Fine powders of diborides of groups IV–VI transition metals (TiB2, HfB2, NbB2, TaB2, CrB2, Mo2B5, and W2B5) were produced by mechanochemical synthesis and low-temperature heat treatment of activated charges. The particle size of the powders was ≤1 μm. It is revealed that processes of formation of diborides of groups IV–VI transition metals differ within period and group. Diborides Ti and Nb formed discontinuously. Diborides Hf and Ta formed after the formation of lower boride phases, whereas higher borides Cr, Mo, and W formed in almost a single-phase state only after a low-temperature treatment of preliminarily mechanoactivated charges. The differences were analyzed from the point of view of the donor-acceptor capacity of the atoms of boron and diboride-forming transition metals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their high hardness, heat resistance, refractoriness, durability, and un-reactiveness, borides of groups IV–VI transition metals are used either independently or as components of composite materials intended to resist high temperatures, high mechanical stresses, and corrosive media. In particular, TiB2, ZrB2, CrB2 are used in cutting-tool, wear-resistant, oxidation-resistant, and creep-resistant alloys and in protective coatings. The resistance of these borides to molten aluminum, copper, iron, and steel allows producing a variety of melting crucibles, thermocouple tubes, and special drills. Molybdenum boride Mo2B5 is used in radio engineering. Studying and designing processes for the synthesis of borides are of scientific and applied interest [1].
A common method to produce refractory borides is synthesis from elements in normal conditions at temperatures 1600–1700°C accompanied by intensive heat liberation, resulting in the formation of strong cakes that should be thoroughly ground [2].
To obtain fine and nano-dispersed powders of refractory compounds, the reaction should be run at low temperatures in conditions that are far from equilibrium. Mechanochemical synthesis and mechanical activation followed by low-temperature synthesis have become very popular recently. Mechanical synthesis produces solid-phase compounds. High-energy milling causes plastic deformation of and structural defects in the original material. Such a maximally disordered structure relaxes, forming a new phase. The energy barrier is created by the interatomic interaction between the original components and, therefore, depends on their electronic structure and the type of chemical bond in the substances formed [3, 4].
The mechanochemical synthesis of transition-metal boride powders is addressed in [5–16], where various approaches to describing the formation of boride phases are discussed: structural correspondence of metal and its boride [7], special selection of conditions for mechanical synthesis [5]; diffusion mechanism of the processes [6, 10], etc.
The purpose of this study is (i) to obtain fine diborides of groups IV–VI transition metals by mechanochemical synthesis of elements, (ii) to identify the differences in their formation, and (iii) to discuss these differences in terms of electronic structure of the metals and boron atoms.
Materials and Experimental Procedure
Borides TiB2, HfB2, NbB2, TaB2, CrB2, Mo2B5, and W2B5 were produced in the same conditions of high-energy milling. Commercial metal powders and amorphous boron with a purity of 99.8% and a particle size of ~1 μm were used as original agents.
Characteristics of the Original Metal Powders | |
Metal, grade | Specific surface area, m2/g |
Titanium PTE . . . . . . . . . . . . . . . . . . . . . | 1.4 |
Hafnium GFN-1 . . . . . . . . . . . . . . . . . . . | 0.7 |
Niobium PNB-1 . . . . . . . . . . . . . . . . . . . | 0.4 |
Tantalum TaPA. . . . . . . . . . . . . . . . . . . . | 0.9 |
Chrome PKh-1 . . . . . . . . . . . . . . . . . . . . | 0.2 |
Molybdenum MPCh . . . . . . . . . . . . . . . . | 0.4 |
Tungsten PV1. . . . . . . . . . . . . . . . . . . . . | 1.2 |
The mechanical treatment of Me–B mixtures intended for producing diboride and Me2B5 phases in the relevant systems was performed in an AIR planetary mill with a centrifugal acceleration of 25g in steel drums with steel balls charged with boron to reduce the iron ground. The ball-to-powder weight ratio was 20 : 1; with a ball diameter of 9.5 mm and degree of mill fill 1 : 3. The mechanical treatment was carried out in argon for 2 min, followed by cooling for 5 min. The total treatment time was 5, 10, 15, 30, and 60 min. After 30 min of mechanical activation in the conditions indicated above, the powder mixtures were subject to heat treatment in an SShV vacuum furnace at 1000°C for 1 h.
The results of mechanical synthesis were analyzed using a DRON-3 diffractometer. The specific surface area was determined by thermal desorption of nitrogen. The morphology and particle size were studied using a Jeol Superprobe 733 scanning electron microscope.
Experimental Results
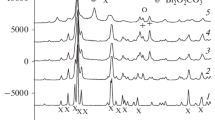
Mechanical Synthesis of Diborides of Group IV Metals. After high-energy milling of a mixture of titanium and boron powders for 5 min, the products of mechanical synthesis contain original α-Ti with hardly changed lattice periods and a small amount of titanium diboride. Milling for 10 min results in a single-phase product—the Xray pattern shows only the reflection lines of hexagonal TiB2 (Table 1; Fig. 1).
These results are in agreement with the data in [5], where the formation of titanium diboride is considered to be “explosive” synthesis.
The structure HfB2 forms after 10 min of milling of an Hf–B powder mixture. The high intensity of the reflection from the plane (100) may indicate that the diboride forms across this plane. In addition to the main phase HfB2, the products have a trimetric boride phase HfB that, according to [2], is stabilized by gas admixtures such as nitrogen and oxygen. Being metastable, this phase could be formed in non-equilibrium conditions of mechanochemical synthesis. Also, the phase HfB is still present after 30 min of mechanical treatment. A single-phase HfB2 composition powder is also observed after 60 min of mechanical treatment (Fig. 2; Table 1). Unlike TiB2, hafnium diboride forms for a longer time and after the formation of boride phase HfB.
Mechanical Synthesis of Diborides of Group V Metals. According to the X-ray analysis, after 5 and 10 min of treatment of the original mixtures Nb–B and Ta–B, the powders contain only the original metals Nb and Ta, respectively. If the mechanical treatment of Nb–B mixture is performed for 15 min, the X-ray patters show all reflection lines of hexagonal NbB2 (Fig. 3). After 30 min of mechanical treatment, all X-ray reflection lines are broader, which may result from the deformation and dispersion of niobium diboride. At the same time, the specific surface area increases up to 5.3 m2/g (Table 1).
During 10 and 15 min of high-energy milling of Ta–B mixture, the metal tantalum remains and its lines are broader. Should the milling time is increased up to 30 min, a mixture of boride phases Ta2B, Ta3B4, and TaB2 appears in the reaction products. After 60 min of milling, a single-phase powder of hexagonal tantalum diboride TaB2 forms (Table 1).
Mechanical Synthesis of Diborides of Group VI Metals. The formation of boride phases during mechanical treatment of mixtures of group VI metals (Cr, Mo, W) with boron is very complicated.
After 60-min mechanical treatment of the mixture Cr–B, along with reflection lines of the main phase (metallic chromium), the X-ray patterns display weak lines of chromium boride of the CrB2 composition (Table 1). The study of the phase formation in mechanically activated system Cr–B in [6] has established that no boride phases form during mechanical activation in conditions similar to our experimental procedure. However, they did form after subsequent heat treatment of the activated charge.
During mechanical treatment in the systems Mo–B and W–B, boride phases did not form. However, the lattice periods of metals increased for both systems with increasing the duration of mechanical treatment from 5 to 60 min (Table 1). This, apparently, results from the dissolution of boron atoms in the metal lattice in the special conditions of high-energy milling.
Low-Temperature Treatment of Activated Powder Mixtures. Since it is not possible to produce boride phases of chromium, molybdenum, and tungsten by mechanochemical synthesis, the mixtures Cr–B, Mo–B, and W–B (preliminarily milled for 30 min) were subjected to a low-temperature treatment in vacuum. The optimum temperature (1000°C) and holding time (1 h) were chosen based on the results of heat treatment of chromium diboride (Table 2). Table 2 shows that the powders consist of higher boride phases with a small admixture of lower borides. All powders were produced at temperatures lower by 500–600°C than the temperature of conventional synthesis of borides, which promoted their fineness. However, the boride CrB2 produced is not a phase of stoichiometric composition, but is located in the homogeneity range of the phase CrB2, as confirmed by the value of its interplanar spacings.
According to an electron-microscopic analysis, the products of mechanochemical synthesis of TiB2, HfB2, NbB2, and TaB2, and powders produced by heat treatment widely vary in particle size. The powders are composed of particles ≤1 μm that make agglomerates of 5–20 μm in size, which explains their small specific surface area. Figure 4 shows micrographs of the single-phase powders TiB2, HfB2, and NbB2 produced by mechanochemical synthesis.
Discussion of the Results
The study on the mechanochemical synthesis of diborides of groups IV–VI transition metals has revealed differences in formation under the same conditions of high-energy milling. The difference in the time and way the compounds form is observed within both period and group of metals. Titanium and niobium diborides are formed easily and quickly, which is in good agreement with published data. Hafnium and tantalum diborides are formed after the formation of lower boride phases, while higher borides of chromium, molybdenum, and tungsten (not resulting from the mechanical treatment) are formed only after additional low-temperature heat treatment of preliminarily mechanically activated original charges .
It can be assumed that the difference in diboride formation results from the electronic structure of metals and boron.
During boride formation, the electron interaction of boron atoms in the boric sublattice (whose structure becomes more complex with increasing B/Me atomic ratio) is essential. Having one unpaired electron in the outer electronic shell, the boron is seeking to form stable sp-states, utilizing both its own valence electrons and electrons of the partner. In diborides, boron forms graphite-like layers with strong covalent bond between the atoms and acts as an electron acceptor [17]. The charge state of boron and metals in borides was assessed using X-ray photoelectron spectroscopy [18].
Interacting with boron during diboride formation, groups IV–VI transition metals transfer their d-electrons for the formation of the Me–B bond and strengthening of the B–B bonds, which is confirmed by the study of the magnetic and electromagnetic properties of diborides [2]. However, the donor-capacity of these metals decreases with higher energy stability of d-phases resulting from the increase in the number of electrons in the d-orbitals and their principal quantum number [19]. This is also witnessed by the increased electronic work function of metals during transition from titanium to niobium and chromium, for which it makes 3.84, 3.95, and 4.5 eV, respectively [20]. Quantum-mechanical calculations of the diboride electronic structure made it possible to evaluate the transfer of electrons from the shells of isolated atoms to the orbitals of their compounds and B–B bond energy in the diboride structure:
Results of Quantum-Mechanical Calculations of Diboride Electronic Structure [17] | ||
Filling of d-states, % | Energy of B–B bond, eV | |
Ti. . . . . . . . . . . . 43 | TiB2. . . . . . . . . . .21.1 | TiB2. . . . . . . . . . . . . . 443.5 |
V. . . . . . . . . . . . 81 | VB2. . . . . . . . . . 56.8 | VB2. . . . . . . . . . . . . . . 412.5 |
Cr. . . . . . . . . . . . 84 | CrB2. . . . . . . . . . . 79.5 | CrB2. . . . . . . . . . . . . . 358.3 |
The above results allow examining the differences in the diboride formation from the point of view of donor-acceptor capacity of metals and boron.
Thus, during mechanochemical synthesis, titanium having with two electrons in the d-shell and tending to d 0-configuration easily transfers its electrons to boron, which promotes its intensive interaction with boron and formation of diboride.
Hafnium is in the same group with titanium, but in a lower period. This hampers the transition of electrons to the outer electronic shell, complicating its interaction with boron during the formation of the diboride phase, which is manifested as longer mechanical synthesis.
Niobium and tantalum (group V metals) demonstrate a lower donor capacity than the group IV metals. Therefore, to form diborides, a higher energy of mechanical activation should be accumulated to break through the energy barrier of the interatomic interaction of the original components. This is also manifested aslonger mechanical synthesis and additional formation (in the case of TaB2) of lower borides.
Among the metals under consideration, chromium, molybdenum, and tungsten possess the lowest donor capacity due to the presence of a stable half-filled d-shell. Under normal conditions, there are no diborides at all in the systems Mo–B and W–B, in which the most likely boride phases are Me2B5. Due to the insufficient donor capacity of chromium, molybdenum, and tungsten and, therefore, low reactivity with boron, we failed to obtain higher boride phases during high-energy milling: the energy of mechanical activation was not enough. To obtain boride phases, preliminarily activated original charges should be subjected to heat treatment. To obtain CrB2, Mo2B5, and W2B5 composition borides, a heat treatment at 1000°C for 1 h turned out to be sufficient. These results are consistent with the published data [6, 11].
Conclusions
Fine powders TiB2, HfB2, NbB2, and TaB2 with a grain size of ≤1 μm have been produced by mechanochemical synthesis.
To produce powders of boride phases CrB2, Mo2B5, and W2B5, a low-temperature treatment of preliminarily mechanoactiated charges was required. A heat treatment at temperatures lower by 500–600°C than the temperature of conventional synthesis of borides ensures their fineness.
Differences in periods and groups during the formation of diborides of groups IV–VI transition metals have been revealed: diborides TiB2 and NbB2 form discontinuously, while diborides HfB2 and TaB2 form after the formation of lower boride phases, whereas higher borides Cr, Mo, and W form in almost a single-phase state only after an additional low-temperature treatment of preliminarily mechanoactiated charges. The established differences have been discussed from the point of view of the donor-acceptor capacity of atoms of transition metals and boron.
References
M. S. Kovalchenko, “Metal-like refractory compounds and materials,” in: G. G. Gnesin and V. V. Skorokhod (eds.), Inorganic Materials Science [in Russian], Vol. 2, Book 1, Naukova Dumka, Kiev (2008), pp. 657–664.
T. I. Serebryakova, V. A. Neronov, and P. D. Peshev, High-Temperature Borides [in Russian], Metallurgiya, Chelyab. Otd., Moscow (1991), p. 367.
P. Yu. Butyagin, “On the dynamics of mechanochemical synthesis,” Dokl. AN USSR, 319, No. 2, 384–388 (1991).
P. Yu. Butyagin, “Development of mechanochemistry: problems and prospects,” Usp. Chim., 63, No. 12, 1031–1043 (1994).
K. B. Shelimov and P. Yu. Butyagin, “On the explosive mechanochemical synthesis of refractory compounds” Dokl. AN USSR, 316, No. 6, 1439 (1991).
E. P. Shelekhov, O. N. Pripisnov, and C. I. Rupasov, “Diffusion and phase formation in mechanoactivated systems Cr–C, Cr–Si, Cr–B,” Izv. Vuz. Tsvet. Metallurg., No. 6, 66–71 (2001).
M. P. Savyak, V. V. Skorokhod, V. Yu. Mel’nik, et al., “Structural transformations in titanium ground in a planetary mill in the presence of non-metallic additives of carbon and boron,” in: Research Notes [in Ukrainian], Issue 32, Luts. Nats. Tech. Univer., Lutsk (2011), pp. 369–374.
R. Ricceri and P. Matteazzi, “A fast and low-cost room temperature process for TiB2 formation by mechanosynthesis,” J. Mater. Sci., 379, No. 1–2, 341–346 (2004).
M. Moallem, M. H. Abbasi, and F. K. Laden, “Synthesis and characterization of TiB2 nanocrystalline powder by mechanical alloying,” Int. J. Mod. Phys. Conf., Ser. 05, 204 (2012).
K. Kudaka, K. Iizumi, T. Sasaki, and S. Okada, “Synthesis of MoB2 and Mo2B5,” J. Alloys Compd., 315, No. 1–2, 104–107 (2001).
H. E. Camurlu, “Preparation of single phase molybdenum boride,” J. Alloys Compd., 509, No. 17, 5431– 5436 (2011).
B. Akgün, H. E. Çamurlu, Y. Topkaya, and N. Sevinç, “Mechanochemical and volume combustion Synthesis of ZrB2,” Int. J. Refract. Met. Hard Mater., 29, No. 5, 601–607 (2011).
E. Bilgi, H. E. Camurlu, B. Akgun, et al., “Formation of TiB2 by volume combustion and mechanochemical process,” Mater. Res. Bull., 43, No. 4, 873–881 (2008).
S. Coskun and M. L. Ovecoglu, “Room-temperature mechanochemical synthesis of W2B5 powders,” Met. Mater. Trans. A., 44, No. 4, 1805–1813 (2013).
H. E. Çamurlu, “Preparation of single phase molybdenum boride,” J. Alloys Compd., 509, No. 17, 5431–5436 (2011).
L. Light, C. Hettige, J. Lau, et al., “Nano VB2 synthesis from elemental vanadium and boron,” Electrochem. Sol.-State Lett., 15, A12–A14 (2012).
G. V. Samsonov, B. A. Kovenskaya, and V. I. Matkevich (ed.), The Electronic Structure of Boron Compounds. Boron and Refractory Borides, Springer-Verlag, Berlin–Heidelberg–New York (1977), pp. 1–18.
I. I. Lyakhovskaya, T. M. Zzimkina, and V. A. Fomichev, “K-spectra in diborides of transition metals and LaB6, BaB6, and AsB compounds,” Fiz. Tverd. Tel., 12, 174–181 (1970).
G. V. Samsonov, I. F. Pryadko, and L. F. Pryadko, Configuration Model of Substances [in Russian], Naukova Dumka, Kiev (1971), p. 230.
CRC Handbook of Chemistry and Physics, Springer-Verlag, Berlin–Heidelberg–New York (2008), pp. 12–114.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Poroshkovaya Metallurgiya, Vol. 53, No. 9–10 (499), pp. 24–32, 2014.
Rights and permissions
About this article
Cite this article
Makarenko, G.N., Krushinskaya, L.A., Timofeeva, I.I. et al. Formation of Diborides of Groups IV–VI Transition Metals During Mechanochemical Synthesis. Powder Metall Met Ceram 53, 514–521 (2015). https://doi.org/10.1007/s11106-015-9645-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11106-015-9645-3