A process was developed for the production of agglomerated carbonyl nickel powders with spherical particles ranging from 45 to 71 μm. The powders are intended to make spongy oxide/nickel cathodes. The idea relied on the separation of integral and local compaction effects in the sintering of agglomerated powders. The morphology and particle size of the powders were examined by scanning electron microscopy using a Superprobe-733 analyzer. The nickel powders were chemically tested to determine their carbon content with automatic coulometric titration by pH values employing an AN-7529U rapid analyzer. The agglomerated carbonyl nickel powders with spherical 45–71 μm particles were produced from PNK-1L5 carbonyl nickel powders with average particle sizes of 4 μm by annealing without mechanical grinding. The optimum process of producing nickel powders with 45–71 μm particles involves stage-by-stage annealing of agglomerated particles at 400, 500, and 600°C for 0.5 h with intermediate sifting through sieves 071 and 045. Testing of the spongy oxide/nickel cathodes produced in compliance with the agglomeration process for the fine PNK-1L5 nickel powders showed that they could be used to replace the PNK-2K10 nickel powders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The oxide cathodes that are effectively used in various ultrahigh-frequency vacuum-tube devices represent classic cathode materials.
A spongy (or sintered) oxide cathode is one of the cathode types used for magnetrons and pulsed klystrons. A spongy cathode consists of a porous nickel powder layer grown on and sintered to a nickel core, the nickel layer pores being filled with an emissive substance. The chemical composition, morphology, and sizes of nickel powder particles are important factors that determine the functional and process properties of oxide cathodes. The content of admixtures such as silicon, manganese, and iron should not exceed 0.01% and that of sulfur, lead, and zinc should not exceed 0.005%. The properties of a cathode material depend on its carbon content or, particularly, on the carbon content of the carbonyl nickel powder. The allowed carbon content of the nickel powders is within 0.3% [1, 2]. Since a nickel layer grows when the powder is poured freely and its particles fuse together at 0.88Tmelt without shrinkage, the particle size and morphology are crucial. The use of coarse 45–71 μm particles does not lead to volume shrinkage, and morphological features—spherical particles consisting of fine grains ≤2 μm—promote a uniform structure when the powder is poured and the particles are actively sintered to each other.
To make spongy oxide/nickel cathodes employing the conventional technique, a carbonyl nickel powder of PNK-2K10 grade with 45–71 μm particles (as per GOST 9722-97) was used. Coarse fractions of the PNK-2K9 and PNK-2K10 carbonyl nickel powders can be produced in insignificant amounts because the technique it complex and requires a special reactor. To coarsen the carbonyl nickel powder, it is preliminary heated to 200–230°C and then fed together with nickel tetracarbonyl to the decomposition chamber where the tetracarbonyl vapors dissociate on the heated nickel particles and cause them to grow [3].
Should there be no nickel powders of required particle size on sale, they are produced employing various techniques at plants’ cathode departments. Two methods find the widest application [1]. One method is to reduce nickel oxide resulting from the decomposition of nickel oxalate at 700–800°C for 1–2 h in a hydrogen atmosphere. With the other method, coarse nickel powder fractions are obtained by oxidation of the fine nickel powder at 400–700°C and subsequent reduction of the nickel oxide powder in hydrogen at 750–850°C. In both cases, the resultant nickel powder is ground in drums and separated into fractions. However, the morphology of these powders differs from that of the PNK-2K10 powder because the particles are distorted by plastic deformation after the conglomerates are mechanically ground. Moreover, powder grinding introduces a significant number of unwanted and uncontrolled admixtures.
Note that electrolytic nickel powders of this particle size cannot be used for oxide cathodes because, first, of the presence of unwanted impurities affecting the cathode emissive properties and, second, of the dendritic particle shape. In contrast, carbonyl nickel powders have agglomerated strong spherical particles consisting of superfine grains.
Our objective is to produce agglomerated carbonyl nickel powders with spherical particles varying from 45 to 71 μm in size using the starting fine PNK-1L5(6, 7, 8) carbonyl nickel powders with particles smaller than 20 μm by annealing without mechanical grinding.
EXPERIMENTAL PROCEDURE
For the experiments, we used the PNK-2K10 and PNK-1L5 carbonyl nickel powders (as per GOST 9722–97). The nickel content of the PNK-2K10 and PNK-1L5(6, 7, 8) powders is at least 99.7% and the admixture content does not exceed the standard (Table 1).
According to GOST 9722–97, PNK-2K10 carbonyl nickel powder particles vary from 45 to 71 μm in size, and the content of particles in other sizes is no more than 20% by weight. The particles of the PNK-1L5(6, 7, 8) carbonyl nickel powders should be smaller than 20 μm and the content of particles larger than 20 μm should not exceed 15% by weight. The nickel powders were separated into size fractions by sifting through sieves with 280, 71, and 45 μm meshes (GOST 6613–86). The powders were annealed and sintered in dried hydrogen with a dew point not lower than –60°C.
The powder morphology was examined with a Superprobe-733 scanning electron microscope. The pore sizes in porous nickel powder bodies were determined in accordance with GOST 26849–86 [4]. The nickel powders were chemically tested to determine their carbon content with automatic coulometric titration by pH values employing an AN-7529U rapid analyzer.
The key stage in the production process is to grow a spongy porous layer with a homogeneous structure on a nickel core by annealing at 1250°C. The homogeneity of the porous layer is due to the use of nickel powder with a relatively narrow particle size range of 45–71 μm. Figure 1 shows morphology of the starting PNK-2K10 nickel powder. The powder morphology and fine-grained structure promote excellent fluidity and sintering between the particles.
Prior to being used, the PNK-2K10 carbonyl nickel powder is annealed in a hydrogen atmosphere at 600°C for 0.5 h to decrease its carbon content. However, Fig. 2 shows that the fine-grained nickel particles become somewhat coarser after annealing. An annealed powder layer of sieved 45–71 μm size fraction is applied to the nickel core and sintered to form a strong nickel skeleton and fuse the powder layer to the nickel core in the following mode: temperature rise to 1250°C within 20 and subsequent holding for 20 min. Figure 3 shows a skeletal structure of the sintered porous nickel powder layer. The structure was formed as the nickel particles fused to each other (Fig. 3a, b) and fine grains coalesced within the particles (Fig. 3c). The porosity of this layer is 80%. The pores are filled with triple barium, strontium, and calcium carbonate of KTA-1-6 grade, whose content should constitute 10%.
To produce 45–71 μm agglomerates, the PNK-1L5 nickel powder was used. Electron microscopy analysis indicated that the average size of the starting PNK-1L5 nickel powder particles was 4 μm and the particles consisted of finer grains, ≤1 μm (Fig. 4a). These powders hardly differ in chemical composition from the PNK-2K10 powders but show smaller amounts of tin, lead, and antimony (Table 1).
The idea was to separate the volume (integral) and local compaction effects in the sintering of agglomerated powders. Agglomerated powders should be annealed at temperatures that do not lead to volume shrinkage of the loose powder but involve only local shrinkage in the granules, which thus become stronger.
To determine the annealing temperature range to produce agglomerated PNK-1L5 nickel powders, we found the volume and local shrinkage in the sintering of the starting powder compacts (Table 2). To calculate the local shrinkage (ΔV/V)local involving changes in the pore diameter and the volume shrinkage of compacts, we used Skorokhod and Solonin’s equation [5]:
where D0 and Θ0 are the initial pore diameter and porosity; D and Θ are the final pore diameter and porosity; and ΔV/V0 is the volume (integral) shrinkage. The initial pore diameter and porosity of the compacts were 1.42 μm and 35 ± 1%.
Table 2 indicates that the volume shrinkage begins at 500°C. Nevertheless, active local shrinkage begins at 300°C and already reaches 25.1% at 500°C, which is also confirmed by Fig. 4d, showing virtually poreless nickel particles.
The experiments testify that the nickel powder that was agglomerated to the required sizes should be annealed preliminarily at 400°C because there is almost no volume shrinkage and 11.2% local shrinkage. The sieved 45–71 μm agglomerates are again annealed at 500°C to induce further local shrinkage. With this annealing mode, volume shrinkage is avoided and the powders are produced without grinding and retain near-spherical agglomerate shape.
To obtain powders with low carbon content, the nickel powder wetted with a 50 vol.% glycerin–50 vol.% ethyl alcohol solution was sifted through sieve 027. The glycerin decomposition temperature is 290°C.
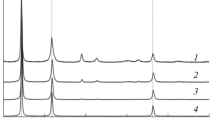
The morphology of the agglomerated PNK-1L5 nickel powder particles annealed in the range 400–700°C testifies that particles in the agglomerates densify with increasing temperature (Fig. 5).
The experimental findings were used to develop an optimal process for producing 45–71 μm nickel powders. The process involves stage-by-stage annealing of the agglomerated powder particles at 400, 500, and 600°C for 0.5 h and their intermediate sifting through sieves 071–045. This results in 25–30% of the 45–71 μm nickel powders because the starting granules are not strong, and the residual nickel size fractions smaller than 45 μm can be reused.
Figure 6 shows the structure of a sintered porous layer of the agglomerated PNK-1L5 nickel powder on a nickel core, corresponding to the structure of a porous layer of the agglomerated PNK-2K10 nickel powder.
CONCLUSIONS
The process developed to produce 45–71 μm nickel powders using PNK-1L5 carbonyl nickel with an average particle size of 4 μm includes agglomeration of the nickel powder wetted with a 50 vol.% glycerin–50 vol.% ethyl alcohol solution by sifting through sieve 027 and stage-by-stage annealing at 400, 500, and 600°C with intermediate sifting through sieves 071–045 without mechanical grinding.
The testing results for spongy oxide/nickel cathodes produced employing the agglomeration process for the fine PNK-1L5 nickel powders have shown that they can replace the PNK-2K10 nickel powders.
References
G.A. Kudintseva, A.I. Melnikov, A.V. Morozov, and B.P. Nikonov, Thermionic Cathodes [in Russian], N.D. Deviatkov (ed.), Energiya, Moscow–Leningrad (1966), p. 368.
A.B. Kiselev, Metal Oxide Cathodes Based on Electron Devices [in Russian], Izd. MFTI, Moscow (2001), p. 240.
V.G. Syrkin and V.N. Babin, Gas Grows Metals [in Russian], Nauka, Moscow (1986), p. 190.
GOST 26849–86, Pore Size Determination Method. Powder Materials [in Russian], intr. March 26 (1986).
V.V. Skorokhod and Yu.M. Solonin, “Relationship between integral and local densification in the sintering of porous solids,” Powder Metall. Met. Ceram., 22, No. 12, 985–989 (1983).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkova Metallurgiya, Vol. 60, Nos. 7–8 (540), pp. 16–23, 2021.
Rights and permissions
About this article
Cite this article
Hetman, O., Izunova, O., Samelyuk, A. et al. Highly Active Granular Nickel Powders for Multi-Batch Production of Spongy Oxide Cathodes. Powder Metall Met Ceram 60, 396–402 (2021). https://doi.org/10.1007/s11106-021-00252-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-021-00252-y