoxin-NADP+-reductase), the PQ pool is only partially reoxidized. The main part of the DF increase, in this time scale, is probably due to the photoinduced proton gradient but not to the reopening of PS II reaction centers (Goltsev et al. 2003).
The PQ pool maintains some level of reduction after PS I activation. Further, a sharp decrease in the ms lifetime can be observed that coincides with the activation of the Calvin–Benson cycle enzymes and reopening of the PS II reaction centers. It is evident that the PF decrease after the maximal level Fp (the P level) is due to non-photochemical quenching rather than PQ pool reoxidation. This is in agreement with the work of Briantais et al. (1980), who showed that P-to-S decay in PF is a result of energy-dependent quenching by photoinduced lumen acidification.
Relationship between prompt and delayed fluorescence
Information about the state of the photosynthetic machinery can be obtained from the shape of the DF induction curve and from the analysis of its correlation with the simultaneously recorded PF transient.
For a better understanding of the nature of the maxima that are seen in the DF induction curve, this curve is often compared to the PF transient placed on the same time scale (Govindjee and Papageorgiou 1971; Krause and Weis 1991; Malkin et al. 1994). The DF and PF induction curves reflect the same changes of the redox state of the PS II reaction center (Itoh 1980). The comparative analysis of PF and DF allows the investigation of the routes of utilization of the absorbed energy and photosynthetic activity. A close correlation between DF and PF was shown in leaves with inactive PS II (plants grown under the regime of 1 ms flash given after every 15 min of darkness). During continuous illumination of such leaves, the rise of the photosynthetic activity and the oxygen evolution rate paralleled the appearance of DF induction and variable fluorescence transients (Srivastava et al. 1999). The simultaneous study of PF and DF was carried out mainly with isolated chloroplasts, which do not display the complex induction behaviour that is characteristic of intact leaves. The ms DF with a lifetime of 2–3 ms does not correlate with the changes of PF, while for the longer DF components, such a correlation is observed (Clayton 1969; Malkin and Barber 1978).
The problem of comparison of the DF with PF transient is that the ms DF usually includes overlapping fast and slow kinetic components, which behave in a different way during the induction period (Mar et al. 1975).
In summary, the ms DF induction reflects the changes in the redox level of P680 and QA at low and room temperatures (Itoh 1980). The induction curves of the ms DF and PF transients have an anti-parallel relation (except for the time immediately after the start of illumination) at different experimental conditions—the ms DF is inversely proportional to the QA − concentration (Itoh 1980). After continuous illumination, the S-states of the oxygen evolving complex are unsynchronized (approximately equally distributed) and the S-state transitions have presumably a small effect on the DF induction transitions, although the different S-states yield a different DF intensity at room temperature or at over −20°C (Velthuys and Amesz 1975).
The slow-decaying DF is observed only at room temperature and has an entirely different induction curve, which is parallel to the PF induction curve (Clayton 1969; Malkin and Barber 1978). The slow-decaying DF consists of components that are due to the reversal of the electron transfer at later stages of the electron transfer process (Itoh 1980). The parallel course of PF and the slow DF components is even more apparent in the presence of the uncoupler valinomycin (Satoh and Katoh 1983).
As the PF and DF are controlled by the same processes that take place during the transition from a dark-adapted to a light-adapted state, the relationship between them can be studied theoretically.
Analyzing the intensities of DF and PF recorded simultaneously using the phosphoroscopic method, Goltsev et al. (2003) related the ratio L/Fv to the ratio of the oxidized and reduced states of QA (B) and to the energization of the thylakoid membrane
where, E a is the activation energy of radiative recombination, Δψ is the membrane potential; φF o and φF m are the quantum yield of fluorescence from PS II with open and closed RC, respectively, k is the Boltzmann constant and the other symbols have their usual meaning.
A new term was introduced—luminescence potential (U L), which is the sum of the potentials that lead to the formation of DF. If we assume U L is equal to the logarithm of the ratio L/F v, then U L would be proportional to the sum of the potentials:
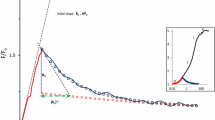
The phase diagrams V(U L) and L(U L) presented in Fig. 6 show the changes of the variable chlorophyll fluorescence (V) and DF intensity (L) on the U L. This figure illustrates the effect of photosynthetic parameters (redox potential of the PS II acceptors and the thylakoid membrane energization) on PF and DF.
The course of the changes of relative variable chlorophyll fluorescence, V (a) and delayed fluorescence, L (b) during dark to light transition as a function of luminescence potential (U L ~ E’ + ΔμH+) in barley leaves. Grey circles represent characteristic points of PF and DF induction curves, as in Fig. 4. All data are normalized to the corresponding maximal values. Experimental conditions were as in Fig. 1 in Goltsev et al. (2003). Inset: part of the experimental points from D2 to I4 are presented on a semi-logarithmic scale. The straight line shows linear regression of the experimental points. The figure is redrawn from Goltsev et al. (2003)
We propose that in the linear regions, only one of the two components of U L (redox term E′ = ln((1−B)/B) or ΔμH+) changes the PF or DF, and the other component remains relatively constant. The slopes of the linear regions reflect the sensitivity of the PF or DF to the corresponding U L component (Goltsev et al. 2003).
In this way, the I–P (Fi–Fp) section of the PF transient reflects the photo-induced reduction of the acceptor side of PS II, and the slope represents a proportionality coefficient between the amount of reduced acceptors of PS II and the increase of PF. The slope of the O–I (Fo–Fi) section is much smaller than the one of the I–P section and is close to the one of the P–S phase that is related to the energization of the thylakoid membrane.
Why do we measure DF?
Plants do not use all the quanta they absorb with 100% efficiency. Some of the absorbed energy is emitted as prompt or delayed chlorophyll fluorescence, or as heat. Each DF quantum has its pre-history—how many and which of the forward and backward reactions have lead to the formation of the excited state of the antenna chlorophylls. Each emitted quantum carries a bit of information about these reactions.
The delayed fluorescence emission of PS II is a useful tool to quantitatively study the light-induced electron transfer and related processes (e.g., proton movement) which are associated with a free-energy drop (Buchta et al. 2007). This method provides insights in the functionally important inner-protein proton movements, which are hardly detectable by conventional spectroscopic approaches (Buchta et al. 2007).
Based on DF, a rationale for the determination of the free energy differences between the excited-antenna state and the radical-pair state, reached at a given time after the flash, has been proposed (Grabolle and Dau 2005). Differences in energies of several PS II redox states can be calculated reasonably precisely. Based on the measured DF decays from oxygen-evolving PS II membrane particles, an attempt was made to obtain redox-potentials of the electron carriers involved (Grabolle and Dau 2005).
The ability of one single equipment to measure simultaneously light emission (both PF and DF) and absorption changes, for example at 820 nm (Srivastava et al. 1999) and at 320 nm (characterizing the redox states of P700 and QA) is valuable in obtaining information about the function and, to some extent, about the structure of the photosynthetic machinery in the same sample. Such instruments are beginning to be available now.
One of the major advantages of DF is the possibility to measure it in native samples. The high sensitivity of photosynthesis to environmental factors, and the sensitivity of DF to changes in the different photosynthetic processes have made DF a useful tool for testing the plant reactions under stress conditions (Bilger and Schreiber 1990; Gaevsky and Morgun 1993). Using a suitable experimental approach, DF has already been used for the analysis of the effects of chemical and physical factors: high and low temperatures (Itoh 1980; Goltsev et al. 1987; Yordanov et al. 1987; Bilger and Schreiber 1990), light stress (Valikhanov et al. 2002) and UV irradiation (Zhang et al. 2007a), drought and high salinity (Mladenova et al. 1998; Zhang and Xing 2008), herbicides (Lambrev and Goltsev 2001), heavy metals (Plekhanov and Chemeris 2003), harmful gases (Ellenson and Amundson 1982). The ms DF can also be used to monitor effects of viral and bacterial diseases on plants (Milanov et al. 1997; Christov et al. 2001), and even to a change in the physiological state of the plant cell during its ontogenetic development, greening and different hormonal levels (Srivastava et al. 1999; Zhang et al. 2007b; Yordanov et al. 2008).
The DF-imaging is highly useful for the visualization of the spatial differences in the physical state of leaves due to mosaic distribution of the viral and bacterial infections on them (Björn and Forsberg 1979; Ellenson and Amundson 1982).
In micro-algal populations, DF has been used as a measure of total photosynthetic activity (Monti et al. 2005), reflecting also the algal biomass (Yacobi et al. 1998; Katsumata et al. 2006). The algal cells are very sensitive to toxic compounds and the DF measurements of algal systems are used as ecotoxicological bioassays, which allow the rapid estimation of the effects of inhibitors of algal growth (Katsumata et al. 2006; Berden-Zrimec et al. 2008).
Prompt fluorescence has been used as a tool to obtain quantitative information about the photosynthetic process in plants, algae and cyanobacteria (Govindjee et al. 1986; Papageorgiou and Govindjee 2004). For example, Reto Strasser and co-workers (Strasser et al. 1995; Tsimilli-Michael and Strasser 2008) have quantitatively related induction transients of PF with the activities of photosystems I and II and with the electron transfer between them. Regardless of the fact that the PF and DF are emitted by the same population of antenna chlorophylls of PS II, they carry different, complementary information about the quantitative characteristics of the photosynthetic process. At the present time, equipment for the simultaneous measurement of induction transients of PF, DF, decay curves of DF and changes in light absorption at 820 nm (P700 +) with μs time resolution, is available (see Figs. 2 and 4). For the processing and evaluation of the large information that is obtained by such an equipment, mathematical models must be evolved to describe the photosynthetic reactions and their relation to the fluorescence characteristics. Such models describe the induction transients of PF and DF (Goltsev and Yordanov 1997; Markovic et al. 2001; Kalauzi et al. 2006), and the curves of DF decay (Goltsev et al. 2004; Li et al. 2007; Guo and Tan 2009). When the models are fitted to the experimental curves, we expect to obtain the values of rate constants of different photosynthetic reactions. The combination of the modelling with the design of artificial neuron networks for specific global physiological properties of the plant (Chernev et al. 2006) would further increase the applicability of the method of delayed chlorophyll fluorescence in plant biology and agriculture.
Abbreviations
- Chl* :
-
Excited state of a chlorophyll molecule
- DF:
-
Delayed (chlorophyll) fluorescence
- ET:
-
Electron transport
- Fo :
-
Fluorescence intensity when all the reaction centers are assumed to be open, at the “O” level
- Fj :
-
Fluorescence intensity at ~3 ms
- Fi :
-
Fluorescence intensity at ~30 ms
- Fp :
-
Maximal measured fluorescence intensity, at the “P” level
- IC:
-
Induction curve
- L:
-
Delayed fluorescence intensity
- P700 :
-
Primary (chlorophyll) electron donor of PS I
- P680 :
-
Primary (chlorophyll) electron donor of PS II
- Pheo:
-
Pheophytin on D1 protein of PS II
- PF:
-
Prompt (chlorophyll) fluorescence
- PQ:
-
Plastoquinone
- PS:
-
Photosystem
- QA :
-
Primary quinone (electron) acceptor of PS II
- QB :
-
Secondary quinone (electron) acceptor of PS II
- RC:
-
Reaction center
- Z (also called Yz):
-
Secondary (electron) donor in PS II, Tyr-161 of the D1 protein of PS II
References
Aartsma T, Matysik J (eds) (2008) Biophysical techniques in photosynthesis, vol II. Volume 26, Advances in photosynthesis and respiration (series ed, Govindjee). Springer, Dordrecht
Amesz J, Hoff AJ (eds) (1996) Biophysical techniques in photosynthesis. Volume 3, Advances in photosynthesis and respiration (series ed, Govindjee). Springer, Dordrecht
Amesz J, Van Gorkom HJ (1978) Delayed fluorescence in photosynthesis. Annu Rev Plant Physiol 29:47–66
Arnold W, Thompson J (1956) Delayed light production by blue-green algae, red algae, and purple bacteria. J Gen Physiol 39:311–318
Arthur WE, Strehler BL (1957) Studies on the primary process in photosynthesis. I. Photosynthetic luminescence: Multiple reactants. Arch Biochem Biophys 70:507–526
Barber J, Kraan GPB (1970) Salt induced light emission from chloroplasts. Biochim Biophys Acta 197:49–95
Barber J, Neumann J (1974) An energy-conservation site between H2O and DBMIB: Evidence from msec delayed light and chlorophyll fluorescence studies in chloroplasts. FEBS Lett 40:186–189
Berden-Zrimec M, Drinovec L, Molinari I, Zrimec A, Umani SF (2008) Delayed fluorescence as a measure of nutrient limitation in Dunaliella tertiolecta. J Photochem Photobiol B 92:13–18
Bilger W, Schreiber U (1990) Chlorophyll luminescence as an indicator of stress-induced damage to the photosynthetic apparatus. Effects of heat-stress in isolated chloroplasts. Photosynth Res 25:161–171
Björn LO, Forsberg AS (1979) Imaging by delayed light emission (phytoluminography) as a method for detecting damage to the photosynthetic system. Physiol Plant 47:215–222
Briantais J-M, Vernotte C, Picaud M, Krause GH (1980) Chlorophyll fluorescence as a probe for the determination of the photoinduced proton gradient in isolated chloroplasts. Biochim Biophys Acta 591:198–202
Buchta J, Grabolle M, Dau H (2007) Photosynthetic dioxygen formation studied by time-resolved delayed fluorescence measurements: method, rationale, and results on the activation energy of dioxygen formation. Biochim Biophys Acta 1767:565–574
Buchta J, Shutova T, Samuelsson G, Dau H (2008) Time-resolved delayed chlorophyll fluorescence to study the influence of bicarbonate on a green algae mutant Photosystem II. Allen JF, Gantt E, Golbeck JH, Osmond B (eds) pp 35–38. Springer, Dordrecht
Chernev P, Goltsev V, Zaharieva I, Strasser RJ (2006) A highly restricted model approach quantifying structural and functional parameters of Photosystem II probed by the chlorophyll a fluorescence rise. Ecol Eng Environ Protect 5:19–29
Christen G, Reifarth F, Renger G (1998) On the origin of the ‘35-ms kinetics’ of P680 .+ reduction in photosystem II with an intact water oxidising complex. FEBS Lett 429:49–52
Christen G, Steffen R, Renger G (2000) Delayed fluorescence emitted from light harvesting complex II and Photosystem II of higher plants in the 100 ns–5 мs time domain. FEBS Lett 475:103–106
Christov IK, Stefanov D, Goltzev VN, Abrasheva P (2001) Effects of grapevine fanleaf and stem pitting viruses on the photosynthetic activity of grapevine plants grown in vitro. Russ J Plant Physiol 48:473–477
Clayton RK (1969) Characteristics of prompt and delayed fluorescence from spinach chloroplasts. Biophys J 9:60–76
Dau H (1994) Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol 60:1–23
Dau H, Hansen U-P (1989) Studies on the adaptation of intact leaves to changing light intensities by a kinetic analysis of chlorophyll fluorescence and oxygen evolution as measured by the photoacoustic signal. Photosynth Res 20:59–83
Dau H, Sauer K (1996) Exciton equilibration and photosystem II exciton dynamics—a fluorescence study on photosystem II membrane particles of spinach. Biochim Biophys Acta 1273:175–190
Desai TS, Rane SS, Tatake VG, Sane PV (1983) Identification of far-red induced relative increase in the decay of delayed light emission from photosynthetic membranes with thermoluminescence peak V appearing at 321 K. Biochim Biophys Acta 724:485–489
Ellenson JL, Amundson RG (1982) Delayed light imaging for the early detection of plant stress. Science 215:1104–1106
Evans EH, Crofts AT (1973) The relationship between delayed fluorescence and the H+ gradient in chloroplasts. Biochim Biophys Acta 292:130–139
Fleishman DE (1971) Luminescence in photosynthetic bacteria. Photochem Photobiol 14:277–286
Gaevsky NA, Morgun VN (1993) Use of variable and delayed chlorophyll fluorescence for the study of plant photosynthesis (in Russian). Fiziol Rast 40:136–145
Gekhman A (1988) Study of the kinetic characteristics of plant photoluminescence (research methods, equipment and results) (in Russian). Krasnoiarsk IBF SO AN USSR
Goltsev V, Yordanov I (1997) Mathematical model of prompt and delayed chlorophyll fluorescence induction kinetics. Photosynthetica 33:571–586
Goltsev V, Ortoidze TV, Sokolov ZN, Matorin DN, Venediktov PS (1980) Delayed luminescence yield kinetics in flash illuminated green plants. Plant Sci Lett 19:339–346
Goltsev V, Yordanov I, Stojanova T, Popov O (1987) High temperature damage and acclimation of the photosynthetic apparatus. II. The effect of mono- and divalent cations and pH on the temperature sensitivity of some functional characteristics of chloroplasts isolated from heat-acclimated and non-acclimated bean plants. Planta 170:478–488
Goltsev V, Traikov L, Hristov V (1998) Effects of exogenous electron acceptors on kinetic characteristics of prompt and delayed fluorescence in atrazine inhibited thylakoid membranes. In: Garab G (ed) Photosynthesis: mechanisms, effects. Kluwer Academic Publishers, The Netherlands, pp 3885–3888
Goltsev V, Zaharieva I, Lambrev P, Yordanov I, Strasser R (2003) Simultaneous analysis of prompt and delayed chlorophyll a fluorescence in leaves during the induction period of dark to light adaptation. J Theor Biol 225:171–183
Goltsev V, Zaharieva I, Chernev P, Strasser RJ (2004) Kinetics of millisecond delayed chlorophyll fluorescence as a probe of photosystem II function. In: Zrimec M, Zrimec A, Drobne D, Milani M (eds) Weak photon emission from living tissues: delayed luminescence—facts & perspectives. Institute of Physical Biology, Grosuplje, Slovenia, pp 18–23
Goltsev V, Chernev P, Zaharieva I, Lambrev P, Strasser R (2005) Kinetics of delayed chlorophyll a fluorescence registered in milliseconds time range. Photosynth Res 84:209–215
Govindjee, Papageorgiou G (1971) Chlorophyll fluorescence and photosynthesis: fluorescence transients. In: Giese AC (ed) Photophysiology. Academic Press, New York, pp 1–46
Govindjee, Amesz J, Fork DC (eds) (1986) Light emission by plants and bacteria. Academic Press, Orlando
Grabolle M, Dau H (2005) Energetics of primary and secondary electron transfer in Photosystem II membrane particles of spinach revisited on basis of recombination-fluorescence measurements. Biochim Biophys Acta 1708:209–218
Grigoryev Y, Morgun V, Gold V, Gaevsky N (1982) Study of light-induced changes of ms-delayed light emission in pea chloroplasts (in Russian). Biofizika 27:973–976
Guo Y, Tan J (2009) A kinetic model structure for delayed fluorescence from plants. Biosystems 95:98–103
Harbinson J, Hedley CL (1993) Changes in P-700 oxidation during the early stages of the induction of photosynthesis. Plant Physiol 103:660–694
Hideg E, Kobayashi M, Inaba H (1990) Ultraweak photoemission from dark-adapted leaves and isolated chloroplasts. FEBS Lett 275:121–124
Hideg E, Kobayashi M, Inaba H (1991) The far red induced slow component of delayed light from chloroplasts is emitted from photosystem-II-evidence from emission-spectroscopy. Photosynth Res 29:107–112
Hipkins MF, Barber J (1974) Estimation of the activation energy for millisecond delayed fluorescence from uncoupled chloroplasts. FEBS Lett 42:289–292
Ireland CR, Long SP, Baker NR (1984) The relationship between carbon dioxide fixation and chlorophyll a fluorescence during induction of photosynthesis in maize leaves at different temperatures and carbon dioxide concentration. Planta 160:550–558
Itoh S (1980) Correlation between the time course of millisecond delayed fluorescence and that of prompt fluorescence at low temperature in uncoupled spinach chloroplasts. Plant Cell Physiol 21:873–884
Itoh S, Murata N (1973) Correlation between delayed light emission and fluorescence of chlorophyll a in system II particles derived from spinach chloroplasts. Photochem Photobiol 18:209–218
Itoh S, Murata N, Takamiya A (1971) Studies on the delayed light emission in spinach chloroplasts. I. Nature of two phases in development of the millisecond delayed light emission during intermittent illumination. Biochim Biophys Acta 245:109–120
Jeans C, Schilistra MJ, Klug DR (2002) The temperature dependence of P680 + reduction in oxygen-evolving Photosystem II. Biochemistry 41:5015–5023
Joliot P, Joliot A, Bouges B, Barbieri G (1971) Studies of System II photocenters by comparative measurements of luminescence, fluorescence, and oxygen emission. Photochem Photobiol 14:287–305
Jursinic P (1986) Delayed fluorescence: current concepts and status. In: Govindjee, Amesz J, Fork DJ (eds) Light emission by plants and bacteria. Academic Press, Orlando, pp 291–328
Jursinic P, Govindjee (1977) Temperature dependence of delayed light emission in the 6 to 340 microsecond range after a single flash in chloroplasts. Photochem Photobiol 26:617–628
Jursinic P, Govindjee (1982) Effects of hydroxylamine and silicomolybdate on the decay of delayed light emission in the 6–100 μs range after a single 10 ns flash in pea thylakoids. Photosynth Res 3:161–177
Jursinic P, Govindjee, Wraight CA (1978) Membrane potential and microsecond to millisecond delayed light emission after a single excitation flash in isolated chloroplasts. Photochem Photobiol 27:61–71
Kalauzi A, Markovic D, Radenovic C (2006) Transients of delayed fluorescence induction signal and photosynthetic antennas: A possible relationship. Mathematical modeling approach. Russ J Plant Physiol 53:289–297
Katsumata M, Koike T, Nishikawa M, Kazumura K, Tsuchiya H (2006) Rapid ecotoxicological bioassay using delayed fluorescence in the green alga Pseudokirchneriella subcapitata. Water Res 40:3393–3400
Katsumata M, Takeuchi A, Kazumura K, Koike T (2008) New feature of delayed luminescence: preillumination-induced concavity and convexity in delayed luminescence decay curve in the green alga Pseudokirchneriella subcapitata. J Photochem Photobiol B 90:152–162
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Lambrev P, Goltsev V (2001) pH dependence of the effects of diuron, atrazine and dinoseb on the luminescent properties of thylakoid membranes. Bulg J Plant Physiol 27:80–95
Lang M, Lichtenthaler HK (1991) Changes in the blue-green and red fluorescence emission spectra of beech leaves during the autumnal chlorophyll breakdown. J Plant Physiol 138:550–553
Lavorel J (1973) Kinetics of luminescence in the 10–6 to 10–4 s range in Chlorella. Biochim Biophys Acta 325:213–229
Lavorel J (1975) Luminescence. In: Govindjee (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 223–317
Lavorel J, Dennery T (1982) The slow component of Photosystem II luminescence. A process with distributed rate constant? Biochim Biophys Acta 680:281–289
Lavorel J, Lavergne J, Etienne AL (1982) A reflection of several problems of luminescence in photosynthetic systems. Photobiochem Photobiophys 3:287–314
Lavorel J, Breton J, Lutz M (1986) Methodological principles of measurment of light emitted by photosynthetic systems. In: Govindjee, Amesz J, Fork DJ (eds) Light emission by plants and bacteria. Academic Press, Orlando, pp 57–98
Lazar D (1999) Chlorophyll a fuorescence induction. Biochim Biophys Acta 1412:1–28
Li Q, Xing D, Jia L, Wang J (2007) Mechanism study on the origin of delayed fluorescence by an analytic modeling of the electronic reflux for photosynthetic electron transport chain. J Photochem Photobiol B 87:183–190
Malkin S (1979) Delayed luminescence. In: Trebst A, Avron M (eds) Photosynthesis I. Photosynthetic electron transport and photophosphorilation. Acad.Press, NY, pp 473–491
Malkin S, Barber J (1978) Induction patterns of delayed luminescence from isolated chloroplasts. I. Response of delayed luminescence to changes in the prompt fluorescence yield. Biochim Biophys Acta 502:524–541
Malkin S, Bilger W, Schreiber U (1994) The relationship between luminescence and fluorescence in tobacco leaves during the induction period. Photosynth Res 39:57–66
Mar T, Brebner J, Roy G (1975) Induction kinetics of delayed light emission in spinach chloroplasts. Biochim Biophys Acta 376:345–353
Markovic DZ, Kalauzi A, Radenovic CN (2001) Delayed fluorescence induction transients: mathematical modelling based on the chosen kinetic models. Gen Physiol Biophys 20:303–313
Milanov G, Stefanov D, Goltsev V, Batchvarova R (1997) Changes in the submilli- and millisecond kinetics of dark relaxation of delayed fluorescence in tobacco leaves under conditions of bacterial infection by Pseudomonas syringae pv. Tabaci. Bulg J Plant Physiol 23:35–42
Miloslavina Y, Szczepaniak M, Muller M, Sander J, Nowaczyk M, Rцgner M, Holzwarth AR (2006) Charge separation kinetics in intact Photosystem II core particles is trap-limited. A picosecond fluorescence study. Biochemistry 45:2436–2442
Mimuro M, Akimoto S, Akimoto S, Tomo T, Yokono M, Miyashita H, Tsuchiya T (2007) Delayed fluorescence observed in the nanosecond time region at 77 K originates directly from the photosystem II reaction center. Biochim Biophys Acta 1767:327–334
Mladenova I, Maini C, Mallegni P, Goltsev V, Vladova R, Vinarova K, Rocheva S (1998) Siapton—an amino-acid-based biostimulant reducing osmostress metabolic changes in maize. Agro-Food-Industry Hi-Tech 9:18–22
Monti M, Zrimec A, Beran A, Berden-Zrimec M, Drinovec L, Kosi G, Tamberlich F (2005) Delayed luminescence of Prorocentrum minimum under controlled conditions. Harmful Algae 4:643–650
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht
Pavlova E, Matorin D, Venedictov P (1978) Investigation of delayed fluorescence in leaves of woody plants grown under different illumination conditions. Soviet Plant Physiol 25:71–78
Plekhanov SE, Chemeris IK (2003) Early toxic effect of zinc, cobalt, and cadmium on photosynthetic activity of green alga Chlorella pyrenoidosa Chick S-39. Izvestiia Akademii nauk Ser Biol/RAN 5:610–616
Pospisil P, Dau H (2002) Valinomycin sensitivity proves that light-induced thylakoid voltages result in millisecond phase of chlorophyll fluorescence transients. Biochim Biophys Acta 1554:94–100
Radenovic C, Markovic D, Jeremic M (1994) Delayed chlorophyll fluorescence in plant models. Photosynthetica 30:1–24
Robinson HH, Crofts AR (1983) Kinetics of the oxidation-reduction reactions of the photosystem II quinone acceptor complex, and the pathway for deactivation. FEBS Lett 153:221–226
Rottenberg H (1977) Proton and ion transport across the thylakoid membranes. In: Trebst A, Avron M (eds) Photosynthesis I. Electron transport and photophosphorilation. Springer-Verlag, Berlin, pp 338–349
Ruby RH (1976) Delayed fluorescence from Chlorella: II. Effects of electron transport inhibitors DCMU and NH2OH. Photochem Photobiol 26:293–298
Rutherford AW, Inoue Y (1984) Oscillation of delayed luminescence from PS II: recombination of S2QB − and S3QB −. FEBS Lett 165:163–170
Rutherford AW, Govindjee, Inoue Y (1984) Charge accumulation and photochemistry in leaves studied by thermoluminescence and delayed light emission. Proc Natl Acad Sci USA 81:1107–1111
Satoh K, Katoh S (1983) Induction kinetics of millisecond-delayed luminescence in intact Bryopsis chloroplasts. Plant Cell Physiol 24:953–962
Schansker G, Srivastava A, Govindjee, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schansker G, Toth SZ, Strasser RJ (2006) Dark recovery of the Chl a fluorescence transient (OJIP) after light adaptation: the qT-component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim Biophys Acta 1757:787–797
Schatz GH, Brock H, Holzwarth AR (1988) Kinetic and energetic model for the primary processes in Photosystem II. Biophys J 54:397–405
Schmidt W, Senger H (1987) Long-term delayed luminescence in Scenedesmus obliquus I. Spectral and kinetic properties. Biochim Biophys Acta 890:15–22
Schreiber U, Schliwa U (1987) A solid-state, portable instrument for measurment of chlorophyll luminescence induction in plants. Photosynth Res 11:173–182
Schreiber U, Bilger W, Hormann H, Neubauer C (2000) Chlorophyll fluorescence as a diagnostic tool: basics and some aspects of practical relevance. In: Raghavendra AS (ed) Photosynthesis: a comprehensive treatise. Cambridge University Press, Cambridge, pp 320–336
Shuvalov VA, Klimov VV (1976) The primary photoreactions in the complex cytochrome-P-890 P-760 (bacteriopheophytin 760) of Chromatium minutissimum at low redox potentials. Biochim Biophys Acta 440:587–599
Shuvalov VA, Litvin FF (1969) Mechanism of delayed light emission of plans leaves and energy storage in photosynthetic centers. Mol Biol (Moscow) 3:45–56
Sonneveld A, Rademaker H, Duysens LNM (1980a) Microsecond delayed fluorescence of photosystem II of photosynthesis in various algae: emission spectra and uphill energy transfer. FEBS Lett 113:323–327
Sonneveld A, Duysens LNM, Moerdijk A (1980b) Magnetic field-induced increase in chlorophyll a delayed fluorescence of photosystem II: a 100- to 200-ns component between 4.2 and 300 K. Proc Natl Acad Sci USA 77:5889–5893
Srivastava A, Strasser RJ, Govindjee (1999) Greening of peas: parallel measurements of 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. Photosynthetica 37:392
Stacy WT, Mar T, Swenberg CE, Govindjee (1971) An analysis of a triplet exciton model for the delayed light in Chlorella. Photochem Photobiol 14:197–219
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strehler BL, Arnold W (1951) Light production by green plants. J Gen Physiol 34:809–820
Tsimilli-Michael M, Strasser RJ (2008) In vivo assessment of stress impact on plants’ vitality: applications in detecting and evaluating the beneficial role of Mycorrhization on host plants. In: Varma A (ed) Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. Springer, Dordrecht, pp 679–703
Tyystjarvi E, Vass I (2004) Light emission as a probe of charge separation and recombination in the photosynthetic apparatus: relation of prompt fluorescence to delayed light emission and thermoluminescence. In: Papageorgiou G, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 363–388
Valikhanov KM, Zakhidov EA, Zakhidova MA, Kasymdzhanov MA, Kurbanov SS, Nematov SK, Khabibullaev PK (2002) Kinetics of photoinhibition and delayed fluorescence in the plant photosynthetic system. Doklady Biochem Biophys 387:331–334
Van Best JA, Duysens LNM (1977) A one microsecond component of chlorophyll luminescence suggesting a primary acceptor of system II of photosynthesis different from Q. Biochim Biophys Acta 459:187–206
Van Gorkom HJ, Donze M (1973) Charge accumulation in the reaction center of photosystem 2. Photochem Photobiol 17:333–342
Velthuys BR, Amesz J (1975) Temperature and preillumination dependence of delayed fluorescence of spinach chloroplasts. Biochim Biophys Acta 376:162–168
Venediktov PS, Goltsev VN, Shinkarev VP (1980) The influence of electric diffusion potential on delayed fluorescence light curves of chloroplasts treated with DCMU. Biochim Biophys Acta 593:125–132
Veselovskii V, Veselova T (1990) Plant luminescence: theoretical and practical aspects (in Russian). Nauka, Moscow
Wong D, Govindjee, Jursinic P (1978) Analysis of microsecond fluorescence yield and delayed light emission changes after a single flash in pea chloroplasts: effects of mono-and divalent cations. Photochem Photobiol 28:963–974
Wraight CA, Crofts AT (1971) Delayed fluorescence and the high-energy state of chloroplasts. Eur J Biochem 19:386–397
Yacobi YZ, Gerhardt V, Gonen-Zurgil Y, Sukenik A (1998) Delayed fluorescence excitation spectroscopy: a rapid method for qualitative and quantitative assessment of natural population of phytoplankton. Water Res 32:2577–2582
Yordanov I, Goltsev V, Stojanova T, Venediktov P (1987) High temperature damage and acclimation of the photosynthetic apparatus. I. Temperature sensitivity of some photosynthetic parameters of chloroplasts isolated from acclimated and non-acclimated bean leaves. Planta 170:471–477
Yordanov I, Goltsev V, Stefanov D, Chernev P, Zaharieva I, Kirova M, Gecheva V, Strasser RJ (2008) Preservation of PS II electron transport from senescence-induced inactivation in primary leaves after decapitation and defoliation of bean plants. J Plant Physiol 165:1954–1963
Zaharieva I, Goltsev V (2003) Advances on Photosystem II investigation by measurement of delayed chlorophyll fluorescence by a phosphoroscopic method. Photochem Photobiol 77:292–298
Zaharieva I, Velitchkova M, Goltsev V (1998) Effect of cholesterol and benzyl alcohol on prompt and delayed chlorophyll fluorescence in thylakoid membranes. Kluwer Academic Publishers, Netherlands, pp 1827–1830
Zaharieva I, St Taneva, Goltsev V (2001) Effect of temperature on the luminescent characteristics in leaves of arabidopsis mutants with decreased unsaturation of the membrane lipids. Bulg J Plant Physiol 27:3–18
Zankel KL (1971) Rapid delayed luminescence from chloroplasts: kinetic analysis of components; the relationship to the O2 evolving system. Biochim Biophys Acta 245:373–385
Zhang L, Xing D (2008) Rapid determination of the damage to photosynthesis caused by salt and osmotic stresses using delayed fluorescence of chloroplasts. Photochem Photobiol Sci 7:352–360
Zhang L-R, Xing D, Wang J-S, Zeng L-Z, Li Q (2007a) Light-induced delayed fluorescence as an indicator for UV-B radiation environment stress on plants. J Optoelectron Laser 18:878–881
Zhang L, Xing D, Wang J, Li L (2007b) Rapid and non-invasive detection of plants senescence using a delayed fluorescence technique. Photochem Photobiol Sci 6:635–641
Acknowledgements
We thank the Bulgarian National Science Fund, Project No. DO 02-137/15.12.2008 for the financial support. R.J.S acknowledges support by the Swiss National Science Foundation, Project Nr: 200021-116765. We thank Detelin Stefanov for critical reading of the manuscript. The paper was written while I.Z. was working at the Free University in Berlin on an Alexander von Humboldt fellowship. This review was edited by Govindjee. We thank him for many suggestions that have improved this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goltsev, V., Zaharieva, I., Chernev, P. et al. Delayed fluorescence in photosynthesis. Photosynth Res 101, 217–232 (2009). https://doi.org/10.1007/s11120-009-9451-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-009-9451-1