Abstract
The psbZ gene of Synechocystis sp. PCC 6803 encodes the ∼6.6 kDa photosystem II (PSII) subunit. We here report biophysical, biochemical and in vivo characterization of Synechocystis sp. PCC 6803 mutants lacking psbZ. We show that these mutants are able to perform wild-type levels of light-harvesting, energy transfer, PSII oxygen evolution, state transitions and non-photochemical quenching (NPQ) under standard growth conditions. The mutants grow photoautotrophically; however, their growth rate is clearly retarded under low-light conditions and they are not capable of photomixotrophic growth. Further differences exist in the electron transfer properties between the mutants and wild type. In the absence of PsbZ, electron flow potentially increased through photosystem I (PSI) without a change in the maximum electron transfer capacity of PSII. Further, rereduction of P700+ is much faster, suggesting faster cyclic electron flow around PSI. This implies a role for PsbZ in the regulation of electron transfer, with implication for photoprotection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chloroplast-encoded psbZ gene (formerly ycf9, orf62) encodes a ∼6.6 kDa, highly conserved, hydrophobic protein containing two putative membrane-spanning segments. psbZ is present in all chloroplast genomes sequenced so far (Rochaix 1997; Douglas and Penny 1999; Sato et al. 1999; Turmel et al. 1999; Hupfer et al. 2000; Lemieux et al. 2000) except for the peridinin-containing dinoflagellates (Koumandou et al. 2004). In tobacco, the psbZ gene is located downstream of the psbD and psbC genes and is part of the transcription unit formed by these three genes (Yao et al. 1989). In the alga, Chlamydomonas reinhardtii, psbZ is found close to the psbM gene of photosystem II (PSII).
Several groups have studied tobacco psbZ knockout mutants, resulting in ambiguous results. Since psbZ knockout leads to reduced amounts of the minor antenna protein, CP26, it was first suggested (Ruf et al. 2000) that PsbZ plays a role in the stable integration of CP26 into the PSII antenna complex. However, PsbZ was later shown to be a PSII component in tobacco and C. reinhardtii (Swiatek et al. 2001) and more recently, its peripheral location in PSII were confirmed (Ferreira et al. 2004; Arteni et al. 2005; Rokka et al. 2005). Proteomic analysis of a Synechocystis sp. PCC 6803 (hereafter Synechocystis PCC 6803) PSII preparation also revealed the presence of PsbZ in cyanobacterial PSII (Kashino et al. 2002). Further, the presence or absence of CP26 in psbZ knockout plants was shown to be due to growth conditions (Baena-Gonzalez et al. 2001). Thus, it has also been concluded that, rather than a role in light-harvesting, PsbZ functions in regulating the mode of electron transfer in plant chloroplasts (Baena-Gonzalez et al. 2001). Although the PSII core complex of cyanobacteria highly resembles its plant counterparts, the light-harvesting system differs greatly (MacColl 1998), with a membrane-peripheral phycobilisome complex rather than the integral membrane complex. Importantly, CP26 is not found in the cyanobacterial thylakoids. In cyanobacteria, both oxygenic photosynthesis and cell respiration take place in the same cell compartment: photosynthetic electron transfer exclusively in the thylakoid membranes, while the respiratory electron transfer components can be found both in the thylakoid and cytoplasmic membranes (Molitor and Peschek 1986). Some common components like plastoquinone, cytochrome b6f (cyt b6f), soluble electron transfer components, and NADH dehydrogenase, are shared between the photosynthetic and respiratory electron transfer (Mi et al. 1995). However, the respective roles of photosynthesis and respiration in cyanobacterial metabolism are currently poorly understood.
To investigate the role of the psbZ gene in Synechocystis PCC 6803 disruption and deletion mutants of psbZ were generated. Examination of the Synechocystis PCC 6803 genome (Kaneko et al. 1996) confirms that there are no other photosynthetic genes in the proximity of the psbZ gene. Thus, phenotypes arriving from mutations in the psbZ orf of Synechocystis PCC 6803 can be examined without the potential for an indirect effect. Biophysical, biochemical and in vivo characterization of the cyanobacterial psbZ mutants reveal no obvious phenotype under standard growth conditions: the mutants are able to perform light-harvesting, photosynthesis, state transitions and non-photochemical quenching (NPQ). However, their growth is impaired under low-light intensity; the mutants have lost the glucose tolerance phenotype of the wild-type Synechocystis sp. PCC 6803 strain used in this study; and differences exist in the electron transfer properties of the mutant when compared to the wild type.
Materials and methods
Cell material, growth conditions and determination of growth rates
Synechocystis PCC 6803 cells were grown in BG-11 medium (Stanier et al. 1971) under low light (7 μmol photons m−2 s−1; LL) or growth light (40 μmol photons m−2 s−1; HL) conditions at 32°C and used during the logarithmic growth phase. Spectrophotometric determination of growth rates was performed according to Mäenpää et al. (1998). Selection and maintenance of the psbZ mutants was performed in the presence of 100 μg ml−1 kanamycin. Where indicated, BG-11 was also supplemented with 5 mM glucose. Spot tests were carried out on agar plates: a 10 μl aliquot of the appropriate culture in log phase was spotted onto a plate, and then incubated under low light at 32°C.
Plasmid construction, cell transformation and selection of mutant lines
The psbZ orf, or orf sll1281, together with 452 bp 5′ and 245 bp 3′-flanking sequences was amplified by polymerase chain reaction (PCR) of genomic DNA isolated from wild-type Synechocystis PCC 6803 using primers 5′-GCCAGGGAAATTGATCGTTGGAGTG-3′ and 5′-CACATCAATGCCATGACGTTTGAGGC-3′. The 886 bp PCR product was then cloned into the HincII site of the plasmid vector pUC19 generating pSpsbZ. Present within the PCR product were two BsmFI sites at positions 429 (23 bp upstream of the psbZ ATG) and 564 (within the psbZ orf). The 1,291 bp HincII fragment from pUC4K (Amersham Pharmacia Biotech) containing the kanamycin-resistance cassette was legated into the BsmFI sites, producing two different constructs. The first, pSpsbZ2, was the result of the insertion of the kanamycin-resistance cassette at position 429. This was used to generate the mutant Kn2 in which the transcription of psbZ is disrupted by the insertion of the cassette. In the second, pSpsbZ5, the kanamycin-resistance cassette replaced the 429–564 BsmFI fragment and was used to generate the mutant Kn5 in which most of the psbZ coding region is deleted. DNA sequence analysis confirmed the integrity of the plasmids together with insert orientations. The kanamycin-resistance cassette was present in the opposite orientation to psbZ orf in both plasmids. Wild-type Synechocystis PCC 6803 was transformed with pSpsbZ2 and pSpsbZ5, and transformants selected on the basis of kanamycin resistance. Transformant homoplasmicity was confirmed using Southern analysis. The wild type used in this study was the glucose-tolerant strain developed by Williams (1998).
Isolation of thylakoid membranes, gel electrophoresis, immunoblotting and chlorophyll determination
Cell membranes were isolated at 4°C as described (Tyystjärvi et al. 1994). The chlorophyll a content of the cell suspensions and isolated membranes was determined according to the method of Porra (1989). The proteins were separated by using 15% PAGE containing 6 M urea (Laemmli 1970), with samples loaded on an equal chlorophyll basis. For detection of PsbZ, thylakoid polypeptides were separated on Tricine-SDS PAGE (Schägger and von Jagow 1987) and stained with silver nitrate. Polyclonal antibodies against the D1 and D2 polypeptides were purchased (Research Genetics, Inc.). Other antibodies were gifted as follows: CP1 (Dr Hundal), CP 43, α-Rieske and Photosystem I (PSI) (Dr Barbato), Cyt b6f (Dr Wollman), ndhH (Dr Peltier) and ndhK (Dr Appel). Immunoblotting was performed using standard methods.
Measurements of oxygen evolution in vivo
Oxygen evolution at 32°C was measured with a Clark-type oxygen electrode under saturating red light. For each measurement, 1 ml of cell suspension corresponding to 10 μg chl ml−1 was re-suspended in 1 ml of BG-11. 0.5 mM 2,6-dichloro-p-benzoquinone (DCBQ) in the presence of 0.25 mM ferricyanide was used as electron acceptor for measurements of PSII activity. Oxygen evolution was also measured using 0.6 mM bicarbonate as the electron acceptor for measurements of photosynthetic capacity of the cells.
Dark re-reduction of P700
The redox state of the reaction centre chlorophyll of PSI (P700) in isolated thylakoids was determined from the absorbance of the oxidised form of P700 (P700+) at 810 nm, using 860 nm as a reference. Absorbance changes were monitored using an ED-P700DW unit attached to the PAM 101 fluorometer. Measurements were done under anaerobic conditions in a temperature-regulated cuvette (25°C) containing 0.5 ml of buffer (50 mM Tricine (pH 7.5), 5 mM MgCl2, 6 mM glucose, 2 mM NH4Cl, 400 U ml−1 catalase, 50 μM ferredoxin) (Scheller 1996). The mixture was flushed with nitrogen, and 2 U of glucose oxidase and thylakoids (50 μg chl) were added. An initial illumination period (1,000 μmol photons m−2 s−1) of 30 s was applied to reduce ferredoxin. After that, the samples were kept in darkness for 10 s. Thereafter, 30 cycles of actinic light (1.2 s) and darkness (8.8 s) were applied and the average post-illumination change in the P700+ signal of the 30 repetitions was resolved in the sum of two exponentials. The results reported considers only the fast component of the signal.
Electron paramagnetic resonance (EPR) analysis
EPR analysis was performed as in Bishop et al. (2003) on whole cell samples concentrated from 200 ml of exponentially growing culture. The pellet was re-suspended in TES buffer (5 mM Tris (pH 8.5), 50 mM NaCl, 5 mM EDTA). Samples were dark-adapted then frozen, and analysed using a Jeol REIX spectrometer with an Oxford Instruments liquid helium cryostat. The EPR conditions were microwave power 10−3 mW, modulation amplitude 0.2 mT and temperature 10 K.
Analysis of state transition by 77 K fluorescence emission spectroscopy
This measurement enables energy transfer to and between PSI and PSII to be determined. Seventy-seven Kelvin fluorescence emission spectra were taken using a Perkin Elmer LS50 luminescence spectrometer with excitation and emission slit widths of 5 and 10 nm, respectively. Measurements were taken following excitation at 435 nm (chlorophyll a) and 600 nm (allophycocyanin). Results were analysed using Sigma Plot for Windows Version 5.0.
Analysis of state transition
Synechocystis PCC 6803 cells were grown photoautotrophically under low-light conditions and whole cell samples at 5 μM chlorophyll prepared. Cells were dark-adapted at room temperature for a minimum of 5 min (state 2). Transition from state 2 to state 1 was initiated by excitation at 600 nm (phycocyanin absorption) after 60 s. Cells were illuminated for a further 140 s (transition to state 1), and then allowed to return to state 2. Fluorescence emission was measured at 680 nm (PSII chlorophyll a).
Results
Verification and phenotype of the mutant strains
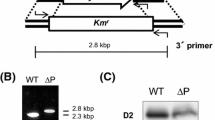
Two different Synechocystis PCC 6803 transformant lines were generated in which psbZ was inactivated. In the Kn2 transformants, the transcription of psbZ was disrupted by insertion of the kanamycin-resistance cassette immediately upstream of the psbZ translational start. In the Kn5 transformants, 112 bp of psbZ were deleted by insertion of the cassette within the gene. Transformant homoplasmicity was confirmed using Southern analysis (data not shown).
Preliminary growth comparisons of the transformants Kn2 and Kn5 with wild type were performed using spot tests. Four types of plates were used: BG-11, BG-11 with 5 mM glucose, BG-11 with 100 μg ml−1 kanamycin or BG-11 with glucose and kanamycin. Wild type Synechocystis PCC 6803 cells were capable of growth on BG-11 plates and BG-11 plates with glucose, but did not grow in the presence of kanamycin, as expected (Fig. 1A). The mutants Kn2 and Kn5 were able to grow on BG-11 plates and BG-11 plates with kanamycin, showing that they were capable of photoautotrophic growth and that the antibiotic-resistance cassette was functional. Unexpectedly, however, the mutants did not grow in the presence of glucose.
Growth analysis of wild-type Synechocystis PCC 6803 and the psbZ mutants. (A) Spot tests for growth on BG-11, BG-11 supplemented with glucose (5 mM), BG-11 supplemented with kanamycin (100 μg ml−1) and BG-11 supplemented with glucose and kanamycin. An aliquot of each culture was spotted onto the corresponding plate, then grown at 30°C and 7 μmol photons m−2 s−1. (B) Median growth rates of wild type and Kn2 mutant strains in liquid BG-11 under growth light (gl) and low light (LL) conditions. Note: both Kn2 and Kn5 showed the same growth response (data not shown)
Under standard growth conditions (40 μmol photons m−2 s−1) the psbZ mutants grew slightly slower than wild-type cells (Fig. 1B). Growth under low-light conditions (7 μmol photons m−2 s−1) did not affect the wild-type growth rate. In contrast, the mutants were further impaired at this lower light intensity—wild-type cells had a doubling time of 2.8 days under both growth and low-light conditions, whilst the doubling time for the mutant under growth light was 3.4 days. This was further reduced to 4.3 days under low-light conditions.
Thylakoid protein composition and oxygen evolution properties in vivo
Silver staining of polyacrylamide gels containing separated thylakoid polypeptides confirmed the absence of PsbZ in the mutant lines Kn2 and Kn5 (data not shown). Immunoblot analysis did not reveal significant changes in the amounts of specific subunits of PSI, PSII, cyt b6f complex, ndhH polypeptide and the ATP synthase following loss of PsbZ (Fig. 2). There is no molecular reason to suppose that the Kn2 and Kn5 mutants would not have the same phenotype.
Electron transfer from H2O to either DCBQ (PSII oxygen evolution) or to bicarbonate (photosynthetic capacity) was measured in the psbZ mutants and wild-type cells. The light-saturated PSII activities of wild type and the mutants did not differ being (218 ± 20 μmol O2 (mg of chlorophyll)−1 h−1). However, the oxygen evolution activity obtained from the photosynthetic capacity measurement of the mutant strains was significantly increased (129 ± 8 μmol O2 (mg of chlorophyll)−1 h−1) when compared to the wild-type value (89 ± 9 μmol O2 (mg of chlorophyll)−1 h−1).
Re-reduction of P700+ in vitro
Post-illumination re-reduction rates of P700+ were determined from isolated thylakoid membranes. Figure 3 demonstrates that the reduction of P700+ occurred at a much faster rate in both psbZ mutants strains when compared to wild-type Synechocystis PCC 6803, suggesting an increased flow of electrons to P700+, referring to activated cyclic electron flow around PSI.
EPR analysis of the psbZ mutants
Next, EPR spectra were obtained for dark-adapted wild-type cells together with the psbZ mutants Kn2 and Kn5 and a PSII-deficient mutant generated by the deletion of psbH (O’Connor et al. 1998; Fig. 4A). A typical \( {\hbox{Y}}_{\hbox{D}}^ \cdot \) spectrum was produced by the wild-type cells, showing that the PSII core complex was assembled and active. For both the psbZ mutants similar spectra were observed, indicating that the PSII core complex was not compromised in these cells. The detection of the \( {\hbox{Y}}_{\hbox{D}}^ \cdot \) signal also confirmed that PSII was able to oxidise YD via P680. As expected, the control PSII-deficient cells lacked the \( {\hbox{Y}}_{\hbox{D}}^ \cdot \) signal.
EPR analysis of wild-type Synechocystis PCC 6803, the psbZ mutants and a PSII− mutant. (A) EPR spectra of the \( {\hbox{Y}}_{\hbox{D}}^ \cdot \)
in wild-type Synechocystis PCC 6803, Kn2, Kn5 and PSII− dark-adapted cells. (B) EPR analysis of P700+ in wild-type Synechocystis PCC 6803, Kn2 and Kn5 cells. The spectra are the illuminated spectrum (at 10 K) minus the dark spectrum. See Materials and methods for further details
Figure 4B shows the P700+ photoinduced EPR spectra for wild type and the psbZ mutants. The spectra confirm that PSI was also functional in all three cell types. Comparative quantitative analysis was achieved by normalising the EPR signals to the same chlorophyll concentration. This showed that the levels of PSII (measured by the \( {\hbox{Y}}_{\hbox{D}}^ \cdot \) signal) relative to PSI (measured by the P700+ signal) were slightly lower in the Kn2 and Kn5 mutants, with respect to wild type. This reduction, although not striking, was consistently observed when measurements were compared for several independent wild type and mutant samples (data not shown).
State transition assay of the psbZ mutants
There is a high degree of similarity in the PSII of oxygenic photosynthetic organisms. However, one of the major differences is the light-harvesting system that is present. In cyanobacteria, the phycobilisomes are responsible for capturing light, whilst in higher plants and green algae, chlorophyll containing LHC performs this function. Consequently, the interaction of antenna systems with the PSII core complex will be different in prokaryotes and eukaryotes. Swiatek et al. (2001) proposed that PsbZ might play a role in the interaction between the LHC and PSII. If this is the case, it is plausible that state transitions in the psbZ mutants may give a difference in light-harvesting between eukaryotes and prokaryotes. Therefore, we examined whether the loss of PsbZ in Synechocystis PCC 6803 had any effect on cyanobacterial state transitions (Fig. 5).
Room temperature fluorescence time course for dark-adapted (state 2) wild-type Synechocystis PCC 6803 and the psbZ mutants. Excitation was at 600 nm (phycocyanin absorption) with fluorescence measured at 680 nm (PSII chlorophyll a). The samples were illuminated at 60 s to induce a transition to state 1, then the light switched off at 200 s. The spectra from the two Kn mutants are very similar. The distinct wild-type spectrum is labelled
Dark-adapted wild-type cells (state 2—PBS associated with PSI) produced a stable level of relative fluorescence. Following illumination of the cells for 60 s there was a slight, but temporary, decrease in the relative fluorescence, resulting from the rapid oxidation of the plastoquinone pool. This was followed by a rise in fluorescence as the PBS moves from PSI to PSII (transition to state 1), equilibrating at 200 s. When the cells were returned to the dark after 140 s of illumination, the sharp increase in relative fluorescence was followed by a gradual decrease as the PBS return to PSI. Examination of the mutants Kn2 and Kn5 produced the same overall pattern, confirming that in the absence of PsbZ cells were still capable of performing state transitions. The decrease in fluorescence observed in wild type at 60 s is absent from both of the mutant spectra, indicating that the plastoquinone pool was not as reduced (i.e. more oxidised) in the mutant cells, relative to wild type. This was typical of a shift in the ratio of delivery of excitation energy between PSII and PSI, consistent with previous observations.
To follow the energy transfer to and between the photosystems, 77 K fluorescence emission spectroscopy was performed on wild type and psbZ mutant cells grown under low-light conditions (3 μmol photons m−2 s−1) and adjusted to 10 μM chlorophyll. Figure 6 shows the resulting spectra for dark-adapted (state 2) cells following excitation at 435 nm (chlorophyll a; Fig. 6A) and 600 nm (allophycocyanin; Fig. 6B). Examination of the 435 nm fluorescence emission spectra revealed a decrease in the peaks at 685 nm (fluorescence of chlorophyll molecules associated with PSII) and 695 nm (fluorescence of chlorophyll molecules specifically associated with CP47). The reduction was greatest for the Kn2 mutant. The same fluorescence measurements were performed for the cells in state 1. Excitation at 435 and 600 nm confirmed the findings from the state 2 measurements (data not shown). Thus, while energy transfer between PSII and PSI is not compromised in the mutants, the absence of PsbZ appears to result in an increase in the antenna compliment relative to wild type.
Seventy-seven Kelvin fluorescence emission spectroscopy of dark-adapted (state 2) wild-type Synechocystis PCC 6803 (solid line) and the psbZ mutants Kn2 (thick dashed line) and Kn5 (thin dashed line). (A) Excitation at 435 nm or (B) at 600 nm. Both spectra are normalised to the PSI maximum at ∼725 nm
Discussion
The PsbZ protein has been shown to be an intrinsic component of PSII of higher plants and C. reinhardtii (Swiatek et al. 2001). In a study with tobacco, PsbZ was connected to CP26 (Ruf et al. 2000), a component of plant light-harvesting antenna of PSII. However, the presence of PsbZ was also revealed in cyanobacterial PSII (Kashino et al. 2002), whose light-harvesting complex lacks any component resembling CP26. This makes Synechocystis PCC 6803 an interesting model to study the role of PsbZ. Here, we have employed reverse genetics to unravel the function of the PsbZ protein in Synechocystis PCC 6803.
Although psbZ mutants of Synechocystis PCC 6803 were capable of photoautotrophic growth, the growth rates were impaired when compared to wild type. Surprisingly, in contrast to wild type, the psbZ mutants were not capable of growth in the presence of glucose. Photomixotrophic growth conditions are known to produce excess photooxidative damage (Hihara and Sonoike 2001). Such damage to the thylakoid membrane can usually be avoided by dispersal of the excess excitation energy via photoprotection mechanisms, which are intimately linked through the xanthophyll cycle in plants. There are a number of photosynthetic organisms, like cyanobacteria, that do not perform the xanthophyll cycle, that do not accumulate zeaxanthin, or lack both processes (Demmig-Adams 1990; Horton 1996). However, these organisms still express PsbZ and are capable of photoprotection. It is, therefore, possible that PsbZ plays a role in protection against photoinhibition. It is interesting that a similar role has been proposed for psbK following the isolation of glucose-sensitive psbK-distruptants of Synechocystis PCC 6803 (Kobayashi et al. 2005).
To reveal the reasons for reduced growth in mutants we analysed the partial reactions of photosynthetic electron transfer. EPR spectroscopic analysis of the psbZ mutants showed a normal \( {\hbox{Y}}_{\hbox{D}}^ \cdot \) spectrum. Further, the oxygen-evolution capacity of PSII was similar for wild type and mutants, confirming that the absence of PsbZ does not significantly affect the assembly of the PSII reaction centre core and that PsbZ is not required for the oxidation of YD via P680+. Examination of PSI function, on the other hand, showed some abnormality. However, reasons for the significantly accelerated post-illumination reduction of P700+ are difficult to evaluate: in addition to the linear PSI electron transfer and the PSI back reaction, the water–water cycle (Makino et al. 2002), the NDH-1 (Zhang et al. 2004) and a PGR5-dependent cyclic electron pathway (Munekage et al. 2002) may be involved. It is possible that these accelerated electron transfer pathways, in the absence of PsbZ, results in strong protection around PSI. Under normal light conditions this change has little impact; however, under low-light conditions when linear electron transfer will be slower and NADPH production reduced, the influence of such an increase in protection is expected to be more conspicuous.
Consistent with the tobacco mutants, the Synechocystis PCC 6803 psbZ mutants presented a phenotype similar to wild type under standard growth conditions, but showed impaired growth at low irradiances. In one case it was concluded that the tobacco psbZ knockout had reduced light-harvesting capacity relative to wild type. These plants were shown to have reduced amounts of the CP26 light-harvesting antenna component (Ruf et al. 2000). In contrast, Baena-Gonzalez et al. (2001) showed that, under low-light conditions, where the mutant phenotype became obvious, CP26 clearly accumulates in plants lacking PsbZ. In cyanobacteria, the light-harvesting antenna of PSII differs from that of plants (Sidler 1994) and lacks any subunits homologous to CP26. Importantly, despite the retarded growth at low irradiance, no differences in the percentage decrease of PSII activities between the psbZ mutants and the wild type was observed when the measurements were performed at limiting light intensities. Further, comparison with the corresponding activities under saturating light conditions, argues against impairment of the efficiency of energy transfer from the light-harvesting antenna to the reaction centre in the absence of PsbZ in Synechocystis PCC 6803. Similarly, energy transfer to and between the photosystems takes place in the same way in the mutants and the wild type.
Retarded growth at low irradiances of cyanobacterial psaE (Zhao et al. 1993) and ndhF (Schluchter et al. 1993) mutants has been suggested to result from an inability to produce enough ATP under those conditions. Interestingly, the tobacco) psbZ mutant (Baena-Gonzalez et al. 2001) had defects in electron transfer routes alternative to linear photosynthetic electron transfer (Bendall and Manasse 1995), which may result in impairment of growth under sub-optimal conditions. The slight change in the PSII/PSI ratio in favour of PSI in the Synechocystis PCC 6803 psbZ mutants may reflect an adjustment of the ATP/NADPH ratio necessary for mutant growth under sub-optimal conditions such as low-light intensities. Acclimation of plants to low light conditions is known to involve reduction of the PSII/PSI ratio (Anderson et al. 1995) aiming at adjustment of the ATP/NADPH ratio through cyclic electron flow (Finazzi et al. 1999).
In summary, we present the characterisation of Synechocystis PCC 6803 psbZ mutants supporting a model in which PsbZ functions in the regulation of electron transfer activity through the two photosystems, and particularly in dissecting the electron flow in PSI.
Abbreviations
- CP1:
-
Major chlorophyll a-protein of PSI
- cyt b6f:
-
Cytochrome b6f
- DCBQ:
-
2,6-Dichloro-p-benzoquinone
- EPR:
-
Electron paramagnetic resonance
- LHC:
-
Light-harvesting complex
- NPQ:
-
Non-photochemical quenching
- orf :
-
Open reading frame
- P700:
-
Reaction centre chlorophyll of photosystem I
- P700+ :
-
Oxidized P700
References
Anderson JM, Chow WS, Park Y-I (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Arteni AA, Nowaczyk M, Lax J, Kouril R, Rogner M, Boekema EJ (2005) Single particle electron microscopy in combination with mass spectrometry to investigate novel complexes of membrane proteins. J Struct Biol 149:325–331
Baena Gonzalez E, Gray JC, Tyystjärvi E, Aro E-M, Mäenpää P (2001) Abnormal regulation of photosynthetic electron transport in a chloroplast ycf9 inactivation mutant. J Biol Chem 276:20795–20802
Bendall DS, Manasse RS (1995) Cyclic photophosphorylation and electron transport. Biochim Biophys Acta 1229:23–38
Bishop CL, Purton S, Nugent JHA (2003) Molecular analysis of the Chlamydomonas nuclear gene encoding PsbW and demonstration that PsbW is a subunit of photosystem II, but not photosystem I. Plant Mol Biol 52:285–289
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Douglas SE, Penny SL (1999) The plastid genome of the cryptophyte alga, Guillardia theta: complete sequence and conserved synteny groups confirm its common ancestry with red algae. J Mol Evol 48:236–244
Ferreira KN, Iverson TM, Magjlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838
Finazzi G, Furia A, Barbagallo RP, Forti G (1999) State transitions, cyclic and linear electron transport and photophosphorylation in Chlamydomonas reinhardtii. Biochim Biophys Acta 1413:117–129
Hihara Y, Sonoike K (2001) Regulation, inhibition and protection of photosystem I. In: Anderson B, Aro EM (eds) Advances in photosynthesis XI, regulation of photosynthesis. Kluwer Academic, The Netherlands, pp 507–531
Horton P (1996) Nonphotochemical quenching of chlorophyll fluorescence. In: Jennings RC, Zucchelli G, Ghetti F, Colombetti G (eds) Light as energy source and information carrier in plant physiology. Plenum Press, New York, pp 99–112
Hupfer H, Swiatek M, Hornung S, Herrmann RG, Maier RM, Chiu WL, Sears B (2000) Complete nucleotide sequence of the Oenothera elata plastid chromosome, representing plastome I of the five distinguishable euoenothera plastomes. Mol Gen Genet 263:581–585
Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E et al (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res 3:109–136
Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmars J, Satoh K, Pakrasi HB (2002) Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 41:8004–8012
Kobayashi M, Katsuhiko O, Ikeuchi M (2005) A suppressor mutation in the phycocyanin gene in the light/glucose-sensitive phenotype of the psbk-disruptant of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 46:1561–1567
Koumandou VL, Nisbet RER, Barbrook AC, Howe CJ (2004) Dinoflagellate chloroplasts—where have all the genes gone? Trends Genet 20:261–267
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lemieux C, Otis C, Turmel M (2000) Ancestral chloroplast genome in Mesostigma viride reveals an early branch of green plant evolution. Nature 403:649–652
MacColl R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124:311–334
Makino A, Miyake C, Yokota A (2002) Physiological functions of the water–water cycle (Mehler reaction) and the cyclic electron flow around PSI in rice leaves. Plant Cell Physiol 43:1017–1026
Mi H, Endo T, Ogawa T, Asada K (1995) Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol 36:661–668
Molitor V, Peschek GA (1986) Respiratory electron transport in plasma and thylakoid membrane preparations from the cyanobacterium Anacystis nidulans. FEBS Lett 195:145–150
Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110:361–371
Mäenpää P, Sippola K, Rokka A, Aro E-M (1998) Substitution of Ala-251 of the D1 reaction centre polypeptide with a charged residue results in impaired function of photosystem II. Plant Mol Biol 38:1191–2000
O’Connor HE, Ruffle SV, Cain AJ, Deak Z, Vass I, Nugent JHA, Purton S (1998) The 9 kDa phosphoprotein of photosystem II. Generation and characterisation of Chlamydomonas mutants lacking PSII-H and a site-directed mutant lacking the phosphorylation site. Biochim Biophys Acta 1364:63–72
Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents. Biochim Biophys Acta 975:384–394
Rochaix JD (1997) Chloroplast reverse genetics: new insights into the function of plastid genes. Trends Plant Sci 2:419–425
Rokka A, Suorsa M, Saleem A, Battchikova N, Aro E-M (2005) Synthesis and assembly of thylakoid protein complexes: multiple assembly steps of photosystem II. Biochem J 388:159–168
Ruf S, Kossel H, Bock R (2000) A small chloroplast-encoded protein as a novel architectural component of the light-harvesting antenna. J Cell Biol 139:95–102
Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S (1999) Complete structure of the chloroplast genome of Arabidopsis thaliana. DNA Res 6:283–290
Scheller HV (1996) In vitro cyclic electron transport in barley thylakoids follows two independent pathways. Plant Physiol 110:187–194
Schluchter WM, Zhao J, Bryant DA (1993) Isolation and characterization of the ndhF gene of Synechococcus sp. PCC 7002 and initial characterization of an interposon mutant. J Bacteriol 175:3343–3352
Schägger H, von Jagow G (1987) Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 166:368–379
Sidler WA (1994) Phycobilisome and phycobiliprotein structures. In: Bryant DA (ed) The molecular biology of cyanobacteria. Kluwer Academic Publishers, The Netherlands, pp 139–216
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35:171–205
Swiatek M, Kuras R, Sokolenko A, Higgs D, Olive J, Cinque G, Muller B, Eichacker LA, Stern DB, Bassi R, Herrmann RG, Wollmann F-A (2001) The chloroplast gene ycf9 encodes a photosystem II (PSII) core subunit, PsbZ, that participates in PSII supramolecular architechture. Plant Cell 13:1347–1367
Turmel M, Otis C, Lemieux C (1999) The complete chloroplast DNA sequence of the green alga Nephroselmis olivacea: insights into the architechture of ancestral chloroplast genomes. Proc Natl Acad Sci USA 96:10248–10253
Tyystjärvi T, Aro E-M, Jansson C, Mäenpää P (1994) Changes of amino acid sequence in PEST-like area and QEEET motif affect degradation rate of D1 polypeptide in photosystem II. Plant Mol Biol 25:517–526
Yao WB, Meng BY, Tanaka M, Sugiura M (1989) An additional promoter within the protein-coding region of the psbD–psbC gene cluster in tobacco chloroplast DNA. Nucleic Acids Res 17: 9583–9591
Williams JGK (1988) Construction of specific mutations in the photosystem II photosynthetic reaction center by genetic engineering methods in the cyanobacterium Synechocystis 6803. Methods Enzymol 167:766–778
Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, Aro E-M (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16:3326–3340
Zhao J, Snyder WB, Muhlenhoff U, Rhiehl E, Warren PV, Goldbeck JH, Bryant DA (1993) Cloning and characterization of the psaE gene of the cyanobacterium Synechococcus sp. PCC 7002: characterization of a psaE mutant and overproduction of the protein in Escherichia coli. Mol Microbiol 9:1283–1294
Acknowledgements
The work in the U.K. was supported by funding from the U.K. Biotechnology and Biological Sciences Research Council and the Leverhulme Trust. The Finnish group acknowledges research grant from the Academy of Finland (PM), from Turku University Foundation (PM) and from CIMO (SU). We are grateful to Conrad Mullineaux for his advice and assistance with the 77 K fluorescence spectrometry and Yagut Allahverdiyeva for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bishop, C.L., Ulas, S., Baena-Gonzalez, E. et al. The PsbZ subunit of Photosystem II in Synechocystis sp. PCC 6803 modulates electron flow through the photosynthetic electron transfer chain. Photosynth Res 93, 139–147 (2007). https://doi.org/10.1007/s11120-007-9182-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9182-0