Abstract
Undoubted lines of evidence point out that members of CYCLOIDEA (CYC) 2 clade are essential players to control flower symmetry and, amusingly, also are determinants of capitula architecture (pseudanthium). In several species, CYC-like genes influence the androecium patterning, but to date, the function of these genes in the development of gynoecium organs is less clear. In this review, we first reported details about floral symmetry and an overview of genes and molecular mechanisms regulating the development of zygomorphism in different angiosperm lineages (e.g., basal and core eudicots and monocots). Then, we paid emphasis on the role of CYC-like genes in the development of heterogamous inflorescence of sunflower as well as other Asteraceae and some species within the Dipsacaceae family. Helianthus annuus is particularly attractive because it represents a useful model to study the role of CYC-like genes on shaping floral corolla as well as the differentiation of reproductive organs in different flowers of pseudanthia. A special attention was reserved to inflorescence morphology mutants of sunflower (i.e., Chrysanthemoids2 and tubular ray flower) because they provide useful information on the role of CYC-like genes in the radiate capitulum evolution. Finally, we discuss data from literature to suggest that CYC-like genes are also co-opted to regulate stamen and carpel differentiation likely throughout their interaction with the cell cycle and flower organ identity genes. The recruitment of reproductive organs in ray flowers also supports the phylogenetic origin of a radiate inflorescence of sunflower from a discoid capitulum and suggests that in sterile zygomorphic ray flower primordia the latent identity to differentiate both microsporangium and macrosporangium was conserved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Floral Symmetry Genes in the Origin of Zygomorphism
In the Cretaceous, one key evolutionary novelty that appeared in some major angiosperm lineages was a change in floral symmetry, from radially symmetrical actinomorphic flowers to bilaterally symmetrical zygomorphic flowers (Friedman 2009). The morphological diversity of zygomorphic flowers allows more specific interactions with pollinators with bilateral vision, such as insects. Accordingly, some of the most speciose taxa harbor monosymmetric flowers, in core eudicots (e.g., Fabaceae, Asteraceae) or monocots (e.g., Zingiberales, Orchidaceae) (Damerval and Nadot 2007).
Symmetry is generally defined for the perianth, reflecting human eye perception. The conventional terms of floral symmetry according to the number of symmetry planes are “asymmetric” (without any symmetry plane); “monosymmetric,” “zygomorphic” (with one symmetry plane); “disymmetric” (with two symmetry planes); and “polysymmetric,” “actinomorphic” (with several symmetry planes) (Endress 1999, 2001).
In zygomorphic flowers, in contrast to actinomorphic flowers, the organs within a whorl are not identical. Typically, organs in the dorsal (upper, adaxial) region of the flower, which are nearer to the stem, are structurally different from the organs in the ventral (lower, abaxial) region of the flower (Carpenter and Coen 1990; Coen and Nugent 1994; Coen et al. 1995). Moreover, lateral organs can often be distinguished structurally from the dorsal- and ventral-most organs. In comparative developmental studies, it appears that floral monosymmetry is expressed at different stages depending on the systematic group (Tucker 1999), and that even in polysymmetric flowers, there may be transient monosymmetric stages (Endress 1999; Buzgo and Endress 2000).
Early progresses in the molecular network operating in flower symmetry have been reached in the asterid Antirrhinum majus (Lamiales) and rosid Lotus japonicus (Fabaceae). In snapdragon, floral symmetry is controlled by the interaction between genes that regulate flower development within the dorsal region, CYCLOIDEA (CYC), RADIALIS (RAD), and DICHOTOMA (DICH), and the ventral region, DIVARICATA (DIV) (Coen 1996; Luo et al. 1996, 1999; Almeida et al. 1997; Galego and Almeida 2002; Almeida and Galego 2005; Corley et al. 2005). CYC and the paralog DICH code for transcription factors (TFs) of the TCP family, a group of genes that have been associated to the control of growth and development such as cell cycle, axillary shoot outgrowth, and leaf development (Luo et al. 1996, 1999; Doebley et al. 1997; Cubas et al. 1999a; Theissen 2000; Nath et al. 2003; Krizek and Fletcher 2005). The TCP acronym stands for the first three identified members: TEOSINTE BRANCHED1 (TB1) in Zea mays, CYCLOIDEA (CYC) in A. majus, and PROLIFERATING CELL FACTOR (PCF) 1 and 2 in Oryza sativa (Cubas et al. 1999a). These TFs are characterized by a noncanonical basal helix-loop-helix (bHLH) domain of circa 60 residues, called the TCP domain (Cubas et al. 1999a; Aggarwal et al. 2010; Martín-Trillo and Cubas 2010; Uberti Manassero et al. 2013; Hileman 2014a, b). Based on the TCP motif, members of this TF family have been classified into PCF (TCP-P or class I) and CYC/TB1 (TCP-C or class II) subfamilies (Cubas et al. 1999a). Class II TCP TFs have an additional domain, called R domain, which is circa 20 residues long motif rich in arginine (Cubas et al. 1999a). Phylogenetic and sequence analysis allows class II to be split into two clades, the CYC/TB1-like and the CINCINNATA (CIN)-like clades. The first clade, called the “ECE” clade, is further divided into CYC1, 2, and 3 subclades, which have evolved due to two duplication events (Howarth and Donoghue 2006; Preston and Hileman 2009; Martín-Trillo and Cubas 2010; Uberti Manassero et al. 2013; Hileman 2014b). ECE refers to a conserved short motif (glutamic acid-cysteine-glutamic acid) between the TCP and R domains that have been found in many members of this clade (Howarth and Donoghue 2006).
In Antirrhinum, together with DICH, CYC was expressed in the dorsal domain of young floral meristem, resulting in retarded growth of petals and stamens. At later stage, CYC expression persisted throughout the dorsal domain where it promoted petal lobe growth while it repressed stamen development (Luo et al. 1996). DICH expression was restricted to the dorsal-most part of the dorsal petals, participating in their internal symmetry (Luo et al. 1999). DIV and RAD encode for MYB-type TFs (Almeida et al. 1997; Galego and Almeida 2002; Corley et al. 2005). The RAD gene was activated by CYC and DICH in the dorsal region of flowers where it antagonized the DIV protein (Corley et al. 2005). RAD is a small protein with 93 amino acids and a single MYB domain that is 52 % identical to the N-terminal MYB domain of DIV (Corley et al. 2005). Thus, rather than acting as a DNA-binding TF, RAD operated through a mechanism involving protein-protein interactions (Raimundo et al. 2013). RAD negatively regulated DIV function in dorsal parts of the flower by competing with DIV proteins to form heterodimers with common target MYB proteins, termed DRIF1 and DRIF2 (DIV- and RAD-interacting factors) (Raimundo et al. 2013). div mutants have a ventral petal with lateral identity (Almeida et al. 1997; Galego and Almeida 2002). In Antirrhinum, cyc mutants have partially ventralized flowers, with dorsal and lateral petals and stamens resembling their more ventral counterparts. dich mutants have dorsal petals with reduced internal symmetry. The phenotype of the cyc;dich double mutant is more extreme: radially symmetrical flowers with extra floral organs and fully ventralized petals and stamens (Luo et al. 1996, 1999). Thus, the functions of CYC and DICH were to establish the dorsal fate of petals and stamens also controlling the activity of both DIV and RAD genes (Luo et al. 1996, 1999). In this context, the zygomorphic flowers of Antirrhinum required an interplay between TCP and MYB TFs (Corley et al. 2005).
Genes homologous to the CYC2 clade and involved in the evolution of floral symmetry have been amply recognized in many angiosperm families (Cubas et al. 1999b; Citerne et al. 2000, 2003, 2013; Smith et al. 2004; Costa et al. 2005; Howarth and Donoghue 2005; Howarth et al. 2011; Feng et al. 2006; Damerval et al. 2007; Chapman et al. 2008, 2012; Wang et al. 2008; Fambrini et al. 2011, 2014a, b; Tähtiharju et al. 2012; Yang et al. 2012, 2015; Hileman 2014a). However, in some monocot and dicot species, it has been proven that also CYC1 and/or CIN clade genes played a role in the development of flower zygomorphy (Yuan et al. 2009; Bartlett and Specht 2011; Preston and Hileman 2012; Bartlett et al. 2015; De Paolo et al. 2015). Analogous to Antirrhinum, most of the CYC2 clade genes were specifically expressed in dorsal or dorsal plus lateral regions of developing flowers (Costa et al. 2005; Damerval et al. 2007; Preston and Hileman 2009; Citerne et al. 2013); however, in Asteraceae and monocot, some exceptions in the expression pattern of CYC-like genes have been observed (e.g., expression in ventral region of floral organs) (Bartlett and Specht 2011; Broholm et al. 2014; Juntheikki-Palovaara et al. 2014; Garcês et al. 2016). Therefore, the origin and evolution of zygomorphism have been correlated to asymmetric CYC2-like gene expression. Gene expression can be influenced by the following mechanisms: (i) cis-regulatory changes due to variations in enhancer or promoter sequences; (ii) changes in the transcribed region, or changes in chromatin structure or trans-regulatory elements; and (iii) changes generated by genetic and epigenetic modifications affecting the activity or availability of proteins and RNAs (Wittkopp et al. 2008). In particular, changes in cis-regulatory elements of CYC2 genes arose multiple times in angiosperms, which could explain the independent origin of floral zygomorphy (Yang et al. 2012; Della Pina et al. 2014). Additionally, segmental chromosome duplication is a leading mechanism for acquiring new genes that can be co-expressed, or shift in functional divergence (i.e., neofunctionalization or subfunctionalization) creating genetic novelty in organisms. In various organisms, many new gene functions have evolved through these mechanisms that have contributed tremendously to the evolution of developmental programs (Van De Peer et al. 2009). In plants, further genes originated by whole-genome duplication (polyploidy), a ubiquitous mechanism among angiosperm that has played a major role in plant evolution (Comai 2005). The tendency for gene and whole-genome duplication in plants gives raw genetic material that after their subsequent divergence plays an important function in organismal complexity and radiative divergence. There are lines of evidence, from both MADS-box and TCP gene families, that extensive gene duplication, and subsequent modification in various lineages, have resulted in diversified gene expression and protein functions associated with the development of new floral shape as well as inflorescence morphologies (Gübitz et al. 2003; Reeves and Olmstead 2003; Howarth and Donoghue 2005, 2006; Irish and Litt 2005; Chapman et al. 2008; Carlson et al. 2011; Howarth et al. 2011; Heijmans et al. 2012; Yang et al. 2012, 2015; Specht and Howarth 2015).
Recent studies of phylogeny of CYC2-like genes across the Lamiales have suggested that at the base of Lamiales, there was a single ancestral CYC-like gene subjected to extensive duplication and functional divergence, no less than six times in core Lamiales nearly the Cretaceous-Paleogene boundary, and seven more in early diverging clades relatively more recently (Zhong and Kellogg 2015a, b). Duplications and losses of CYC2-like paralogs are prevalent, but Zhong and Kellogg (2015b) pointed the attention to changes in both cis-regulatory elements and coding domains of CYC2-like genes as key events for the evolution of zygomorphism in Oleaceae and Tetrachondraceae (Lamiales). Actually, it has been shown that cis-regulatory changes in the CYC/TB1 locus that participate in the regulation of asymmetric perianth protein activities arose in both eudicot (Luo et al. 1999; Feng et al. 2006; Busch and Zachgo 2007; Damerval et al. 2007; Wang et al. 2008; Jabbour et al. 2014) and monocot (Bartlett and Specht 2011; Preston and Hileman 2012) lineages.
Within Lamiales, Gesneriaceae have diverse forms of zygomorphic flowers (Cubas 2004). Several studies have shown that the spatial-temporal expression alterations of CYC-like genes are highly correlated with the different morphologies of zygomorphic flowers in Gesneriaceae (Du and Wang 2008; Gao et al. 2008; Song et al. 2009; Yang et al. 2012). In Petrocosmea glabristoma and Petrocosmea sinensis, it was demonstrated that the dorsal specific expression level of both CYC1C and CYC1D is negatively correlated with the dorsal petal size (Yang et al. 2015). Notably, a differential expression of CINCINNATA1-(CIN1)-like genes in all petals of the two species with distinct petal morphology was also shown (Yang et al. 2015). The results suggested that CYC1C, CYC1D, and CIN1 coordinately promoted the morphological shape of the dorsal petals, characterizing the different types of zygomorphic flowers of P. glabristoma and P. sinensis. Additionally, through allele-specific expression analyses, Yang et al. (2015) showed that the expression differentiation of CYC1C was mainly caused by cis-regulatory changes, while that of CYC1D was largely under the control of trans-acting factors.
As previously reported, in angiosperms, especially in eudicots, the CYC2 clade genes have undergone recurrent duplications that gave rise to multiple copies of CYC2 genes in most members of zygomorphic clades (Howarth and Donoghue 2006). Of these, a pair of CYC2 genes are usually involved in controlling the dorsal petals redundantly in zygomorphic flowers, with others having no or transient expression signals (Luo et al. 1996, 1999; Feng et al. 2006; Gao et al. 2008; Wang et al. 2008; Song et al. 2009; Yang et al. 2012). In Primulina heterotricha (Gesneriaceae), Yang et al. (2012) revealed that a double positive autoregulatory feedback loop evolved for a pair of CYC2 genes to maintain their expression in developing flowers.
Yang et al. (2012) also suggested that the required CYC DNA-binding sites are absent from the promoter sequences of CYC homologues in species with radially symmetrical flowers (e.g., Arabidopsis and tomato), but present in soybean (Fabaceae), characterized by bilateral flower symmetry. However, as reviewed by Hileman (2014a), this intriguing theory is not universally valid.
A key role of CYC and/or DIV homologues on floral symmetry has been recognized for other eudicots, such as Fabales (Citerne et al. 2003; Fukuda et al. 2003; Feng et al. 2006; Wang et al. 2008; Weng et al. 2011), Ranunculales (Damerval and Nadot 2007; Citerne et al. 2013; Jabbour et al. 2014), and Asterales (Abbott et al. 2003; Broholm et al. 2008; Fambrini et al. 2011; Chapman et al. 2012; Garcês et al. 2016), suggesting that the function of CYC-like genes has been widely conserved. CYC-like genes have been isolated from several species of Fabales (Rosids) and phylogenetic analyses have revealed that, as in the Lamiales, repeated duplication events of a single ancestral CYC-like gene (LEGCYC) have taken place. In particular, before the evolution of the Papilionoideae (a subfamily of Leguminosae with flower weakly to strongly zygomorphic), an early duplication of LEGCYC gave rise to LEGCYC I and LEGCYC II genes (Citerne et al. 2003; Fukuda et al. 2003). Later, early in the diversification of the Papilionoideae, a duplication of LEGCYC I generated LEGCYC IA and LEGCYC IB suggesting that they might have assumed divergent functions. Most of the analyzed papilionoid species had these three types of genes (Citerne et al. 2003). In L. japonicus, Feng et al. (2006) demonstrated a role for LjCYC2 in establishing dorsal identity in zygomorphic flowers by altering both its expression in transgenic plants and analyzing its mutant allele squared standard 1 (squ1). Further work suggested that KEELED WINGS IN LOTUS 1 (KEW1) was a specific factor that controlled lateral petal identity and interacted with LjCYC2 in determining floral bilateral symmetry (Feng et al. 2006). SQU and KEW activities were precisely modulated at both transcription and posttranscriptional levels, which might link the dorsal-ventral asymmetric flower development to different physiological status and/or signaling pathways (Xu et al. 2016). In particular, SQU possessed both activation and repression activities, while KEW, which encoded for TCP TF (Wang et al. 2008), acted only as an activator. They formed homo- and heterodimers and then collaboratively regulated their expression at the transcriptional level (Xu et al. 2016). Furthermore, two types of posttranscriptional modifications (phosphorylation and ATP/GTP binding) that could affect their transcriptional activities were identified (Xu et al. 2016).
In the Ranunculaceae, two paralogous lineages have also been found and seem to originate from a duplication event independent from the one that has occurred in the Papaveraceae (Citerne et al. 2013). In an extensive phylogenetic analysis performed in 48 species of Ranunculaceae, 109 CYC-like sequences were identified (Jabbour et al. 2014). The phylogenetic tree was consistent with the hypothesis of two paralogous lineages originating from a duplication predating the divergence of Lardizabalaceae and Ranunculaceae. In Ranunculaceae, there was a single evolutionary transition from actinomorphy to zygomorphy in the stem lineage of the tribe Delphinieae (Jabbour et al. 2014). Transcription analyses in two species of Delphinieae, Aconitum carmichaelii and Consolida regalis, also indicated that the CYC-like paralogs were transcribed early in floral buds and that the level of their activity diverged between species, with differences correlating with their specific perianth architecture, and paralog class (Jabbour et al. 2014). The results suggested that within Ranunculaceae the regulation of CYC-like expression involved gene duplication events and then species divergence. Therefore, Jabbour et al. (2014) remarked that the regulation of CYC-like genes among the Ranunculales demonstrated large flexibility and evolvability.
A complex case of CYC2 recruitment occurs in members of the Asteraceae and Dipsacaceae families characterized by species with radiate inflorescences originated independently (Abbott et al. 2003; Broholm et al. 2008; Chapman et al. 2008, 2012; Kim et al. 2008; Busch and Zachgo 2009; Carlson et al. 2011; Fambrini et al. 2011; Garcês et al. 2016). Asteraceae and Dipsacaceae appear to have the greatest numbers of CYC2 genes (Chapman et al. 2008; Carlson et al. 2011; Juntheikki-Palovaara et al. 2014; Garcês et al. 2016) with differential CYC transcription linked to changes in floral symmetry and inflorescence architecture (Broholm et al. 2008; Carlson et al. 2011; Garcês et al. 2016). In Asteraceae, the molecular dissection of radiate inflorescence (e.g., in Senecio, Helianthus, and Gerbera) has demonstrated that modification of the ancestral network of TCP factors has, through gene duplications, led to the establishment of new expression domains and to functional diversification (Broholm et al. 2008; Chapman et al. 2008, 2012; Kim et al. 2008; Tähtiharju et al. 2012; Juntheikki-Palovaara et al. 2014; Garcês et al. 2016). For example, the gerbera GhCYC2 transcription followed the radial organization of the inflorescence, being upregulated in the marginal ray flowers, while no expression was detected in the centermost disc flowers (Broholm et al. 2008). However, with the exception of GhCYC2 being absent from dorsal petals, the other CYC2 clade genes showed largely overlapping gene expression patterns being redundantly transcribed in all five petals as well as in carpels of both ray and disc flowers (Juntheikki-Palovaara et al. 2014). At the level of single flower, their expression domain in petals showed a spatial shift from the dorsal pattern known so far in species with bilaterally symmetrical flowers, suggesting that this change in expression may have evolved after the Asteraceae origin (Juntheikki-Palovaara et al. 2014). Functional analysis in transgenic gerbera revealed that in addition to GhCYC2 (Broholm et al. 2008), GhCYC3 and GhCYC4 exhibited redundant functions in the regulation of ray flower identity and promoted corolla development until the final size and shape of the petals are reached (Juntheikki-Palovaara et al. 2014). Instead, GhCYC5 operated in regulating the rate of flower initiation in the inflorescence. Therefore, modification of the ancestral network of TCP TFs after gene duplications led to the establishment of new expression domains and to functional diversification (Juntheikki-Palovaara et al. 2014). In Senecio vulgaris, Garcês et al. (2016) demonstrated how recruitment of floral symmetry regulators into dynamic networks between CYC (RAY1, RAY2, and RAY3) and MYB (SvDIV and SvRAD) TFs was crucial to originate the complex and elaborate structure of the Senecio capitulum.
Dipsacaceae (Dipsacales) is one of relatively few families outside of Asteraceae that contains species with both radiate and discoid capitula (Carlson et al. 2011). Howarth and Donoghue (2005) have verified that gene duplication events yielded five major copies of CYC-like genes prior to the origin of Dipsacaceae (DipsCYC1, DipsCYC2A, DipsCYC2B, DipsCYC3A, and DipsCYC3B), with a subsequent duplication occurring in the DipsCYC2B gene (2Ba, 2Bb). The number and activity of CYC-like genes correlated with the corolla form of Dipsacaceae as well as other Dipsacales (e.g., Morinaceae and Adoxaceae) (Howarth and Donoghue 2005). More recently, Carlson et al. (2011) identified several additional copies in the DpcCYC1 (i.e., DipsCYC1) and DpcCYC2B (i.e., DipsCYC2B) clades. Notably, the pattern of CYC-like gene diversification appeared also correlated with the inflorescence morphology. Groups with radiate capitula are represented in the major clades and subclades of DpcCYC, while the discoid groups (e.g., Bassecoia and Dipsacus) appeared to have only one copy of DpcCYC1, 2Ba, and 2Bb (Carlson et al. 2011). In Knautia macedonica, multiple copies of CYC-like genes were differentially expressed among petal types and between internal and external flowers indicating that subtle changes across multiple paralogs correlated with the change in the degree of zygomorphism (Berger et al. 2016). Analogously, a shift to bilaterally symmetrical flowers at the base of the Caprifoliaceae (Dipsacales) was accompanied by a duplication of the DipsCYC2 gene, resulting in DipsCYC2A and DipsCYC2B and by loss of expression of both of these copies in the ventral petal (Howarth et al. 2011). In addition, DipsCYC2B persisted to be expressed in the dorsal and lateral lobes, while DipsCYC2A expression was restricted to the two dorsal lobes (Howarth et al. 2011). Similar transcription patterns in which one duplicate gene is more dorsally restricted than the other were detected in several eudicots [e.g., A. majus (Luo et al. 1996), Pisum sativum (Wang et al. 2008), and Malphigiaceae (Zhang et al. 2010)]. This suggested that an analogous pattern of differentially restricted expression occurred independently in the evolution of zygomorphic flowers from actinomorphy ancestors (Specht and Howarth 2015).
Twelve CYC/TB1 genes (CYCLs) were identified by in silico analysis or isolated by PCR approach from basal angiosperm [Amborella trichopoda (Amborellaceae), Nuphar advena, Nuphar lutea, and Nymphaea alba (Nymphaeales)] and within magnoliid families (Horn et al. 2015). In particular, the CYCL gene identified in the sequenced genome of Amborella demonstrated that the CYC/TB1 clade evolved in the earliest diverging angiosperms, and it was likely that the common ancestor of all angiosperms harbored one CYC/TB1 gene (Horn et al. 2015). Flower monosymmetry is uncommon in the magnoliids, where it has exclusively evolved in the Aristolochiaceae (Piperales). Expression analyses demonstrated that in Saruma henryi, during floral organ differentiation, ShCYCL1 expression was stronger in the distal parts of petals (Horn et al. 2015). This result was according to the observation that in other species the late petal growth was mainly mediated by repeated cell divisions in the petal tip (Reale et al. 2002; Dinneny et al. 2004), and suggested that ShCYCL1 employed a similar activity (Horn et al. 2015). In Iberis, petal size differences were mainly realized at later flower stages (Busch and Zachgo 2007), and strong adaxial IaTCP1 expression around anthesis likely mediated differential petal growth (Busch and Zachgo 2007). By contrast, in Aristolochia arborea, a differential AarCYCL expression in the abaxial perianth was observed only in later stages, after perianth monosymmetry had already been established. Therefore, the enhanced abaxial CYC-like expression might only contribute to the final growth processes of the mature monosymmetric perianth (Horn et al. 2015). By contrast, a strong CYC-like expression was detected in epidermal layers of the mushroom mimicry structure (MMS), a particular Aristolochia organ evolved to attract flies, indicating a possible function of the CYC-like gene in the formation of this specific epidermis (Horn et al. 2015).
In contrast to dicots, few studies are available on the involvement of CYC/TB1-like genes in the evolution and maintenance of bilateral symmetry for monocots (Yuan et al. 2009; Bartlett and Specht 2011; Preston and Hileman 2012; Hoshino et al. 2014; Bartlett et al. 2015; De Paolo et al. 2015). Indeed, monocots do not have a strict CYC gene ortholog (Howarth and Donoghue 2006); however, the description that the class II TCP gene RETARDED PALEA1 (REP1) in Oryza sativa was expressed only in the dorsally positioned palea suggested further recruitment of TCP genes in the evolution of bilateral symmetric flowers (Yuan et al. 2009). Furthermore, disymmetric flowers of Costus spicatus (Zingiberales, Cotaceae) and Heliconia stricta (Heliconiaceae) displayed asymmetric TCP gene (CsTB1a and HsTBL2b, respectively) expression at early to late stages of flower development (Bartlett and Specht 2011). Notably, analogous to Gerbera hybrida (Broholm et al. 2008), CsTB1a and HsTBL2b expression was restricted to the ventral side of the flower (Bartlett and Specht 2011). In Orchis italica, the class II CYC/TB1-like gene did not appear to be involved in zygomorphic flower evolution. By contrast, one CIN-like group II transcript (comp5062) was an excellent candidate gene for zygomorphism. It was expressed in two different flower stages and significantly correlated to a microRNA (miR)319 that in Arabidopsis showed a critical role in petal and stamen development (De Paolo et al. 2015).
Floral Symmetry in the Pseudanthium of Sunflower: Gene Expression Patterns and Phylogenetic Relationship
During evolution, zygomorphy has very likely arisen from actinomorphy many times independently within the flowering plants, producing several major zygomorphic clades, such as Fabales, Lamiales, Asterales, and Orchidales (Donoghue et al. 1998; Cubas et al. 2001; Cubas 2004; Endress 2001; Rudall and Bateman 2004; Citerne et al. 2010; Hileman 2014b). Really, the ancestral flower in the Asteraceae family was actinomorphic, and a deeply five-lobed and zygomorphic one is considered derived (Harris 1995). This is in contrast with Asteraceae inflorescences from Eocene Patagonia fossils of 47.5 Ma that showed flowers relatively large, with a developed ligule or lip, which support the hypothesis of an ancestral Asteraceae radiate capitulum (Barreda et al. 2010). Nevertheless, reversal to actinomorphic from zygomorphic ancestor flowers also frequently occurred (Donoghue et al. 1998; Cubas 2004; Citerne et al. 2010; Hileman 2014b; Specht and Howarth 2015). It is likely that in Asteraceae the radiate capitulum evolved from a discoid inflorescence. The pseudanthium of sunflower originated after a shift of the more external whorl of actinomorphic flowers in zygomorphic flowers. The great evolutionary success of zygomorphy lies probably in the promotion of pollinator specificity and in the efficient control of pollinator behavior by the plant (Cronk and Möller 1997; Neal et al. 1998; Sargent 2004). In zygomorphic flowers, pollinators are restricted in the directionality of approach and movement within and between flowers to enhance the accurate placement of pollen on the body of the pollinator, independently by pollinator type (Cronk and Möller 1997; Nikkeshi et al. 2015). However, specialized monosymmetric flowers probably evolved to make heterogamous heads of sunflower more conspicuous under pollinator-mediated selection, as evidenced in Helianthus grosseserratus where a drastic reduction in pollination efficiency followed removal of ray flowers (Stuessy et al. 1986).
The sunflower head (capitulum) is a well-known example of pseudanthium (Fig. 1a). The capitulum is produced by an expanded and flattened meristem which develops into an array of sessile units (flowers) arranged on a flat surface and bordered by a protective wrap of involucral bracts (Palmer and Palmer 1982). In Helianthus annuus inflorescence, parastichies of hermaphrodite flowers with actinomorphic symmetry (disc flowers) are surrounded by a whorl of zygomorphic sterile flowers (ray flowers). Several floral zygomorphic patterns have been described within the Asteridae supertree. For example, Antirrhinum shows the most common 2:3 pattern, in which the two adaxial (dorsal) petals differentiate from the other three petals (two laterals and the medial abaxial petal) (Luo et al. 1996). In Helianthus, the zygomorphic ray flower is made of three petals [0:3 pattern (adaxial:abaxial)] shifted abaxially, forming a short and narrow corolla tube confined to the proximal end (Jeffrey 1977). Actinomorphic disc flowers (tubular flowers) are arrayed in arcs radiating from the center of the head to form distinct left- and right-turning spiral rows. Each disc flower is subtended by a sharp-pointed chaffy bract, and it consists of an inferior ovary carrying a single ovule, two pappus scales (highly modified sepals), and a five-lobed tubular-like corolla. The five anthers are joined together to form a tube, with separate filaments attached to the base of the corolla tube. Inside the anther tube is the style, terminating in a divided stigma with receptive surfaces in close contact in the bud stage before the flower opens (Knowles 1978).
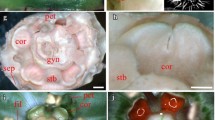
Characteristics of sunflower (Helianthus annuus L.) inflorescences. a The radiate inflorescence of normal sunflower. b The mutant Chrysanthemoides2 (Fambrini et al. 2003, 2014a). c The mutant tubular ray flower (turf) generated by the insertion of a CACTA transposable element (TE), named Transposable element of turf1 (Tetu1) (Fambrini et al. 2011). d A normal (upper) and a tubular-ray flower (under). e A reversion of turf to normal sunflower originated by a perfect excision of Tetu1. f A reversion of turf in a phenotype that resembles hybrid characteristics between ray and disc flowers (arrows) (Fambrini et al. 2014b). g Ovule in ovary of a tubular-ray flower of turf; ov ovary, o ovule, fu funiculus, tc tapetum cells. h Corolla and reproductive structures in the tubular-ray flower of turf; c corolla, a anther with pollen grains, st stigma, s style, f filament. Scale bars are 500 μm in g and 900 μm in h
The pseudanthial phenomenon represents a shared evolutionary trait in several eudicot families (Fig. 2a–d) (Claßen-Bockhoff 1990; Ronse De Craene 2010). In the radiate capitulum of Asteraceae, the corolla of ray flowers is usually strap-shaped with three or fewer teeth (ligule or perianth lamina) at the apex and a short tube at the base. The corolla shape of the disc flowers is actinomorphic, pentamerous, or sometimes tetramerous and characterized essentially by an elongated tube (Jeffrey 1977; Harris 1995). However, in some pseudanthia species, the strap-shaped corolla where the ray flower is positioned is replaced by a tubular corolla with five prominent apical teeth (i.e., Cyanus triumfettii; Fig. 2c). A role of CYC2 gene in the evolution of pseudanthium is well documented. For example, duplication events and expression patterns of CYC-like genes are suggestive for the control of different pseudanthia of Dipsacaceae (Carlson et al. 2011) as well as in morphology development of the Actinodium cunninghamii Schau. (Myrtaceae) inflorescence that represents a novel type of pseudanthium with proximal branches mimicking ray flowers (Claßen-Bockhoff et al. 2013).
In sunflower, ten members of the CYC/TB1 gene family were identified, and phylogenetic analysis also showed that these genes occurred in three distinct clades (Chapman et al. 2008). Therefore, in sunflower, gene duplication has been an outstanding feature in the evolution of the CYC gene family. Additionally, clear evidence of divergence in expression patterns across duplicates within all three clades of sunflower CYC-like genes was discovered (Chapman et al. 2008). Noteworthy, prominent differences in transcription pattern in the CYC2 lineage were detected, in which three genes were expressed in all floral tissues, one was mainly transcribed to ray flowers and, with a lower level also in disc flowers (HaCYC2d) and one was restricted to ray flowers (HaCYC2c). Additionally, positive selection had promoted divergence of the HaCYC2a, HaCYC2b, and HaCYC2c genes (Chapman et al. 2008). Notably, Ree et al. (2004) found evidence for positive selection operating on a CYC paralog in the genus Lupinus (Fabaceae) that corresponded to a shift in floral morphology. A comparative study has been conducted in sunflower and gerbera to explore CYC/TB1 gene family evolution and diversification in Asteraceae (Tähtiharju et al. 2012). In these two species, marginal flowers (ray and trans flowers) showed a strong expression level of the six members of the CYC2 clade in gerbera and the five genes in sunflower. Expression analysis suggested that these genes early functioned in flower-type differentiation and late operated in reproductive organ development (Tähtiharju et al. 2012). Moreover, the null or very low level of expression in disc flowers of one gene in gerbera (GhCYC3) and two in sunflower (HaCYC2d and HaCYC2c) could be indicative to their role in controlling ray flower zygomorphism and evolution of radiate inflorescence (Chapman et al. 2008; Tähtiharju et al. 2012). However, phylogenetic analyses place HaCYC2d and HaCYC2c in separate clades, suggesting independent canalization or expansion of expression domains for sunflower CYC2 genes (Chapman et al. 2008). In S. vulgaris, the role of a CYC2 clade gene (RAY3) as well as two floral symmetry MYB domain regulators (SvRAD and SvDIV1B) was recently investigated by Garcês et al. (2016). The expression analyses demonstrated that after an initial uniform expression in ray flower primordia, the permanent transcription of RAY3 in ventral petals was essential for the development of the zygomorphic form. Additionally, functional analysis showed that RAY3 promoted and SvDIV1B repressed petal growth, confirming their roles in floral zygomorphism. Phylogenetic analysis placed a RAY3 TF in the CYC2 subclade as a HaCYC2b of sunflower and Gerbera GhCYC4 and GhCYC9 (see also Fig. 1h in Garcês et al. 2016). RAY3 expression was consistent with that of GhCYC4 and GhCYC9 (Tähtiharju et al. 2012), but its expression greatly differed from that of HaCYC2b that was transcribed in all tissues analyzed (e.g., leaf, style, disc, and ray flowers) (Chapman et al. 2008; Tähtiharju et al. 2012). Instead, RAY3 transcription was more similar to the sunflower paralogs, HaCYC2c and HaCYC2d (see also Fig. 5 in Chapman et al. 2008).
Here, a phylogenetic analysis of CYC TFs was conducted within some species of Lamiales, Brassicales, Asterales, Ranculales, Fabales, Piperales, and Commellinales (Fig. 3 and Fig. 1S). The phylogenetic tree clearly resolved the three CYC subclades (CYC1, CYC2, and CYC3). According to Garcês et al. (2016), RAY3 (i.e., SvRAY3) was placed within a CYC2 subclade as GhCYC4 and GhCYC9 and HaCYC2b. Instead, HaCYC2c that was mainly expressed in ray flowers (Chapman et al. 2008) was placed within a subclade as GhCYC5 that functional analysis showed regulating the flower density of the gerbera inflorescence (Juntheikki-Palovaara et al. 2014). Therefore, it is likely that different Asteraceae lineages have independently recruited different CYC paralogs for ray flower zygomorphism (Garcês et al. 2016). For other CYC genes, our phylogenetic analysis essentially agreed with the results of Garcês et al. (2016). In addition, according to Horn et al. (2015), relationships between the subclades CYC1/2/3 and Aristolochia arborea CYC-like genes (AaCYCL1 and AaCYCL2) were unclear as there was no suitable resolution in this tree (Fig. 3). In the basal eudicot C. regalis (Ranunculaceae), CrCyL2a expression is mainly asymmetric (i.e., dorsal domain of petals), which is consistent with a role for this gene in determining zygomorphic flowers (Jabbour et al. 2014). However, in our phylogenetic tree, CrCyL2a is placed within the CYC1 clade, which enclosed a TCP TF involved in axillary meristem development (i.e., TCP18 of Arabidopsis) (Aguilar-Martínez et al. 2007) and HaCYC1a and HaCYC1b of sunflower whose expression patterns suggested a slight role in flower zygomorphism (Chapman et al. 2008; Tähtiharju et al. 2012). This result was not surprising. Although most genes that demonstrated to play a role in zygomorphism belong to the CYC2 clade, these are not all orthologs due to the independent duplications in various families (Feng et al. 2006; Broholm et al. 2008; Kim et al. 2008; Wang et al. 2008; Jabbour et al. 2014). Researches in Poaceae, Zingiberales, and Commelinales also suggest a possible role of Tb1-like genes in the independent evolution of zygomorphism (Yuan et al. 2009; Bartlett and Specht 2011; Preston and Hileman 2012). Additionally, a role of CIN-like genes in the complex regulatory network controlling monosymmetry in Petrocosmea spp. (Gesneriaceae) (Yang et al. 2015) and O. italica (De Paolo et al. 2015) has been revealed.
The consensus tree showing the relationship between CYCLOIDEA (CYC) transcription factors (TFs) was obtained with the maximum likelihood method. The species abbreviations are shown as follows: Antirrhinum majus (Am), Arabidopsis thaliana (At), Aristolochia arborea (Aa), Commelina communis (Cc), Consolida regalis (Cr), Gerbera hybrida (Gh), Helianthus annuus (Ha), Lotus corniculatus var. japonicus (Lcj), Mohavea confertiflora (Mc), and Senecio vulgaris (Sv). The Oryza sativa (Os) PCF1 (OsPCF1) amino acid sequence was used as an outgroup. As support for the tree, a bootstrap analysis, with 100 replicates, was performed by the Seqboot program and a consensus tree was obtained by the Consense program. Only bootstrap values higher than 50 are given at the nodes
The TCP domain is predicted to form bHLH structure but shares little sequence similarity with canonical bHLH domain. Therefore, TCP proteins bind to DNA elements that are different from those recognized by bHLH proteins (Kosugi and Ohashi 2002). The TCP domain mediates binding of the TCP protein to GC-rich DNA sequence motifs (Kosugi and Ohashi 2002; Aggarwal et al. 2010; Viola et al. 2012). These motifs have been identified as cis-acting elements in many plant genes, such as cell cycle- and protein synthesis-related genes, seed germination- and bud outgrowth-associated genes, and regulatory genes (Martín-Trillo and Cubas 2010). In the HaCYC2c sequence, a putative CYC-binding site (catGACCCTCtgg) was found 1054 bp upstream of the start codon (GenBank accession number HE604335.1). Putative CYC-binding sites were found in different zygomorphic lineages, and comparative analyses of CYC2 gene expression and function in the core eudicots provide an interpretation for CYC2 clade genes to establish a zygomorphic flower (Yang et al. 2012).
Mutants with altered inflorescence morphology can provide information on the roles of CYC-like genes in radiate inflorescence evolution (Coen et al. 1995). An insertion of a truncated version of CACTA-like transposable element (TE) upstream of the coding region of HaCYC2c (558 bp before the start codon) causes the ectopic expression of the gene and the shift from actinomorphic to zygomorphic disc flowers (Fig. 1b), and the inflorescence looks like a chrysanthemum (Chapman et al. 2012; Fambrini et al. 2014a). The change of floral symmetry displayed by the Chrysanthemoides2 (Chry2) mutant was restricted to disc flowers. The outer whorl of the Chry2 inflorescence was normally composed of ray flowers (Fambrini et al. 2003; Berti et al. 2005). In contrast, the corolla of disc flowers in Chry2 capitula becomes elongated assuming the dimensions and appearance of ray-like flowers. However, zygomorphy was pronounced in the ray-like flowers placed at the periphery (from the second to the fourth whorl) of Chry2 inflorescences, while petal abaxialization decreased in flowers of more internal whorls, where the joining of petals to form a near actinomorphy tubular-like corolla increased to about 80–90 % of the whole corolla length (Fambrini et al. 2003; Berti et al. 2005). In addition, the presence of individual lobes at the distal end of modified corollas indicated the conservation of some typical traits of tubular disc flowers (Berti et al. 2005). Therefore, the corolla of ray-like flowers in Chry2 inflorescence displayed an abaxialization that gradually decreased with a centripetal gradient. The zygomorphic abaxialized phenotype of Chry2 flowers was consistent with the ventral expression of CYC2 clade genes discovered in Asteraceae (Broholm et al. 2008, 2014). Nevertheless, a high level of HaCYC2c transcription was also detected in flowers placed in more internal whorls, and characterized by an elongated corolla with a near actinomorphic shape (Chapman et al. 2012; Fambrini et al. 2014a). Although several hypotheses have been prospected (Fambrini et al. 2014a), this result suggested that a much more complex regulatory system stays behind the Chry2 phenotype.
When the coding region of the HaCYC2c gene is, instead, interrupted by the insertion of a defective CACTA-like TE (Fambrini et al. 2011) or by the insertion of retrotransposons (Chapman et al. 2012), ray flowers switch from zygomorphic to actinomorphic, resembling disc flowers (Fig. 1c, d); this trait is peculiar of tubular ray flower (turf) and tubular-rayed (tub) mutants (Berti et al. 2005; Fambrini et al. 2007, 2011, 2014b; Chapman et al. 2012).
In turf, the origin of androgynous ray flowers as well as the effect of TE excision on flower phenotype gives this material as one of the more exciting mutants of flower symmetry in the Asteraceae family. In the mutant, the TE (5787 bp), named Transposable element of turf1 (Tetu1), is inserted in the basic region of the TCP motif (Fambrini et al. 2011). The Tetu1 integration changed the reading frame of turf-HaCYC2c for the encoded protein and led to a premature stop codon. Tetu1 is nonautonomous because it lacks a transposase coding sequence but still it can be excised thanks to the action of other trans-active transposases (Fambrini et al. 2011; 2014b). Footprints or DNA sequence rearrangements left at the donor site after transposition provide clues to the repair mechanisms of DNA (Weil and Kunze 2000; Oliver et al. 2013). The excision of Tetu1 restored a wild-type (WT) inflorescence, but we witnessed the occurrence of plants that bear ray flowers similar to WT ones (Fig. 1e), ray flowers with a phenotype that resembled hybrid characteristics between ray and disc flowers (Fig. 1f), and ray flowers typical of turf mutants (Fambrini et al. 2007, 2011). We demonstrated that the occurrence of inflorescences with different extents of phenotypes ranging from WT to turf relied on perfect or imperfect excisions of Tetu1 (Fambrini et al. 2014b).
Expression of CYC2 Clade Genes and Development of Reproductive Organs
With respect to the relationship between expression of CYC2-like genes and reproductive organ development, we first summarize our researches in H. annuus. The Chry2 mutation has been found to affect stamen development (Jaranowski et al. 1977; Fambrini et al. 2003), as well as style bifurcation and ovule formation (Fambrini et al. 2003; Berti et al. 2005). In fact, a proportion of the ray-like flowers located between the second and the seventh whorl of Chry2 inflorescences showed features reminiscent of sterile true ray flowers, including the absence of ovules in some ovaries, unbranched styles, and small anthers (Berti et al. 2005). In addition, a reduced number (4) of anthers and filaments in ray-like flowers located between the second and the fourth whorl was frequently detected. In some cases, styles and stigmas displayed homeotic transformation to petaloid structures. The defects of male and female reproductive organs decreased with a progressive reduction of corolla zygomorphism in ray-like flowers placed in more central whorls that however maintained an elongated corolla tube (Berti et al. 2005).
In turf, flowers arranged in the outmost whorl of the inflorescence (i.e., tubular-like ray flowers) maintain their positional identity because they are bigger than the WT polysymmetric disc flowers (Fig. 1c) but achieved hermaphrodite features (Berti et al. 2005; Fambrini et al. 2007, 2011, 2014b; Mizzotti et al. 2015). turf plants differentiated tubular-like ray flowers in which stamen filaments, anthers, style, and ovule displayed some developmental defects (e.g., many styles with a mono- or three-parted stigma, chimeric stamens including petal cells and enlarged deformed filaments), but morphological and genetic analysis demonstrated that both male and female organs were functional (Berti et al. 2005; Fig. 1g, h).
In tubular-like ray flowers, the shift to radial symmetry was coupled to a decrease in size of the corolla, which assumed a characteristic crisp aspect (Fig. 1h), and at maturity, the corolla tube became misshapen and bent abaxially, retaining some zygomorphic symmetry features (Mizzotti et al. 2015). In open-pollinated tubular-like ray flowers, the seed set was low but a noteworthy increase of filled achenes was obtained by hand pollination. Wild-type ray achenes were always empty. Embryos of tubular-like ray flowers were shorter and lighter than the embryos of disc flowers but able to produce fertile plants (Mizzotti et al. 2015).
Although, in asterid eudicots, including Asteraceae, transition from monosymmetry and polysymmetry and vice versa and changes in floral fertility were not supported by phylogenetic analyses (Donoghue et al. 1998; Panero and Funk 2002), within the genus Helianthus, the ray flowers, neuter, or pistillate are always sterile. Thus, sterility could be the result of mutations that arose independently from the shift of floral symmetry. Nevertheless, our results showed that both Chry2 and turf mutations induced alterations in flower symmetry but also affected gynoecium and stamen development. Although from 2003 to 2015 more than 20,000 tubular-like flowers of turf were analyzed, two mutations strictly linked and controlling different traits (i.e., ray flower symmetry and reproductive organ differentiation) could be still presumed. Additionally, in the Chry2 mutant, we analyzed floral organs (i.e., stamen and carpel) in a restricted population, and currently, in this genetic background, the CACTA TE (1034 bp) resulted unable for transposition. However, in turf plants, the excision of Tetu1 always restored the HaCYC2c functionality and a WT inflorescence (i.e., sterile zygomorphic ray flowers), and the occurrence of some inflorescences with different extents of phenotype ranging from WT ray flowers to tubular-like flowers has been related to footprints generated by imperfect excisions of Tetu1 (Fambrini et al. 2014b). In particular, five mutants with a one to four amino acid change at the TCP basic motif were selected; in mutant-1, mutant-2, mutant-3, mutant-4, and mutant-5, modifications of corolla shape were evidenced (Fig. 4a–e), but the differentiation of male reproductive organs was maintained, although malformation and/or homeotic transformation of stamens in petal tissue characterized some ray flowers (Fig. 4h–j). By contrast, in all mutants, ray flowers displayed the lack of ovules suggesting that, despite the modifications at the basic motif of the bHLH domain, the activity of HaCYC2c in these genotypes repressed gynoecium development as in WT (Table 2 in Fambrini et al. 2014b). These data strengthen the assumption of the pleiotropic effects of the HaCYC2c gene, which control both ray flower symmetry and reproductive organ development, likely interacting with cell cycle and/or flower organ identity genes (i.e., genes controlling stamen and carpel development).
Phenotype of mutants (from mutant-1 to mutant-5) generated in sunflower (Helianthus annuus L.) by Transposable element of turf1 (Tetu1) excision. a Inflorescence of mutant-1. b Inflorescence of mutant-2. c Inflorescence of mutant-3. d Inflorescence of mutant-4. e Inflorescence of mutant-5. f Ray flower of mutant-1: the arrow indicates deformed anthers with pollen. g Anthers (arrowhead) and monostigmatic structure (arrow) of mutant-2. h Ray flower of mutant-3; the corolla was partially removed; the arrowhead indicates an anther with pollen and the arrow indicates a monostigmatic structure. i Ray flower of mutant-4; arrows indicate homeotic transformation of stamens in petals. j Ray flower of mutant-5; the arrow indicates short anthers with pollen. Scale bars are 2.0 mm in f, 1.6 mm in g, 1.4 mm in h, 2.9 mm in i, and 1.7 mm in j
The link between changes in floral symmetry and reproductive organ development has been well documented in angiosperms, including other members of Asteraceae. For example, in the interspecific crosses between Layia discoidea and Layia grandulosa, some female ray flowers developed rudimentary anthers, while some “gibbous” ray flowers, with a near actinomorphic symmetry, differentiated hermaphrodite or near hermaphrodite flowers (Ford and Gottlieb 1990). The genes implied in these polymorphic traits are unknown but could be very interesting to evaluate if they are CYC-like genes as in the genus Helianthus. In the cross between the diploid radiate species Senecio squalidus and the tetraploid discoid species S. vulgaris, androecial differentiation was positively associated with the repression of corolla tube growth of ray flowers (Ingram and Taylor 1982). The triploid obtained from the cross S. squalidus × S. vulgaris gave few fertile seeds; nevertheless, some viable progenies occurred as a result of backcrosses with S. vulgaris. Further series of backcrossing are thought to have led to introgression of the radiate trait into some populations of S. vulgaris (Abbott et al. 1992). The resulting polymorphism for the radiate condition in S. vulgaris arose by introgression of a cluster of regulatory genes, the RAY locus, from S. squalidus into S. vulgaris (Kim et al. 2008). The RAY locus includes two tightly linked genes, RAY1 and RAY2, belonging to the CYC2 clade. These genes were expressed in the peripheral regions of the inflorescence meristem, where they promoted flower zygomorphism and, therefore, inflorescence architecture (Kim et al. 2008).
In G. hybrida, two common adaptations of floral morphology, unisexuality and zygomorphy, have been extensively analyzed (Yu et al. 1999; Kotilainen et al. 1999, 2000; Broholm et al. 2008, 2014). G. hybrida inflorescences include bisexual and unisexual flowers that also differ in floral symmetry. The gerbera inflorescence contains ray, trans, and disc flowers. Disc flowers, which are bisexual, contain fertile stamens and an inferior ovary. The lobes of the tubular corolla are similar in size, resulting in a nearly actinomorphic flower. Trans flowers are unisexual due to the abortion of anthers relatively late in development. Unequal growth of the corolla lobes yields a zygomorphic flower. Ray flowers produce sterile stamens and strongly zygomorphic corollas (Yu et al. 1999; Kotilainen et al. 1999, 2000). In sunflower, the incomplete filaments attached at the base of the corolla tube of ray flowers are likely a reminiscent of ancestral stamens. The same outcome is displayed by sterile zygomorphic ray flowers of G. hybrida (Broholm et al. 2008). Kotilainen et al. (2000) demonstrated that abortion programs affected only organs of the appropriate identity. Thus, in some species, the evolution of unisexuality and/or sterility may require the establishment of interactions between the floral homeotic genes and pathway involved in growth arrest. Additionally, the asymmetrical expression pattern of MADS-box genes in young heads of G. hybrida was apparently in response to an unknown signal with a centripetal gradient in the capitula (Yu et al. 1999). It has been suggested that the unequal development of petals in monosymmetric flowers of G. hybrida inflorescences could be a result of this centripetal gradient signal, likely via the action of class B of MADS-box genes (Yu et al. 1999). Also, Broholm et al. (2008) demonstrated that ectopic expression of GhCYC2 in transgenic plants converted disc flowers into ray-like ones with elongated petals and disrupted stamen development. However, suppression of GhCYC2 expression was not sufficient to cause loss of ray flower identity. This indicated that there must be additional genes involved in the regulation of flower-type differentiation. In fact, ectopic activation of GhCYC3 and GhCYC4 caused very similar phenotypes in transgenic gerbera (Juntheikki-Palovaara et al. 2014). All gene activities converted disc flowers into ray-like ones by promoting ligule growth through enhanced cell proliferation and suppressed stamen development. Tähtiharju et al. (2012) showed that the gerbera CYC2 clade proteins have the ability to interact in yeast two-hybrid assays. Therefore, it was postulated that the functional specificity in given tissues (or in various flower types) is connected with the formation of context-specific protein complexes involving CYC2 proteins and their co-regulators that may target different downstream genes (Juntheikki-Palovaara et al. 2014).
In gerbera and sunflower, CYC2 clade genes are likely to have more specialized functions at the level of single flower, including the late functions in floral reproductive organs that may be more conserved across plant families (Tähtiharju et al. 2012). In particular, the expression domains of HaCYC2c were not restricted to the corolla region of ray flowers but included reproductive organs of tubular flowers (stamen, stigma plus style, and ovary) (Tähtiharju et al. 2012).
The extension of expression of a gene that controls a key developmental process would be crucial in generating phenotypic effects (Crews and Pearson 2009). For example, Mohavea confertiflora (Plantaginaceae) has zygomorphic flowers with three (one dorsal and two lateral) stamens aborted instead of the single dorsal stamen aborted in Antirrhinum. These morphological differences were related with alterations in transcription patterns of CYC/DICH-like genes (Hileman et al. 2003). In Mohavea, the orthologs of CYC and DICH are transcribed not only in the dorsal-most stamen primordia but also in the lateral stamen primordia. Therefore, the extension of CYC/DICH-like gene expression into these organs could be responsible for their abortion (Hileman et al. 2003; Cubas 2004; Busch and Zachgo 2009). Primulina heterotricha has a zygomorphic flower with two reduced dorsal petals and abortion of both the dorsal and lateral stamens (Yang et al. 2012). During floral development, when P. heterotricha flowers become dorsoventrally differentiated, CYC2 TCP clade genes, CYC1C and CYC1D, were strongly expressed in the dorsal petals and the dorsal/lateral stamens. Especially, the strong transcription of CYC1C in the lateral staminodes at late stage of development correlated with the abortion of both dorsal and lateral stamens (Yang et al. 2012). Additionally, in rare peloric flowers with radial symmetry, CYC1C and CYC1D were not expressed, indicating that these genes may regulate the dorsal reduction of petal size and the dorsal/lateral abortion of stamens.
CYC/TB1-like genes seem to have a particular role in stamen abortion associated with perianth zygomorphism (Hileman and Cubas 2009; Preston and Hileman 2009). For example, in Opithandra (Gesneriaceae), expression of two CYC2-like genes was correlated with adaxial and abaxial stamen abortion and was negatively correlated with the expression of cyclinD3, a positive regulator of cell division (Gaudin et al. 2000; Song et al. 2009). In snapdragon, CYC and DICH expression was not restricted to petals, but their activity also retarded growth and reduced the number of other organ primordia (sepals and stamens) in the dorsal region of the flower meristem (Luo et al. 1996, 1999), suggesting that CYC can interact in a combinatorial fashion controlling cell cycle genes (e.g., D-cyclin) (Gaudin et al. 2000).
Finally, an open question is if floral symmetry genes are also breeding system genes. The impact of floral symmetry genes on both corolla and androecium development and parallel macroevolutionary patterns involving protandry and floral symmetry is consistent with the hypothesis of an association between bilateral symmetry and protandry (reviewed in Kalisz et al. 2006).
A Poorly Investigated Issue in the Control of Floral Traits: Interaction Between CYC2 Clade Genes and MADS-Box Genes
In gerbera, complementary DNA (cDNA) microarray analyses comparing gene expression in ray and disc flower primordia revealed that similar to CYC2 clade genes, also several MADS-box genes showed differential expression between the flower types (Laitinen et al. 2006; Broholm et al. 2014). In fact, close Arabidopsis homologues to SEPALLATA (SEP) clade MADS-box genes, GERBERA REGULATOR OF CAPITULUM DEVELOPMENT 1 (GRCD1), GRCD5, and GRCD3, as well as GhSOC1 were upregulated during ray flower development compared to the corresponding developmental stage of disc flowers. The gene GRCD1 is necessary for stamen development in whorl 3 and primarily in flowers at the margins of the inflorescence, where they form sterile staminoids (Kotilainen et al. 2000). Analogous to the homologue GRCD1, GRCD2 plays a role in reproductive organ determination (Uimari et al. 2004). Downregulation of both GRCD2 and the Gerbera AGAMOUS (AG) homologues resulted in loss of carpel identity, but only the latter caused indeterminacy in stylar and stigmatic tissues, and only the former resulted in floral reversion, which occurred in the ovaries (Yu et al. 1999). Remarkably, loss of GRCD2 function also altered inflorescence architecture by switching off terminal, determinate growth (Uimari et al. 2004). Moreover, overexpression of GhSOC1 affected the growth of the petals because the ray flower petals were shorter and stamen development in disc flowers was disrupted (Ruokolainen et al. 2011). It has been suggested that GhSOC1 may function upstream in the regulatory cascade imposed to CYC2 clade genes (Broholm et al. 2014). However, gene duplication events during CYC and MADS-box gene evolution have been proposed to be tightly correlated with one another (Howarth and Donoghue 2006).
Three cDNA clones characterized by high sequence similarity with the PISTILLATA (PI), AG, and APETALA3 (AP3) genes from Arabidopsis were isolated from immature inflorescence of sunflower: HaPI, HaAG, and HaAP3 (Dezar et al. 2003). A differential expression for these genes has been observed in fertile tubular flowers and sterile ray flowers. In fact, HaAG accumulated in fertile flowers, mainly in stamens and in developing ovules, while HaPI and HaAP3 were preferentially expressed in ray flowers. An ectopic overexpression of the HaAG gene was also detected in fertile ray flowers of the sunflower mutant L207 (Dezar et al. 2003). In addition, eight full-length cDNAs of HAM (H. annuus MADS) genes were isolated from sunflower inflorescences (Shulga et al. 2008). Notably, expression analysis suggested that the lack of the HAM59 gene activity during ray flower initiation could be essential to determine the structural and functional differences between the ray and tubular flowers. The HAM59 gene encodes for the homeotic C function and controls the pistil and stamen identity (Shulga et al. 2008). Additionally, results obtained in transgenic sunflower indicated that HAM59 in combination with HAM45 was involved in termination of floral meristems, establishment of stamen and pistil identity, and suppression of the cadastral function of A gene activity (Shulga et al. 2015). By contrast, the specification of the reproductive organ identity was performed by HAM59 and HAM45 in the presence of relevant but unknown partners (Shulga et al. 2015).
In Antirrhinum, the CYC gene is expressed asymmetrically in shoot meristems of floricaula mutants and in the terminal flower of centroradialis, indicating that its expression can also be activated in these contexts (Clark and Coen 2002). The early pattern of CYC expression is not affected by mutations in organ identity genes; however, later maintenance of CYC expression in whorl 2 of the inflorescence was controlled by DEFICIENS (DEF) gene (Clark and Coen 2002). Notably, in def mutant, dorsoventral asymmetry was obvious: there were few or no ovules in the dorsal locule of the third whorl, in contrast to the lateral and ventral locules, which contained many ovules. This suggests that CYC expression in carpels may lead to inhibition of ovule development (Clark and Coen 2002). In addition, the analysis of several mutants, as well globosa (glo), plena (ple), and ovulate (ovu), showed that CYC expression can react to a dorsoventral prepattern in flower, shoot, and organ (sepal) development (Clark and Coen 2002). These results most likely reflected interactions of CYC with flower-specific genes, including the MADS-box genes DEF, GLO, and PLE.
In Commellinaceae (Commelinales), a parallel recruitment of TB1-like genes acts in the early stages of floral differentiation, while in later stages, the loss of the expression of a class B gene was detected (i.e., DEF). This combined action of TCP and MADS-box genes established homeotic transformation of inner tepals into outer tepals and the evolution of bilateral symmetry (Preston and Hileman 2012). In maize, the characterization of a B-class mutant, sterile tassel silky ear1 (sts1), showed that sts1 was caused by a loss of function of the maize MADS-box PISTILLATA (PI)/GLO-like gene, Zea mays mads16 (Bartlett et al. 2015). Notably, sts1 mutant displayed novel phenotype that provide insight into two derived aspects of maize flower development: carpel abortion and floral asymmetry.
Lines of evidence collected in plant models suggest that CYC-like genes do not affect flower organ identity determination but rather function downstream of MADS-box genes by either promoting or repressing the growth of the organs (reviewed in Broholm et al. 2014). For example, among SEP3 targets, Kaufmann et al. (2009) showed that 15 TF families were significantly overrepresented, and among them, there were 11 TCP genes. Analogously, Wellmer et al. (2006) found seven TCP genes belonging to class II, among the targets of AP1. It was also supposed that MADS domain TFs operated in concert with TCPs in larger complexes (Dornelas et al. 2010).
PI MADS-box gene of Aristolochia arborea showed analogue expression domain, to the CYC-like gene in inner epidermal layers of the differentiating perianth, but with an additional extension into the petaloid limb region and in the MMS. Thus, activity of the B-class gene PI in the perianth regions that differentiated into petaloid features with attracting function suggests that PI likely contributed to their development (Horn et al. 2015). The expression results also suggested that the CYC-like gene could represent the downstream target of a PI MADS-box B-class gene.
Conclusions and Perspectives
Morphological novelty in angiosperm flowers as well as inflorescences is frequently correlated to changes in the timing, rates, or pattern of gene expression and/or acquisition of new gene functions after extensive gene duplications in both MADS-box and TCP families [for a summary of putative molecular mechanisms involved, see Fig. 5 in Busch and Zachgo (2009)]. In particular, CYC TFs are important to control corolla symmetry during flower development, but it is remarkable that data collected in Asteraceae, Dipsacaceae, and Myrtaceae demonstrated also the recruitment of CYC-like gene regulation at a higher level of hierarchy in the development of different flower types within the flower-like inflorescences. In these “false flowers,” differences in the CYC gene expression dictate radial versus bilateral phenotypes from the center toward the periphery of the inflorescence, and this aspect has a key role to determine the architecture of pseudanthium. For example, in Dipsacaceae, the pattern of CYC-like gene diversification showed correlation with inflorescence form (i.e., radiate vs. discoid inflorescence) (Carlson et al. 2011). The duplication events in Helianthus and radiate Dipsacaceae occurred within the same gene lineage. Therefore, it has been hypothesized that the independent evolution of radiate capitula in Asteraceae and Dipsacaceae may be connected with independent duplication events in orthologous CYC-like genes (Carlson et al. 2011). However, according to Carlson et al. (2011), the studies on gene duplication events in Asteraceae are slightly limited to infer on evolutionary features. However, several data demonstrate that the CYC/TB1 gene family has expanded in Asteraceae, a circumstance that appears to be linked with the increased developmental complexity and evolutionary success of this family (Chapman et al. 2008; Tähtiharju et al. 2012; Garcês et al. 2016). Nevertheless, a comparison of gene expression analyses and phylogenetic relationship suggests that different Asteraceae lineages have independently recruited different CYC paralogs to establish zygomorphism (Garcês et al. 2016). Within the Asteraceae, sunflower represents probably the clearest example of a specie where the complex pattern of expression of the CYC2 clade gene is co-opted to spatial distribution of peripheral sterile flowers (ray flowers) and central fertile flowers (tubular flowers). In fact, in the turf mutant, inactivation of the HaCYC2c gene modified a sterile monosymmetric ray flower into a near actinomorphic hermaphrodite flower (Berti et al. 2005; Fambrini et al. 2011, 2014b; Mizzotti et al. 2015). In particular, the complete gynoecium development, established in all tubular-ray flowers, is a spectacular huge event never reported, in this extent, in other species. The HaCYC2c transcription pattern in stamen, style-stigma, and ovary of tubular flowers at later developmental stages (Tähtiharju et al. 2012) suggests that this TF is also co-opted to regulate reproductive organ growth maybe throughout its interaction with cell cycle and flower organ identity genes as demonstrated in Antirrhinum (Gaudin et al. 2000; Clark and Coen 2002). Information about the role of MADS-box genes in sunflower is reduced with respect to the plant model, and in the future, it could be attractive to investigate the putative interaction between a specific member of the CYC2 clade gene (i.e., HaCYC2c) and identity genes such as HaAG and/or HAM59. Additionally, further identification and functional analyses of related cis-elements of CYC2 clade genes identified in sunflower (Chapman et al. 2008) would be important to decipher how cis-regulatory changes underlie the expression differentiation of these CYC-like genes and subsequent morphological evolution and diversification of flower organs.
The results obtained with HaCYC2c loss-of-function mutation also strictly support the phylogenetic hypothesis that in sunflower the ray flowers originate by actinomorphic fertile flowers (i.e., from a discoid inflorescence). It is not verified if the evolution of a radiate inflorescence and the loss of fertility in ray flowers were contemporary. However, the inactivation of HaCYC2c transcription by insertion of a CACTA TE in the TCP domain revealed the pleiotropic effect of this gene on flower symmetry as well as on flower organ differentiation. Additionally, the result suggests that in zygomorphic ray flower primordia, the latent ability to differentiate both microsporangium and macrosporangium was preserved. In turf and WT sunflower, further studies are required to identify the direct target genes of the HaCYC2c TF required to define the androgynous or sterile ray flower, respectively.
References
Abbott RJ, Ashton PA, Forbes DG (1992) Introgressive origin of the radiate groundsel, Senecio vulgaris L. var. hibernicus Syme: Aat-3 evidence. Heredity 68:425–435
Abbott RJ, James JK, Milne RI, Gillies ACM (2003) Plant introduction, hybridization and gene flow. Philos Trans R Soc B Biol Sci 358:1123–1132
Aggarwal P, Gupta MD, Joseph AP, Chatterjee N, Srinivasan N, Nath U (2010) Identification of specific DNA binding residues in the TCP family of transcription factors in Arabidopsis. Plant Cell 22:1174–1189
Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19:458–472
Almeida J, Galego L (2005) Flower symmetry and shape in Antirrhinum. Int J Dev Biol 49:527–537
Almeida J, Rocheta M, Galego L (1997) Genetic control of flower shape in Antirrhinum majus. Development 124:1387–1392
Barreda VD, Palazzesi L, Tellería MC, Katinas L, Crisci JV, Bremer K, Passalia MG, Corsolini R, Rodríguez Brizuela R, Bechi F (2010) Eocene Patagonia fossils of the daisy family. Science 329:1621
Bartlett ME, Specht CD (2011) Changes in expression pattern of the TEOSINTE BRANCHED1-like genes in the Zingiberales provide a mechanism for evolutionary shifts in symmetry across the order. Am J Bot 98:1–17
Bartlett ME, Williams S, Taylor Z, Deblasio S, Goldshmidt A, Hall DH, Schmidt RJ, Jackson DP, Whipple CJ (2015) The maize PI/GLO ortholog Zmm16/sterile tassel silky ear1 interact with the zygomorphic and sex determination pathways in flower development. Plant Cell 27:3081–3098
Berger BA, Thompson V, Lim A, Ricigliano V, Howarth DG (2016) Elaboration of bilateral symmetry across Knautia macedonica capitula related to changes in ventral petal expression of CYCLOIDEA-like genes. EvoDevo 7:8
Berti F, Fambrini M, Turi M, Bertini D, Pugliesi C (2005) Mutations of corolla symmetry affect carpel and stamen development in Helianthus annuus. Can J Bot 83:1065–1072
Broholm SK, Tähtiharju S, Laitinen RAE, Albert VA, Teeri TH, Elomaa P (2008) A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc Natl Acad Sci U S A 105:9117–9122
Broholm SK, Teeri TH, Elomaa P (2014) Molecular control of inflorescence development in Asteraceae. Adv Bot Res 72:297–333
Busch A, Zachgo S (2007) Control of corolla monosymmetry in the Brassicaceae Iberis amara. Proc Natl Acad Sci U S A 104:16714–16719
Busch A, Zachgo S (2009) Flower symmetry evolution: towards understanding the abominable mystery of angiosperm radiation. BioEssays 31:1181–1190
Buzgo M, Endress PK (2000) Floral structure and development of Acoraceae and its systematic relationships with basal angiosperms. Int J Plant Sci 161:23–41
Carlson SE, Howard DG, Donoghue MJ (2011) Diversification of CYCLOIDEA-like genes in Dipsacaceae (Dipsacales): implications for the evolution of capitulum inflorescences. BMC Evol Biol 11:325
Carpenter R, Coen ES (1990) Floral homeotic mutations produced by transposon-mutagenesis in Antirrhinum majus. Genes Dev 4:1483–1493
Chapman MA, Leebens-Mack JH, Burke JM (2008) Positive selection and expression divergence following gene duplication in the sunflower CYCLOIDEA gene family. Mol Biol Evol 25:1260–1273
Chapman MA, Tang S, Draeger D, Nambeesan S, Shaffer H, Barb JG, Knapp SJ, Burke JM (2012) Genetic analysis of floral symmetry in Van Gogh’s sunflowers reveals independent recruitment of CYCLOIDEA genes in the Asteraceae. PLoS Genet 8(3):e1002628
Citerne HL, Möller M, Cronk QCB (2000) Diversity of cycloidea-like genes in Gesneriaceae in relation to floral symmetry. Ann Bot 86:167–176
Citerne HL, Luo D, Pennington RT, Coen E, Cronk QCB (2003) A phylogenomic investigation of CYCLOIDEA-like TCP genes in the Leguminosae. Plant Physiol 131:1042–1053
Citerne H, Jabbour F, Nadot S, Damerval C (2010) The evolution of floral symmetry. Adv Bot Res 54:85–137
Citerne HL, Le Guilloux M, Sannier J, Nadot S, Damerval C (2013) Combining phylogenetic and syntenic analyses for understanding the evolution of TCP ECE genes in Eudicots. PLoS ONE 8:e74803
Clark JI, Coen ES (2002) The cycloidea gene can respond to a common dorsoventral prepattern in Antirrhinum. Plant J 30:639–648
Claßen-Bockhoff R (1990) Pattern analysis in pseudanthia. Plant Syst Evol 171:57–88
Claßen-Bockhoff R, Ruonal R, Bull-Hereñu K, Marchant N, Albert VA (2013) The unique pseudanthium of Actinodium (Myrtaceae)—morphological reinvestigation and possible regulation by CYCLOIDEA-like genes. EvoDevo 4(1):8
Coen ES (1996) Floral symmetry. EMBO J 15:6777–6788
Coen ES, Nugent JM (1994) Evolution of flower and inflorescences. Development Suppl:S107–S116
Coen ES, Nugent JM, Luo D, Bradley D, Cubas P, Chadwick M, Copsey L, Carpenter R (1995) Evolution of floral symmetry. Philos Trans R Soc B Biol Sci 350:35–38
Comai L (2005) The advantages and disadvantages of being polyploid. Nat Rev Genet 6:836-846
Corley SB, Carpenter R, Copsey L, Coen E (2005) Floral asymmetry involves an interplay between TCP and MYB transcription factors in Antirrhinum. Proc Natl Acad Sci U S A 102:5068–5073
Costa MMR, Fox S, Hana AI, Baxter C, Coen E (2005) Evolution of regulatory interactions controlling floral asymmetry. Development 132:5093–5101
Crews ST, Pearson JC (2009) Transcriptional autoregulation in development. Curr Biol 19:R241–R246
Cronk Q, Möller M (1997) Genetics of floral symmetry revealed. Trends Ecol Evol 12:85–86
Cubas P (2004) Floral zygomorphy, the recurring evolution of a successful trait. BioEssays 26:1175–1174
Cubas P, Lauter N, Doebley J, Coen E (1999a) The TCP domain: a motif found in proteins regulating plant growth and development. Plant J 18:215–222
Cubas P, Vincent C, Coen E (1999b) An epigenetic mutation responsible for natural variation in floral symmetry. Nature 401:157–161
Cubas P, Coen E, Zapater JMM (2001) Ancient asymmetries in the evolution of flowers. Curr Biol 11:1050–1052
Damerval C, Nadot S (2007) Evolution of perianth and stamen characteristics with respect to floral symmetry in Ranunculales. Ann Bot 100:631–640
Damerval C, Le Guilloux M, Jager M, Charon C (2007) Diversity and evolution of CYCLOIDEA-like TCP genes in relation to flower development in Papaveraceae. Plant Physiol 143:759–772
De Paolo S, Gaudio L, Aceto S (2015) Analysis of the TCP genes expressed in the inflorescence of the orchid Orchis italica. Sci Rep 5:16265
Della Pina S, Souer E, Koes R (2014) Arguments in the evo-devo debate: say it with flowers! J Exp Bot 65:2231–2242
Dezar CA, Tioni MF, Gonzalez DH, Chan RL (2003) Identification of three MADS-box genes expressed in sunflower capitulum. J Exp Bot 387:1637–1639
Dinneny JR, Yadegari R, Fischer RL, Yanofsky MF, Weigel D (2004) The role of JAGGED in shaping lateral organs. Development 131:1101–1110
Doebley J, Stec A, Hubbard L (1997) The evolution of apical dominance. Nature 386:485–488
Donoghue MJ, Ree RH, Baum DA (1998) Phylogeny and the evolution of flower symmetry in the Asteridae. Trends Plant Sci 3:311–317
Dornelas MC, Patreze CM, Angenent GC, Immink RGH (2010) MADS: the missing link between identity and growth? Trends Plant Sci 16:89–97
Du ZY, Wang YZ (2008) Significance of RT-PCR expression patterns of CYC-like genes in Oreocharis benthamii (Gesneriaceae). J Syst Evol 46:23–31
Endress PK (1999) Symmetry in flowers: diversity and evolution. Int J Plant Sci 160:S3–S23
Endress PK (2001) Evolution of floral symmetry. Curr Opin Plant Biol 4:86–91
Fambrini M, Bertini D, Pugliesi C (2003) The genetic basis of a mutation that alters the floral symmetry in sunflower. Ann Appl Biol 143:341–347
Fambrini M, Michelotti V, Pugliesi C (2007) The unstable tubular ray flower allele of sunflower: inheritance of reversion to wild type. Plant Breed 126:548–550
Fambrini M, Salvini M, Pugliesi C (2011) A transposon-mediate inactivation of a CYCLOIDEA-like gene originates polysymmetric and androgynous ray flowers in Helianthus annuus. Genetica 139:1521–1529
Fambrini M, Salvini M, Basile A, Pugliesi C (2014a) Transposon-dependent induction of Vincent van Gogh’s sunflowers: exceptions revealed. Genesis 52:315–327
Fambrini M, Basile A, Salvini M, Pugliesi C (2014b) Excisions of a defective transposable CACTA element (Tetu1) generate new alleles of a CYCLOIDEA-like gene of Helianthus annuus. Gene 549:198–207
Feng X, Zhao Z, Tian Z, Xu S, Luo Y, Cai Z, Wang Y, Yang J, Wang Z, Weng L, Chen J, Zheng L, Guo X, Luo J, Sato S, Tabata S, Ma W, Cao X, Hu X, Sun C, Luo D (2006) Control of petal shape and floral zygomorphy in Lotus japonicus. Proc Natl Acad Sci U S A 103:4970–4975
Ford VS, Gottlieb LD (1990) Genetic studies of floral evolution in Laya. Heredity 64:29–44
Friedman WE (2009) The meaning of Darwin’s “abominable mystery”. Am J Bot 96:5–21
Fukuda T, Yokoyama J, Maki M (2003) Molecular evolution of Cycloidea-like genes in Fabaceae. J Mol Evol 57:588–597
Galego L, Almeida J (2002) Role of DIVARICATA in the control of dorsoventral asymmetry in Antirrhinum flowers. Genes Dev 16:880–891
Gao Q, Tao JH, Yan D, Wang YZ, Li ZY (2008) Expression differentiation of CYC-like floral symmetry genes correlated with their protein sequence divergence in Chirita heterotricha (Gesneriaceae). Dev Genes Evol 218:341–351
Garcês HMP, Spencer VMR, Kim M (2016) Control of floret symmetry by RAY3, SvDIV1B and SvRAD in the capitulum of Senecio vulgaris. Plant Physiol 171:2055–2068
Gaudin V, Lunness PA, Fobert PR, Towers M, Riou-Khamlichi C, Murray JA, Coen E, Doonan JH (2000) The expression of D-Cyclin defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the Cycloidea gene. Plant Physiol 122:1137–1148
Gübitz T, Caldwell A, Hudson A (2003) Rapid molecular evolution of CYCLOIDEA-like genes in Antirrhinum and its relatives. Mol Biol Evol 20:537–1544
Harris EM (1995) Inflorescence and floral ontogeny in Asteraceae: a synthesis of historical and current concepts. Bot Rev 61:93–278
Heijmans K, Morel P, Vandenbussche M (2012) MADS-box genes and flower development: the dark side. J Exp Bot 63:5397–5404
Hileman LC (2014a) Bilateral flower symmetry—how, when and why? Curr Opin Plant Biol 17:146–152
Hileman LC (2014b) Trends in flower symmetry evolution revealed through phylogenetic and developmental genetic advances. Phylos Trans R Soc Lond B Biol Sci 369:1648
Hileman LC, Cubas P (2009) An expanded evolutionary role for flower symmetry genes. J Biol 8:90
Hileman LC, Kramer EM, Baum DA (2003) Differential regulation of symmetry genes and the evolution of floral morphologies. Proc Natl Acad Sci U S A 100:12814–12819
Horn S, Pabón-Mora N, Theuß VA, Bush A, Zachgo S (2015) Analysis of the CYC/TB1 class of TCP transcription factors in basal angiosperm and magnoliids. Plant J 81:559–571
Hoshino Y, Igarashi T, Ohhima M, Shinoda K, Murata N, Kanno A, Nakano M (2014) Characterization of CYCLOIDEA-like genes in controlling floral zygomorphy in the monocotyledon Alstroemeria. Sci Hort 169:6–13
Howarth DG, Donoghue MJ (2005) Duplications in CYC-like genes from Dipsacales correlate with floral form. Int J Plant Sci 166:357–370
Howarth DG, Donoghue MJ (2006) Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proc Natl Acad Sci U S A 103:9101–9106
Howarth DG, Martins T, Chimney E, Donoghue MJ (2011) Diversification of CYCLOIDEA expression in the evolution of bilateral flower symmetry in Caprifoliaceae and Lonicera (Dipsacales). Ann Bot 107:1521–1532
Ingram R, Taylor L (1982) The genetic control of a non-radiate condition in Senecio squalidus L. and some observations of the role of ray florets in the compositae. New Phytol 91:749–756
Irish VF, Litt A (2005) Flower development and evolution: gene duplication, diversification and redeployment. Curr Opin Genet Dev 15:454–460
Jabbour F, Cossard G, Le Guilloux M, Sannir J, Nadot S, Damerval C (2014) Specific duplication and dorsoventrallly asymmetric expression patterns of Cycloidea-like genes in zygomorphic of Ranunculaceae. PLoS One 9(4):e95727
Jaranowski JK, Luczkiewicz L, Muszynski A (1977) Inflorescence organogenesis in Helianthus annuus florepleno. Phytomorphology 27:8–12
Jeffrey C (1977) Corolla forms in compositae—some evolutionary and taxonomic speculations. In: Heywood VH, Harborne JB, Turner BL (eds) The biology and chemistry of the compositae, vol 1. Academic, London, pp 111–118
Juntheikki-Palovaara I, Tähtiharjiu Lan T, Broholm K, Rijpkena AS, Ruonala R, Kale L, Albert VA, Teeri TH, Elomaa P (2014) Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae). Plant J 79:783–796
Kalisz S, Ree RH, Sargent RD (2006) Linking floral symmetry genes to breeding system evolution. Trends Plant Sci 11:1360–1385
Kaufmann K, Muiño JM, Jauregui R, Airoldi CA, Smaczniak C, Krajewski P, Angenent GC (2009) Target genes of the MADS transcription factor SEPALLATA3: integration of developmental and hormonal pathways in the Arabidopsis flower. PLoS Biol 7:e1000090
Kim M, Cui M-L, Cubas P, Gillies A, Lee K, Chapman MA, Abbott RJ, Coen E (2008) Regulatory genes control key morphological and ecological trait transferred between species. Science 322:1116–1119
Knowles PF (1978) Morphology and anatomy. In: Carter JF (ed) Sunflower science and technology. ASA, CSSA, SSSA Inc., Madison, pp 55–87
Kosugi S, Ohashi Y (2002) DNA binding and dimerization specificity and potential targets for the TCP protein family. Plant J 30:337–348
Kotilainen M, Helariutta Y, Mehto M, Pöllänen E, Albert VA, Elomaa P, Teeri TH (1999) GEG participates in the regulation of cell and organ shape during corolla and carpel development in Gerbera hybrida. Plant Cell 11:1093–1104
Kotilainen M, Elomaa P, Uimari A, Albert V, Yu D, Teeri TH (2000) GRCD1, an AGL2-like MADS box gene, participates in the C function during stamen development in Gerbera hybrida. Plant Cell 12:1893–1902
Krizek EM, Fletcher JC (2005) Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet 6:688–698
Laitinen RAE, Broholm SK, Albert VA, Teeri TH, Elomaa P (2006) Patterns of MADS-box gene expression mark flower-type development in Gerbera hybrida (Asteraceae). BMC Plant Biol 6:11
Lewin B (1997) Transposons. In: Lewin B (ed) Genes VI. Oxford University Press, New York, pp 563–595
Luo D, Carpenter R, Vincent C, Copsey L, Coen E (1996) Origin of floral asymmetry in Antirrhinum. Nature 383:794–799
Luo D, Carpenter R, Copsey L, Vincent C, Clark J, Coen ES (1999) Control of organ asymmetry in flowers of Antirrhinum. Cell 99:367–376
Martín-Trillo M, Cubas P (2010) TCP genes: a family snapshot ten years later. Trends Plant Sci 15:31–39
Mizzotti C, Fambrini M, Caporali E, Masiero S, Pugliesi C (2015) A CYCLOIDEA-like gene mutation in sunflower determines an unusual floret type able to produce filled achenes at the periphery of the pseudanthium. Botany 93:171–181
Nath U, Crawford B, Carpenter B, Coen E (2003) Genetic control of surface curvature. Science 299:1404–1407
Neal PR, Dafni A, Giurfa M (1998) Floral symmetry and its role in plant-pollinator systems: terminology, distribution, and hypotheses. Annu Rev Ecol Syst 29:345–373
Nikkeshi A, Kurimoto D, Ushimaru A (2015) Low flower-size variation in bilaterally symmetrycal flowers: support for the pollination precision hypothesis. Am J Bot 102:2032–2040
Oliver KR, Mccomb JA, Greene WK (2013) Transposable elements: powerful contributors to angiosperm evolution and diversity. Genome Biol Evol 5:1886–1901
Palmer JM, Palmer JH (1982) Changes in mitotic activity and cell size in the apical meristem of Helianthus annuus L. during the transition to flowering. Am J Bot 69:768–775
Panero JL, Funk VA (2002) Toward a phylogenetic subfamilies classification for the Compositae (Asteraceae). Proc Biol Soc Washington 115:909–922
Preston JC, Hileman LC (2009) Developmental genetics of floral symmetry evolution. Trends Plant Sci 14:147–154
Preston JC, Hileman LC (2012) Parallel evolution of TCP and B-class genes in Commelinaceae flower bilateral symmetry. EvoDevo 3:6
Raimundo J, Sobral R, Bailey P, Azevedo H, Galego L, Almeida J, Coen E, Costa MMR (2013) A subcellular tug of war involving three MYB-like proteins underlies a molecular antagonism in Antirrhinum flower asymmetry. Plant J 75:527–538
Reale L, Porceddu A, Lanfaloni L, Moretti C, Zenoni S, Pezzotti M, Romano B, Ferranti F (2002) Patterns of cell division and expansion in developing petals of Petunia hybrida. Sex Plant Reprod 15:123–132
Ree RH, Citerne HL, Lavin M, Cronk QCB (2004) Heterogeneous selection on LEGCYC paralogs in relation to flower morphology and the phylogeny of Lupinus (Leguminosae). Mol Biol Evol 21:321–331
Reeves P, Olmstead R (2003) Evolution of the TCP gene family in Asteridae: cladistic and network approaches to understanding regulatory gene family diversification and its impact on morphological evolution. Mol Biol Evol 20:1997–2009
Ronse De Craene LP (2010) Floral diagrams: an aid to understanding flower morphology and evolution. Cambridge University Press, Cambridge
Rudall PJ, Bateman RM (2004) Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytol 162:25–44
Ruokolainen S, Ng YP, Albert VA, Elomaa P, Teeri TH (2011) Over-expression of the Gerbera hybrida At-SOC1-like1 gene Gh-SOC1 leads to floral organ identity deterioration. Ann Bot 107:1491–1499
Sargent RD (2004) Floral symmetry affects speciation rates in angiosperms. Proc Royal Soc London Series B Biol Sci 271:603–608
Shulga OA, Shchennikova AV, Angenent GC, Skryabin KG (2008) MADS-box genes controlling inflorescence morphogenesis in sunflower. Russ J Dev Biol 39:2–5
Shulga OA, Neskorodov YB, Shchennikova AV, Gaponenko AK, Skryabin KG (2015) Ectopic expression of the HAM59 gene causes homeotic transformations of reproductive organs in sunflower (Helianthus annuus L.). Dokl Biochem Biophys 461:110–113
Smith JF, Hileman LC, Powell MP, Baum DA (2004) Evolution of GCYC, a Gesneriaceae homolog of CYCLOIDEA, within Gesnerioideae (Gesneriaceae). Mol Phylogenet Evol 31:765–779
Song CF, Lin QB, Liang RH, Wang YZ (2009) Expressions of ECE-CYC2 clade genes relating to abortion of both dorsal and ventral stamens in Opithandra (Gesneriaceae). BMC Evol Biol 9:244
Specht CD, Howarth DG (2015) Adaptation in flower form: a comparative evodevo approach. New Phytol 206:74–90
Stuessy TF, Spooner DM, Evans KA (1986) Adaptive significance of ray corollas in Helianthus grosseserratus (Compositae). Am Midl Naturalist 115:191–197
Tähtiharju S, Rijpkema AS, Vetterli A, Albert VA, Teeri TH, Elomaa P (2012) Evolution and diversification of the CYC/TB1 gene family in Asteraceae—a comparative study in gerbera (Mutisieae) and sunflower (Heliantheae). Mol Biol Evol 29:1155–1166
Theissen G (2000) Evolutionary developmental genetics of floral symmetry: the revealing power of Linnaeus’ monstrous flower. BioEssays 22:209–213
Tucker SC (1999) Evolutionary lability of symmetry in early floral development. Int J Plant Sci 160:S25–S39
Uberti Manassero NG, Viola IL, Welchen E, Gonzalez DH (2013) TCP transcription factors: architectures of plant form. Biomol Concepts 4:111–127
Uimari A, Kotilainen M, Elomaa P, Yu D, Albert VA, Teeri TH (2004) Integration of reproductive meristem fates by a SEPALLATA-like MADS-box gene. Proc Natl Acad Sci U S A 101:15817–15822
Van De Peer Y, Maere S, Meyer A (2009) The evolutionary significance of ancient genome duplications. Nature Rev Genet 10:724–732
Viola IL, Reinheimer R, Ripoli R, Uberti Manassero NG, Gonzalez DH (2012) Determinants of the DNA binding specificity of class I and class II TCP transcription factors. J Biol Chem 287:347–356
Wang Z, Luo Y, Li X, Xu SL, Yang J, Weng L, Sato S, Tabata S, Ambrose M, Rameau C, Feng XZ, Hu XH, Luo D (2008) Genetic control of floral zygomorphy in pea (Pisum sativum L.). Proc Natl Acad Sci U S A 105:10414–10419
Weil CF, Kunze R (2000) Transposition of maize Ac/Ds transposable elements in the yeast Saccharomyces cerevisiae. Nat Genet 26:187–190
Wellmer F, Alves-Ferreira M, Dubois A, Riechmann JL, Meyerowitz EM (2006) Genome-wide analysis of gene expression during early Arabidopsis flower development. PLoS Genet 2:e117
Weng L, Tian Z, Feng X, Li X, Xu S, Hu X, Luo D, Yang J (2011) Petal development in Lotus japonicus. J Integ Plant Biol 53:770–782
Wittkopp PJ, Haerum BK, Clark AG (2008) Regulatory change underlying expression differences within and between Drosophila species. Nat Genet 40:346–350
Xu Z, Cheng K, Li X, Yang J, Xu S, Cao X, Hu X, Yuan L, Ambrose M, Chen G, Mi H, Luo D (2016) Transcriptional and post-transcriptional modulation of SQU and KEW activities in the control of dorsal-ventral asymmetric flower development in Lotus japonicus. Mol Plant 9:722–736
Yang X, Pang H-B, Liu B-L, Qiu Z-J, Gao Q, Wei L, Dong Y, Wang Y-Z (2012) Evolution of double positive autoregulatory feedback loops in CYC2 clade genes is associated with the origin of floral zygomorphy. Plant Cell 24:1834–1847
Yang X, Zhao X-G, Li CQ, Liu J, Qiu Z-J, Dong Y, Wang Y-Z (2015) Distinct regulatory changes underlying differential expression of TEOSINTE BRANCHED1-CYCLOIDEA-PROLIFERATING CELL FACTOR gene associated with petal variations in zygomorphic flower of Petrocosmea spp. of the family Gesneriaceae. Plant Physiol 169:2138–2151
Yu D, Kotilainen M, Pöllänen E, Mehto M, Elomaa P, Helariutta Y, Albert VA, Teeri TH (1999) Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae). Plant J 17:51–62
Yuan Z, Gao S, Xue DW, Luo D, Li LT, Ding SY, Yao X, Wilson ZA, Qian Q, Zhang DB (2009) RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiol 149:235–244
Zhang W, Kramer EM, Davis CC (2010) Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proc Natl Acad Sci USA 107:6388-6393
Zhong J, Kellogg EA (2015a) Stepwise evolution of corolla symmetry in CYCLOIDEA2-like and RADIALIS-like gene expression patterns in Lamiales. Am J Bot 102:1260–1267
Zhong J, Kellogg EA (2015b) Duplication and expression of CYC2-like genes in the origin and maintenance of corolla zygomorphy in Lamiales. New Phytol 205:852–868
Acknowledgments
This work was supported by the Università degli Studi di Pisa. We are indebted to Mariangela Salvini for phylogenetic analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 165 kb)
Rights and permissions
About this article
Cite this article
Fambrini, M., Pugliesi, C. CYCLOIDEA 2 Clade Genes: Key Players in the Control of Floral Symmetry, Inflorescence Architecture, and Reproductive Organ Development. Plant Mol Biol Rep 35, 20–36 (2017). https://doi.org/10.1007/s11105-016-1005-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-016-1005-z