Abstract
Purpose
Plant-soil feedbacks are important drivers of ecosystem dynamics and have been hypothesized to affect woody encroachment in savannas. Woody encroachment is expected to increases savanna soil fertility through deposition of organic matter, favoring further establishment of woody individuals. In this context, we tested if litter input promotes forest seedling growth in dystrophic savanna soils, and if this was accompanied by an increase in microbial activity.
Methods
In a glasshouse experiment, we planted woody seedlings of three forest species in savanna soils either mixed or not (control) with litter from closely related savanna or forest species (10 species). We evaluated the growth of the woody seedlings as well as the response of the soil microbiota activity and biomass to litter addition.
Results
Litter addition had either no effect or negative effects on seedling growth, and different seedling species responded differently to litter addition. However, we did find microbial activity to increase in response to litter addition, especially through the input of litter rich in phosphorus (P) and carbon (C).
Conclusions
Our results indicate that litter input does not favor woody seedlings growth in savanna soil. Instead, litter input showed a potential to hinder seedling growth, especially of fast-growing species. Furthermore, litter input consistently increased soil microbiota activity, mainly through the input of P and C, highlighting the importance of energy and P in the nutrient dynamics of Cerrado. Thus, our results did not support the hypothesis that litter deposition triggers a positive feedback with woody encroachment via increased seedling growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In mesic seasonally dry tropical landscapes, savannas and forests coexist under similar climatic and edaphic conditions (Hirota et al. 2011; Staver et al. 2011; Bueno et al. 2018). The distribution of these vegetation types is regulated by a complex set of feedbacks that involves: fire regime, resource availability and vegetation characteristics (Hoffmann et al. 2012; Dantas et al. 2016; Bueno et al. 2018). According to this model, the fire regime causes thinning of the woody layer, maintaining an open savanna vegetation in regions where the climate and soil are also suitable for forest dominance (Sankaran et al. 2004; Bond et al. 2005; Staver et al. 2011). The persistence of fire reinforces fire-prone conditions and the resulting outcome is a feedback loop, allowing the persistence of open canopy and savanna vegetation (Bond, 2008; Warman and Moles, 2009). In this scenario, the transition of savanna to forest would be dependent on the formation of a closed canopy sufficient to inhibit shade-intolerant grass growth, the main fuel of fire (Hoffmann et al., 2012; Pellegrini, 2016; Warman and Moles 2009). Since savannas and forests are maintained by a complex set of feedbacks, the distribution of these vegetation types is dynamic rather than static. In fact, studies have reported the transition of savannas to forests and vice-versa in some tropical savanna regions (Durigan and Ratter 2006; Silva et al. 2008, 2013; Stevens et al. 2017; Gonçalves et al. 2021).
An important component of forest-savanna dynamics is the woody encroachment into savannas, characterized by the increase of woody biomass in open habitats (Stevens et al. 2017). Besides increasing woody biomass, woody encroachment in open habitats significantly modifies species taxonomic and functional composition, leading to modifications in ecosystem processes, such as nutrient and hydraulic cycles, may also reinforce the maintenance of forests (Parr et al. 2012). In the context of forest-savannas transitions, the accumulation of woody biomass in open habitats may be related to two main factors: i) a reduction or suppression of fires in open habitats, preventing the thinning of the woody layer by fire; and ii) in increasing in woody plant growth rates (e.g., encouraged via improved soil fertility). Thus, environmental and biotic changes following a fire that either inhibit fire or increase the growth rate of woody plants are expected to promote woody encroachment in open habitats.
A key process potentially involved in this dynamic plant-soil feedback is litter accumulation mediated by increased litter input. Litter input is a mechanism by which large amounts of organic matter and nutrients are returned to the ecosystem, with potential impacts on plant growth (Freschet et al. 2013; Hobbie 2015). Litter input affects soil properties such as physical structure and/or chemical composition (Facelli and Pickett 1991; Veen et al. 2019). For instance, litter chemical composition can have both positive or negative effects on nutrient availability to plants, depending on concentration in the litter of both nutrients (increasing nutrient availability for plant uptake) and secondary metabolites (which may inhibit root growth) (Lopez-Iglesias et al. 2014; Veen et al. 2019). Moreover, the physical structure of litter affects seed germination and plant growth, since it filters the amount of radiation that reaches the soil, also maintaining soil humidity and providing some thermal insulation; it may also act as a mechanical barrier for seedlings’ emergence, hindering their development (Facelli and Pickett 1991; Hovstad and Ohlson 2008; Veen et al. 2019). Therefore, modifications in the amount of litter, and in its chemical and physical characteristics may significantly affect plant growth, especially at the stage of recruitment and early growth.
Besides the direct effects in plant growth, litter input can also affect plant-soil feedbacks by influencing that activity of soil microbiota– i.e., via the indirect effects of litter input on plant growth (Van Der Heijden et al. 2008; Zechmeister-Boltenstern et al. 2015). Litter input stimulates the growth of soil microbiota and their overall metabolic activity through the addition of resources that otherwise be limiting (Zechmeister-Boltenstern et al. 2015). In turn, the stimulation of soil microbiota from new litter input can influence the nutrient immobilization-mineralization dynamics. For instance, litter that is rich in limiting resources (usually nutrients) and also harbors low concentrations of defensive and recalcitrant compounds, is rapidly decomposed by soil microbiota (Freschet et al. 2013; Zechmeister-Boltenstern et al. 2015). The decomposition of litter by microorganisms releases the nutrients immobilized in dead tissues, which can be either released in soil solution (making it available for plant uptake) or immobilized in microbial biomass (Bengtson and Bengtsson 2005; Zechmeister-Boltenstern et al. 2015). On the other hand, the stimulation of soil microbiota growth and activity could have negative effects on the growth of seedlings if microbes outcompete plants for limiting soil resources, such as nutrients (i.e., priming effect, Kuzyakov and Xu 2013). Indeed, soil microorganisms have been reported to outcompete plants for nitrogen (N) and phosphorus (P), immobilizing these nutrients in microbial biomass, which becomes unavailable for plant uptake, at least in the short-term (Čapek et al. 2018). Also, the negative effects of microbes on plant growth can be related to the production of phytotoxic compounds that hinder plant development (Jilani et al. 2008). Therefore, to understand the mechanisms by which litter input affects plant growth, it is also important to evaluate the effects of litter input on soil microbiota activity and biomass. Ultimately, soil microbiota might mediate the interaction between litter input and plant growth since microorganisms are responsible for the breakdown of dead tissue and thus drive nutrient mineralization-immobilization dynamics.
In this study we evaluated the effects of litter input in soil microbiota and in the growth of woody seedlings growing in the context of forest-savanna transitions in Brazilian Cerrado (hereafter Cerrado). The Cerrado is an ecoregion of the tropical savanna biome (locally called a “biome”) consisting of savanna-dominated landscapes scattered with grasslands, closed-canopy woodlands and strips of riparian forests along river courses. Overall, it is distributed over a dystrophic soil matrix, with low concentrations of nutrients (especially P and Ca), low pH and high concentration of aluminum (Al; Ratter et al. 1997; Silva et al. 2013; Pellegrini 2016), which makes factors regulating nutrient dynamics especially important for fast growing forest trees. The different vegetation types of the Cerrado are composed of distinct plant species and functional traits (Ribeiro and Tabarelli 2002; Maracahipes et al. 2018). Major distinctions occur between the traits of species typical of closed-canopy environments (hereafter, forest species), with resource-acquisitive strategies (low resource use efficiency, high leaf nutrient concentration, high growth rate and high leaf area indices), and species of sunny environments (i.e., savanna species), which are usually resource-conservative (with the opposite characteristics of forest species) (Dantas et al. 2013; Miatto and Batalha 2016; Maracahipes et al. 2018). These differences likely result in high spatial variability in the amounts and quality of litter produced, with consequences for microbial activity, decomposition and, ultimately, vegetation-soil feedbacks. Indeed, it has been hypothesized that the high input of nutrient-rich and organic matter- rich litter by woody forest species increases soil fertility and favors the further development and expansion of forests into savanna areas (Silva et al. 2008, 2013; Paiva et al. 2015).
In this context, we performed a glasshouse and a field experiment in order to evaluate the effects of litter input on forest seedling growth rates in typical dystrophic Cerrado soils, and to examine the potential mechanisms involved, focusing on those mediated by decomposition and soil microbiota activity. In the glasshouse experiment, we evaluated the effects of litter input in the growth rate of seedlings of forest woody species commonly found in closed-canopy woodlands and/or forests of the Cerrado region. We also evaluated the effects of litter input in soil microbiota activity and biomass, using the same experimental design, but without planting woody seedlings in the pots. The litter used in these experiments was collected in the field and additionally used to determine litter functional traits for each litter species, in order to further assess potential mechanisms relating litter input and seedling growth. Finally, we performed a field experiment evaluating litter decay rates using the litterbag technique. For all these experiments, we used litter from 10 Cerrado species, divided in two groups according to the species’ natural occurrence (in savanna or forest). Through these experiments, we evaluated: i) the overall effect of litter input on forest seedling growth rates; and in soil microbiota activity and biomass; and ii) how well these effects were explained by litter species origin (from savanna or forest), and litter properties (both chemical litter traits and litter decay rates). We expected: i) an overall positive effect of litter input in forest seedling growth rates and soil microbiota biomass and activity due to the input of resources that were previously limiting with thus resulting in higher decomposition rates and nutrient release that positively affect plant growth rate (positive plant-soil feedback); ii) that these effects were associated most strongly with the litter of resource acquisitive species (i.e., forest species), with rapidly decomposing litter and litter with high concentrations of limiting nutrients (especially P and Ca); and iii) that these effects were less pronounced or even reversed (negative litter effect) for seedlings given litter from resource-conservative species (i.e., savanna species), and litter with high concentrations of phytotoxic and recalcitrant compounds, that result in slow decomposition rates.
Material and methods
Study area
Litter collection was carried out at two Cerrado reserves located in the State of São Paulo, Southeastern Brazil: one at the Municipality of São Carlos (21° 58' S and 47° 52' W) and the other at a reserve comprising the Municipalities of Itirapina and Brotas (Experimental and Ecological Station of Itirapina at 22° 00’ S and 47° 45’ W). The first reserve has approximately 125 ha and the second has approximately 2,300 ha, and are formed by gallery forest, typical savanna (locally called ‘cerrado sensu stricto’), wooded grassland (known as ‘campos sujos’) and short closed-canopy woodland (dense cerrados or ‘cerrado denso’). The regional climate of both areas is Cwa, according to the Köppen classification system, with mean annual precipitation of 1450 mm, with well-defined wet (October to March) and dry (April to September) seasons, and mean annual temperature of 20.8 ºC.
Litter was collected in short closed-canopy woodland Cerrado communities, a transitional vegetation of forest and savanna, which harbors a mixed woody flora containing typical shade-intolerant savanna, shade-tolerant forest, as well as more generalist shade-tolerant plant species (Bueno et al. 2018) all growing in the same environment (e.g., soil, microclimate). We collected litter from 10 woody species: five shade-intolerant savanna species and five shade-tolerant species (three forest and two generalist species, hereafter called “forest species” for simplicity), classified according to occurrence records in Sano et al. (2008; see Table 1 and Fig. S1). We chose pairs of shade tolerant and intolerant species from the same genus or family to minimize phylogenetic influences. This approach allowed the inclusion of leaves spanning a large range of light niches, and was directed at maximizing differences in litter traits.
We measured litter traits from freshly-senesced leaves (litter) collected from at least 20 individuals of each species. Individuals were selected systematically along a trail as the first visualized individuals, but respecting a distance of at least 10 m between individuals of the same species. The litter collection was performed at the end of the dry season (August, September and October, 2014). Litter sampling was performed through the placement of collection bags in the branches of the selected plants and also through the collection of freshly senesced litter (when we could unambiguously assign the litter to the selected plant). The collected material was dried at 25ºC for 72 h, and stored in sealed paper bags until use.
Litter chemical traits and decay rates
To study potential mechanisms explaining seedling and soil microbiota responses to the litter input and its characteristics, we measured litter traits related to species nutrient acquisition strategy – i.e., litter phenol, lignin, carbon (C) and nutrient concentrations. For this, we ground 200 g of dried litter collected from three individuals of each plant species using a ball mill (Geno/Grinder 2010 SPEX SamplePrep). The samples were sieved through 5 mm meshes, and stored. Phenol concentration was determined using the Folin-Ciocalteu extraction method (Graça et al. 2007). Lignin concentration was determined using Acid-Detergent method, obtaining the Klason lignin values following Graça et al. (2007). Carbon concentration was determined colorimetrically after oxidation with potassium dichromate (K2Cr2O7; Nelson and Sommers 1996). Nutrient analyses were based on subsamples of 10 g. Nitrogen concentration was determined colorimetrically after Kjeldahl digestion (Bremner 1996). Phosphorus concentration was determined by spectrophotometry after nitric percloric acid digestion (Motomizu and Oshima 1987). Manganese (Mn), potassium (K) and Ca concentrations were determined by atomic absorption spectrophotometry (Malavolta et al. 1997). The means of the three samples per species analyzed were determined and are shown in Table S1.
We also measured the decomposition rates of litter of each species, since these rates are related to the release of nutrients immobilized in dead tissue (Hobbie 2015). To determine the litter decomposition rates of each species, we placed litterbags in closed-canopy woodland Cerrado sites at the Experimental and Ecological Station of Itirapina in December 2014, following Graça et al. (2007). For each species, we added approximately 5 g of unfragmented senesced leaves from different individuals into 10 × 10 cm litterbags of 5 mm mesh. This mesh size allowed the access of litter by microorganisms, as well as meso and macro fauna (Makkonen et al. 2012). To maximize environmental heterogeneity, the bags were set in a randomized block design, setting five blocks with 50 litterbags (five replicates per species), in a total of 250 litterbags. We collected a bag in each block from each species after approximately 3, 4, 6, 8 and 10 months from the beginning of the decomposition experiment. After the collection of the litterbags, their contents were washed and dried at 70 ºC for 48 h to constant mass, and the dry mass recorded. We determined the decomposition rates for each species by applying the Olson (1963) model: Xt = X0 * e(−k * t); to the data, in which Xt is the predicted mass at time t; X0 is the initial mass converted to equivalent dried mass at 70ºC; and k is the Olson decomposition rate constant, a dimensionless parameter (Fig. S2).
Litter effects on seedling growth
In order to evaluate the effects of litter input on seedling growth rates, we performed a glasshouse experiment at the Biology Institute of the State University of Campinas (UNICAMP), Brazil. We used 15 L pots of 45 cm height, filled with soil collected in short closed-canopy woodland at the Experimental and Ecological Station of Itirapina (see Appendix Table S2 for soil features). For this, we prepared a plot of 9 m2 of which we discarded the top layer of soil (0 – 20 cm) and collected the soil from deep layer (20 – 50 cm) and used it for pot preparation. We collected the soil from deep layer to avoid legacy effects (Cuddington 2011), which could influence the seedlings growth rate and hinder the interpretation of the effects of specific litter addition in plants growth. Also, the soil microbiota would be inoculated in each pot through the input of microorganisms present in the surface of litter, colonizing the soil. Doing so, we could evaluate the net effect of specific litter input in seedlings and soil microbiota.
We prepared pots for 10 treatments (with litter of each species) and a control (without the addition of litter). For each treatment, we prepared five pots (replicates) in which we mixed the top soil of the pots with 100 g of litter (approximately 0.2 g of litter by g of soil), whose litter species varied according to the treatment. Since our aim was to investigate whether woody encroachment can specifically influence seedling growth through its chemical traits – i.e., nutrients and secondary metabolites input -, the litter material was manually fragmented prior to mixing in order to simulate a more advanced stage of physical fragmentation. After two weeks since litter input, at the center of each pot, we planted a seedling of one of three fast growing forest species also found in dense cerrado: Croton floribundus Spreng. (Euphorbiaceae), Tapirira guianensis Aubl. (Anacardiaceae) and Inga vera Willd. (Fabaceae). We obtained the plants from a seedling nursery, growing in propagation tubes (approximately 110 mL) with similar substrate. The seedlings had a similar time since germination, with and approximately height of 20 cm and a well-developed root system. The woody seedlings were used as phytometers, in order to evaluate the net effect of litter input in woody plants growth (Dietrich et al. 2014). We did not use savanna species because we were particularly interested in the consequences of litter input in the encroachment of savanna by forest trees and because savanna species are less responsive to differences in soil nutrient availability (Viani et al. 2011).
Plants were grown for approximately six months and were watered daily, simulating an entire growth season. Previous evidence suggest that plants already start to respond to litter addition after 3–4 month in these ecosystems (Villalobos-Vega et al. 2011). During the experiment, temperature and relative air humidity were monitored using a HOBO® U23 temperature and humidity data logger, protected inside a solar radiation shield. The mean glasshouse air temperature was 23.7ºC ± 5.5, and the mean relative air humidity was 68% ± 19.4. Every two weeks we measured seedling stem height (from the surface to the apical meristem) and diameter at soil height (DSH) on each individual. Based on DSH, height and dry total biomass from the seedlings harvested at the end of the experiment, we built allometric equations to estimate seedlings biomass during the experiment. We selected the best fitted equation for each species using Akaike’s Information Criterion (AIC; Fig. S3 and Table S3).
Litter effects on soil microbiota activity and biomass
We also measured the effects of litter addition in the soil microbiota activity and biomass. For this, we prepared pots adopting the same experimental design as described in previous section (see Litter effects on seedling growth), but without planting the seedlings, and used soil respiration rates as a proxy of soil microbiota activity (Singh and Gupta 1977). Soil respiration was estimated based on CO2 evolution (Singh and Gupta 1977) by inserting PVC tubes at the center of each pot to a depth of 5 cm and measuring CO2 efflux every three weeks using an infra-red gas analyzer EGM-4 (PP Systems®). Soil temperature in each pot was measured using the temperature probe of EGM-4 (PP Sytems®). Since soil moisture was kept high under constant and controlled glasshouse conditions, and was similar among pots, we did not monitor this variable. All measurements were performed between mid-day and 3:00 pm. We adjusted a Non-Linear Mixed Effect Model (NLME) on soil respiration rates against time for each litter species using a negative exponential model (Rt = Ri * exp (-R * T). In this model, Ri was the initial soil respiration rate, Rt was the soil respiration rate in day t, R was the soil respiration rate through time, and T was the incubation time. We then calculated the total heterotroph CO2 (referred now on as total C respired) that evolved from soil during the experiment, by plotting the soil respiration rate by time and calculating the area under the adjusted NLME model curve (Meier and Bowman 2008; Fig. S4).
To determine the effects of the litter input on soil microbial biomass, we collected, at the end of the experiment, the top 10 cm soil and sieved it, separating the soil from the remaining litter. Sieved soil was used to quantify C and N in the microbial biomass by the fumigation-extraction method (Vance et al. 1987). The microbial C biomass was determined by potassium sulfate extraction (Vance et al. 1987), and microbial N biomass by the ninhydrin method (Joergensen and Brookes 1990).
Statistical analyses
To evaluate the effects of litter addition on soil microbial activity, we performed an Analysis of Variance (ANOVA), considering the total C respired as response variable and litter identity as predictive variable. Then we performed a post-hoc Tukey’s honestly significant difference test (Tukey HSD) to identity which litter significantly affected the microbial biomass in relation to the control soil. Similar analyses were performed in order to evaluate the effects of litter addition in soil microbial C and N biomass.
A similar analysis was performed to evaluate the overall effects of litter input in seedlings growth. Firstly, we estimated the biomass relative growth rate of each individual. Since relative growth rate was non-linear and did not reach a plateau, we fitted the exponential model: Xt = X0 * e (t*r) (Paine et al. 2012) to the data, in which Xt is the estimated biomass of the seedlings at time t, X0 is the initial seedling estimated biomass, t is time in days and r is the relative growth rate. Then, we performed an ANOVA, considering the seedling biomass relative growth rate as response variable and litter species identity and seedling species as predictive variables. For the ANOVAs in which we have found significant influence of the predictive variables over the response variables, we performed a Tukey HSD in order to identify which litter significantly affected seedlings growth rate in relation to control seedlings.
To evaluate the potential mechanisms by which litter input affected seedlings growth rate and soil microbiota activity, we performed a correlation analysis using the Pearson’s correlation coefficient. We correlated litter chemical traits (C, N, P, K, Ca, Mn, phenol and lignin concentrations) with seedlings growth rate and and the heterotroph soil respiration and the litter decay rate. We did not consider the control seedlings and pots in these analyses.
To evaluate if the origin of litter species (savanna or forest) is related to its effects in seedlings and soil microbiota, we initially performed a principal component analysis (PCA) ordering the litter chemical traits (C, N, P, K, Ca, Mn, phenol and lignin concentrations) to visualize the relations among litter chemical traits and litter species origin. Then, we performed an ANOVA considering seedlings growth rate as response variable and litter origin and seedling species as predictive variables. A similar analysis was performed, considering soil heterotroph C respiration and litter decay rates as response variables, and litter origin as predictive variables. For these analyses we did not consider control pots.The analyses were performed in R v.4.0.0 (R Core Team 2020), using the packages ‘nlme’ (Pinheiro et al. 2020),, ‘emmeans’ (Lenth 2020), ‘ggplot2’ (Wickham 2016), ‘corrplot’ (Wei and Simko 2017) and reshape2 (Wickham 2007).
Results
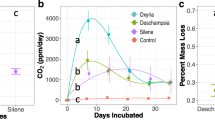
In our short-term glasshouse experiment (of approximately 6 months), litter input increased soil microbial activity (Table 2, Fig. 1a). The addition of litter increased the total heterotrophic C respired from soil for all litter species with the strongest effect in pots to which litter from T. formosa had been added (Fig. 1a). However, despite the increase in soil microbial activity, we did not observe an increase in microbial biomass in relation to control pots (Table 2, Fig. 1b, c). Regarding the effects on seedlings growth, litter input had an overall neutral or negative effect on growth rates in comparison with the control (i.e., no litter addition; Table 3, Fig. 2). In our field experiment, we observed wide variation in litter decay rates (Fig. S2). The decay rate constant (Olson’s k-value) varied from 0.0035, for T. formosa litter, to 0.00041, for V. tucanorum, corresponding to variation in the half-life of the litter from 188 to 1724 days.
Response of microbial activity (total carbon (C) respired from soil) and biomass (C and nitrogen (N)), to the addition of litter from different species. The total C respired from soil was estimated by the soil respiration rates of pots with different litter species input in a six-month glasshouse experiment. The different colors of the boxplot indicate the main occurrence of the litter species: White: Litter species mainly occurs in forests; Grey: Litter species mainly occurs in savannas. While the black boxplot indicates the response of microorganism in control pots (no litter addition). The (*, **) symbols indicates the litter species that significantly affected soil microbiota in relation to control pots, which was calculated through a post-hoc Tukey test. Boxplots with different symbols significantly differed from the others
Relation of the relative growth rate of three forest seedling to the input of litter from different species. A) The response of seedlings of Croton floribundus; B) The response of seedlings of Inga vera; C) The response of seedlings of Tapirira guianensis. The relative growth rate of seedlings was estimated by the adjusted exponential growth curve (Xt = X0 * e (t*r), in which r is the relative growth rate), based on its biomass gain in a six-month glasshouse experiment. The different colors of the boxplot indicate the main occurrence of the litter species: White: Litter species mainly occurs in forests; Grey: Litter species mainly occurs in savannas. While the black boxplot indicates the response of control seedlings (no litter addition). The (*) symbol indicates the litter species that significantly affected seedlings growth in relation to control seedlings, which was calculated through a post-hoc Tukey test
The responses of seedlings to litter input were varied by species. The growth rate of seedlings of I. vera, the fastest growing among the three forest species, was negatively affected by the addition of litter from six out of ten litter species, whilst for seedlings of C. floribundus and T. guianensis litter addition had no effect.
The two main proxies used to evaluate soil microbiota activity, litter decay rates and soil heterotroph respiration, showed a similar relation with litter chemical traits (Fig. 3). Specifically, both litter decay rate and soil heterotrophic respiration were positively related to litter C concentration (respectively: r = 0.94; p-value < 0.001; r = 0.71; p-value = 0.03) and negatively related to litter phenol concentration (respectively: r = -0.68; p-value = 0.04; r = -0.77; p-value = 0.02). However, soil heterotrophic respiration was also positively related with litter P concentration (r = 0.76; p-value = 0.02). On the other hand, the response of seedling growth rate to litter input was weakly correlated with litter traits and the relations between litter traits and seedling growth varied according to species (Fig. 3). For instance, with I. vera growth rates were positively related to litter N concentration (r = 0.30; p-value = 0.04), while with C. floribundus growth rate was negatively correlated with litter K concentration (r = -0.31; p-value = 0.03; Fig. 3); and the growth rate of T. guianensis was not correlated with any of the litter traits measured (Fig. 3).
Heatmap showing the correlations between soil microbiota activity – i.e., soil heterotroph respiration, litter decay rates – and the three species seedlings growth rate -i.e., Croton floribundus, Tapirira guianensis and Inga vera – with litter chemical traits of the ten litter species studied. Asterisks represents significative correlations (Pearson’s r coefficient: * < 0.05), which could be positive (red) or negative (blue)
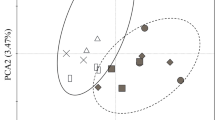
The litter species used cover a wide range of variation in litter chemical traits (Fig. 4). However, the litter chemical traits of savanna and forest species did not differ, as initially expected (Fig. 4), as also seen in the lack of significant difference in the effects of litter from forest vs. savanna species in seedling growth rates (Table 4, Fig. 5) and soil microbial activity and biomass (Table 5, Fig. 6).
Principal component analysis (PCA) ordination of 9 litter traits (arrows) from the 10 studied species. The species were grouped according to their habitat of occurrence. The traits were centered and standardized prior to the analysis. The litter traits used were: [N], nitrogen concentration (mg g−1); [P], phosphorus concentration (mg g−1); [K] potassium concentration (mg g−1); [Ca] calcium concentration (mg g−1); [Mg] magnesium concentration (mg g−1); [C] carbon concentration (mg g−1); [Phenols], phenol concentration (mg g−1); [Lignin], lignin concentration (g g−1). The italic names represent the litter species to which the points correspond
Relation between the relative growth rate of three forest seedling to the input of litter from different origin (savanna and forest). A) The response of seedlings of Croton floribundus; B) The response of seedlings of Inga vera; C) The response of seedlings of Tapirira guianensis. The relative growth rate of seedlings was estimated by the adjusted exponential growth curve (Xt = X0 * e (t*r), in which r is the relative growth rate), based on its biomass gain in a six-month glasshouse experiment. In this analysis we did not consider control pots
Response of microbial activity (total carbon (C) respired from soil) and biomass (C and nitrogen (N)), to the addition of litter from different origins (savanna and forest). The total C respired from soil was estimated by the soil respiration rates of pots with different litter species input in a six-month glasshouse experiment
Discussion
In this study, we evaluated if the input of litter from different species could promote the growth of woody seedlings growing in dystrophic savanna soils, and if the effects of litter input on growth rates were related to the chemical traits in the litter, and to the provenance of the litter species – i.e., savanna or forest. In order to examine a potential mechanism by which litter accumulation and seedling growth rate are related, we also tested for an effect of litter addition on the activity of soil microbiota. Litter input directly affected soil microbiota, promoting activity mainly through the input of C- and P-rich organic matter. On the other hand, litter input had either no effect or hindered the growth of woody seedlings growing in savanna dystrophic soil. Although litter addition could negatively influence forest tree seedling growth, the growth rates of forest tree seedlings were weakly correlated with some litter chemical traits. This suggests that the litter input process did not result in a simple addition of limiting resources or phytotoxic compounds. Instead, the impact on plant growth is likely to have been indirect, potentially mediated by the activities of soil microbiota. Finally, we found no evidence that litter origin influenced the responses of either soil microbiota or plants. Our experimental evidence thus suggests that woody encroachment of new species into savannas vegetation in the Cerrado biome is unlikely to influence nutrient dynamics via the chemical traits of the new accumulated litter.
The effects of litter addition in soil microbial activity and biomass
In our study, litter input stimulated soil microbial activity potentially through the direct input of limiting resources, as litter is an important substrate for soil microbiota (Cotrufo et al. 2013; Zechmeister-Boltenstern et al. 2015). Previous reports for Cerrado and in other ecosystems occurring over dystrophic soils have identified nutrient limitation of soil microbiota, especially for N and P, with metabolic activity stimulated by the input of nutrient-rich litter (Resende et al. 2011; Zechmeister-Boltenstern et al. 2015). In these cases, nutrient-rich litter is preferentially consumed by soil microbiota, decomposing faster than nutrient-poor or recalcitrant litter (Zhang et al. 2008; Cornwell et al. 2008); and we consistently observed soil microbiota activity and litter decay rates to be related to the same litter chemical traits in both the field and the glasshouse experiments.
Our data indicate that soil microbiota activity was not related to litter N and lignin, but was correlated with litter C, P and phenol concentration. This result partially contradicts findings elsewhere of key roles for N and lignin in regulating litter decay rates (Cornwell et al. 2008), but it is consistent with resource availability relating instead to C, P and phenol concentrations being a key driver of litter decay rate and stimulation of soil microbial activity (Zechmeister-Boltenstern et al. 2015). In our study, the observed soil respiration positive correlation with litter C and P, and negative correlation with litter phenol; and litter decay rates positive correlation with litter C and negative correlation with litter phenol, might be related to three non-exclusive mechanisms explaining their control over microbial activity: (1) the soil microbial community is energetically limited and its activity is stimulated by the input of labile C-rich organic matter (Hättenschwiler and Jørgensen 2010; Makkonen et al. 2012; Whitaker et al. 2014); (2) respiration rates are constrained by recalcitrant phenol compounds (Hättenschwiler and Vitousek 2000; Dunn and Freeman 2018); and (3) the soil microbial community is more P-limited than N-limited, as frequently observed in Cerrado (Reich and Oleksyn 2004; Kozovits et al. 2007; Jacobson et al. 2011; Bustamante et al. 2012; Dionizio et al. 2018; Abrahão et al. 2019), with the addition of P-rich litter potentially increasing its activity and the turnover of organic matter.
The effects of litter input in soil microbiota and the relationships with the chemical traits in litter have important implications for nutrient cycling in the Cerrado. Firstly, litter that is rich in C and P is rapidly decomposed, indicating a fast mineralization of P immobilized in plant dead tissues. Secondly, the relation between soil microbiota activity with litter C and P indicates that one of these resources are preferentially re-immobilized in microbial biomass, becoming unavailable for plant uptake until remineralization (Bengtson and Bengtsson 2005; Manzoni et al. 2010; Čapek et al. 2018). Soil microbes are usually limited by either energy or nutrients, and these limitations have distinct consequences to nutrient cycling of the ecosystem (Manzoni et al. 2010; Čapek et al. 2018). Thus, two potential mechanisms of soil microbiota influences on nutrient cycling in Cerrado can be expected based on our observations: i) soil microbiota is mainly energy limited, resulting in preferential immobilization of C in microbial biomass and mineralization of nutrients, such as P; ii) soil microbiota is mainly P limited, resulting in preferential immobilization of P in microbial biomass and mineralization (respiration) of C. We could not evaluate which of these mechanisms are occurring, as we do not have data on mineral nutrients before and after the experiment. Thus, further studies should evaluate how litter input increases P immobilization in soil microbial biomass and the dynamics of re-mineralization of this nutrient in field conditions.
The effects of litter input on the growth of woody seedlings
The overall neutral and/or negative effect of litter input on seedling growth rates is consistent with previous reports (Xiong and Nilsson 1999; Lopez-Iglesias et al. 2014; Gavinet et al. 2018; Sarker et al. 2020). However, the mechanisms related to these effects could not be fully identified based on our experiment. Litter can affect plant growth directly, through the input of phytotoxic compounds that inhibit root growth (Bonanomi et al. 2011; Lopez-Iglesias et al. 2014), or indirectly, through the stimulation of soil microbial activity (i.e., priming effects, Van Der Heijden et al. 2008; Čapek et al. 2018). Even though we could not confirm these mechanisms, our data highlight the potential importance of the soil microbiota in mediating the relation between litter and plants due to the following observations in our experiment: i) the lack of correlation between seedling growth and litter chemical traits, especially phenolic compounds (which would be expected if the litter phytotoxicity accounted for the observed effects); ii) the stimulation of soil microbiota activity by litter input (increased soil heterotroph respiration rate due to the litter addition); iii) the direct correlations between soil microbiota activity and litter traits, specifically, litter C, P and phenol. Further analysis of mineralization-immobilization dynamics and the net mineralization of nutrients (in plant and microbial biomass) should thus enable a better understanding of how litter input affects nutrient availability for plants.
Implications for forest encroachment in dystrophic savannas
Villalobos-Vega et al. (2011) reported an overall null effect of litter manipulation on woody trees in Cerrado, demonstrating that litter input did not influence soil fertility or the growth of five out of six savanna species, even after four years (Villalobos-Vega et al. 2011). Our study was consistent with this by showing that the litter properties from savanna and forest species are not essentially different in their chemical traits or in how they affect plant growth. However, in contrast to our results, Villalobos-Vega et al. (2011) observed that their fastest growing species were positively affected by litter addition, likely reflecting the effect of litter in that study on reducing water loss from soil (Villalobos-Vega et al. 2011; Xiong and Nilsson 1999; Scalon et al. 2014).
The fastest growing species in this study, I. vera, was from forest and was also more responsive (negatively) to litter addition than the slower-growing species. This is consistent with the general pattern of fast-growth species being more responsive to stimuli (Viani et al. 2011; Freschet et al. 2013), but further study is clearly needed to combine nutrient, phytotoxicity and water dynamics with seedling growth strategy to understand the potential impacts of encroachment and changing litter inputs on ecosystem dynamics in dystrophic tropical savannas (Veen et al. 2019; Gundale and Kardol 2021).
Finally, we note that future studies on the role of changing litter properties on forest-savanna dynamics should also include the responses of grasses to new litter input, since the grass layer is essential to keep the ecosystem in its open fire-prone state (Hoffmann et al. 2012). Rapid grass growth in response to new litter would hinder woody encroachment processes but reduced grass growth responses might favour them. Thus, integrating the responses of both woody seedlings and grasses to changing litter inputs will ultimately be needed to understand ecosystem dynamics in this key vegetation transition zone.
Conclusions
In regions in which forest and savanna coexist as landscape mosaics, forests have been shown to produce larger amounts of litter than savannas and with higher concentration of nutrients (Silva et al. 2008, 2013; Paiva et al. 2015). This high input of nutrients and organic matter in forest litter has been suggested to increase soil fertility, which could favor woody encroachment in savannas areas (Silva et al. 2008, 2013; Paiva et al. 2015). Our study tested this process experimentally and contributes to a better understanding of the effects of litter accumulation on the dynamics of nutrients and vegetation.
Our results identify a key role of C and P in nutrient cycling dynamics in the Brazilian Cerrado, although the mechanisms by which they are affected by litter addition appear to be indirect, mediated by the soil microbiota. The positive relation between litter C and P with soil microbial activity, either measured as soil respiration or litter decay rates, indicates a potential C-supply (i.e., energetic) or P-limitation of the soil microbial community. Furthermore, and consistent with earlier work, our results suggest that litter addition alone does not generally benefit woody seedling growth, and may indeed increase fire risk if grass growth rates are enhanced at the same time.
Data availability
Part of the data is available as supplementary electronic material.
Code availability
Not applicable.
References
Abrahão A, de Costa P, B, Lambers H, et al (2019) Soil types filter for plants with matching nutrient-acquisition and -use traits in hyperdiverse and severely nutrient-impoverished campos rupestres and Cerrado in Central Brazil. J Ecol 107:1302–1316. https://doi.org/10.1111/1365-2745.13111
Bengtson P, Bengtsson G (2005) Bacterial immobilization and remineralization of N at different growth rates and N concentrations. FEMS Microbiol Ecol 54:13–19. https://doi.org/10.1016/j.femsec.2005.02.006
Bonanomi G, Cesarano G, Lombardi N et al (2017) Litter chemistry explains contrasting feeding preferences of bacteria, fungi, and higher plants. Sci Rep 7:9208. https://doi.org/10.1038/s41598-017-09145-w
Bonanomi G, Incerti G, Mazzoleni S (2011) Assessing occurrence, specificity, and mechanisms of plant facilitation in terrestrial ecosystems. Plant Ecol 212:1777. https://doi.org/10.1007/s11258-011-9948-5
Bremner JM (1996) Nitrogen—Total. Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatai MA, Johnston CT, Sumner ME, editors. Methods of soil analysis, Part 3— Chemical methods. Wisconsin: Soil Science Society of America, 1085–1121.
Bueno ML, Dexter KG, Pennington RT et al (2018) The environmental triangle of the Cerrado Domain: Ecological factors driving shifts in tree species composition between forests and savannas. J Ecol 106:2109–2120. https://doi.org/10.1111/1365-2745.12969
Bustamante MMC, de Brito DQ, Kozovits AR et al (2012) Effects of nutrient additions on plant biomass and diversity of the herbaceous-subshrub layer of a Brazilian savanna (Cerrado). Plant Ecol 213:795–808. https://doi.org/10.1007/s11258-012-0042-4
Čapek P, Manzoni S, Kaštovská E et al (2018) A plant–microbe interaction framework explaining nutrient effects on primary production. Nat Ecol Evol 2:1588–1596. https://doi.org/10.1038/s41559-018-0662-8
Cornwell WK, Cornelissen JHC, Amatangelo K et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Cotrufo MF, Wallenstein MD, Boot CM et al (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob Change Biol 19:988–995. https://doi.org/10.1111/gcb.12113
Cuddington K (2011) Legacy effects: the persistent impact of ecological interactions. Biol Theory 3:203–210. https://doi.org/10.1007/s13752-012-0027-5
Dantas V de L, Batalha MA, Pausas JG (2013) Fire drives functional thresholds on the savanna–forest transition. Ecology 94:2454–2463. https://doi.org/10.1890/12-1629.1
Dantas V de L, Hirota M, Oliveira RS, et al (2016) Disturbance maintains alternative biome states. Ecol Lett 19:12–19. https://doi.org/10.1111/ele.12537
Dietrich AL, Lind L, Nilsson C, Jansson R (2014) The use of phytometers for evaluating restoration effects on riparian soil fertility. J Environ Qual 43:1916–1925. https://doi.org/10.2134/jeq2014.05.0197
Dionizio EA, Costa MH, Castanho AD de A, et al (2018) Influence of climate variability, fire and phosphorus limitation on vegetation structure and dynamics of the Amazon–Cerrado border. Biogeosciences (Online) 15. https://doi.org/10.5194/bg-15-919-2018
Dunn C, Freeman C (2018) The role of molecular weight in the enzyme-inhibiting effect of phenolics: the significance in peatland carbon sequestration. Ecol Eng 114:162–166. https://doi.org/10.1016/j.ecoleng.2017.06.036
Durigan G, Ratter JA (2006) Successional changes in cerrado and cerrado/forest ecotonal vegetation in western São Paulo state, Brazil, 1962–2000. Edinburgh Journal of Botany 63:119–130. https://doi.org/10.1017/S0960428606000357
Facelli JM, Pickett STA (1991) Plant litter: Its dynamics and effects on plant community structure. Bot Rev 57:1–32. https://doi.org/10.1007/BF02858763
Freschet GT, Cornwell WK, Wardle DA et al (2013) Linking litter decomposition of above- and below-ground organs to plant–soil feedbacks worldwide. J Ecol 101:943–952. https://doi.org/10.1111/1365-2745.12092
Gavinet J, Prévosto B, Bousquet-Melou A et al (2018) Do litter-mediated plant-soil feedbacks influence Mediterranean oak regeneration? A two-year pot experiment. Plant Soil 430:59–71. https://doi.org/10.1007/s11104-018-3711-9
Graça MAS, Bärlocher F, Gessner MO (2007) Methods to study litter decomposition: A practical guide. Springer, Dordrecht
Gonçalves RVS, Cardoso JCF, Oliveira PE, Oliveira DC (2021) Changes in the Cerrado vegetation structure: insights from more than three decades of ecological succession. Web Ecology 21:55–64. https://doi.org/10.5194/we-21-55-2021
Gundale MJ, Kardol P (2021) Multi-dimensionality as a path forward in plant-soil feedback research. J Ecol n/a: https://doi.org/10.1111/1365-2745.13679
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest: Decomposition in a tropical rain forest. J Ecol 98:754–763. https://doi.org/10.1111/j.1365-2745.2010.01671.x
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243. https://doi.org/10.1016/S0169-5347(00)01861-9
Hirota M, Holmgren M, Nes EHV, Scheffer M (2011) Global resilience of tropical forest and savanna to critical transitions. Science 334:232–235. https://doi.org/10.1126/science.1210657
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Hoffmann WA, Geiger EL, Gotsch SG et al (2012) Ecological thresholds at the savanna-forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol Lett 15:759–768. https://doi.org/10.1111/j.1461-0248.2012.01789.x
Hovstad KA, Ohlson M (2008) Physical and chemical effects of litter on plant establishment in semi-natural grasslands. Plant Ecol 196:251–260. https://doi.org/10.1007/s11258-007-9349-y
Jacobson TKB, da Bustamante MM, C, Kozovits AR, (2011) Diversity of shrub tree layer, leaf litter decomposition and N release in a Brazilian Cerrado under N, P and N plus P additions. Environ Pollut 159:2236–2242. https://doi.org/10.1016/j.envpol.2010.10.019
Jilani G, Mahmood S, Chaudhry AN et al (2008) Allelochemicals: sources, toxicity and microbial transformation in soil —a review. Ann Microbiol 58:351–357. https://doi.org/10.1007/BF03175528
Joergensen RG, Brookes PC (1990) Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 m K2SO4 soil extracts. Soil Biol Biochem 22:1023–1027. https://doi.org/10.1016/0038-0717(90)90027-W
Kozovits AR, Bustamante MMC, Garofalo CR et al (2007) Nutrient resorption and patterns of litter production and decomposition in a Neotropical Savanna. Funct Ecol 21:1034–1043. https://doi.org/10.1111/j.1365-2435.2007.01325.x
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Lenth L (2020). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.8. https://CRAN.R-project.org/package=emmeans
Lopez-Iglesias B, Olmo M, Gallardo A, Villar R (2014) Short-term effects of litter from 21 woody species on plant growth and root development. Plant Soil 381:177–191. https://doi.org/10.1007/s11104-014-2109-6
Makkonen M, Berg MP, Handa IT et al (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041. https://doi.org/10.1111/j.1461-0248.2012.01826.x
Malavolta E, Vitti GC, Oliveira SA (1997) Evaluation of plant nutritional status: principles and applications. 2nd ed., POTAFÓS, Piracicaba, Brazil. 319p
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106. https://doi.org/10.1890/09-0179.1
Maracahipes L, Carlucci MB, Lenza E et al (2018) How to live in contrasting habitats? Acquisitive and conservative strategies emerge at inter- and intraspecific levels in savanna and forest woody plants. Perspect Plant Ecol Evol Syst 34:17–25. https://doi.org/10.1016/j.ppees.2018.07.006
Meier CL, Bowman WD (2008) Phenolic-rich leaf carbon fractions differentially influence microbial respiration and plant growth. Oecologia 158:95–107. https://doi.org/10.1007/s00442-008-1124-9
Miatto RC, Batalha MA (2016) Leaf chemistry of woody species in the Brazilian cerrado and seasonal forest: response to soil and taxonomy and effects on decomposition rates. Plant Ecol 217:1467–1479. https://doi.org/10.1007/s11258-016-0658-x
Motomizu S, Oshima M (1987) Spectrophotometric determination of phosphorus as orthophosphate based on solvent extraction of the ion associate of molybdophosphate with malachite green using flow injection. Analyst 112:295–300. https://doi.org/10.1039/AN9871200295
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis, Part 3— Chemical methods. Soil Science Society of America, Wisconsin, pp 961–1010
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331. https://doi.org/10.2307/1932179
Paine CET, Marthews TR, Vogt DR et al (2012) How to fit nonlinear plant growth models and calculate growth rates: an update for ecologists. Methods Ecol Evol 3:245–256. https://doi.org/10.1111/j.2041-210X.2011.00155.x
Paiva AO, Silva LCR, Haridasan M (2015) Productivity-efficiency tradeoffs in tropical gallery forest-savanna transitions: linking plant and soil processes through litter input and composition. Plant Ecol 216:775–787. https://doi.org/10.1007/s11258-015-0466-8
Parr CL, Gray EF, Bond WJ (2012) Cascading biodiversity and functional consequences of a global change–induced biome switch. Diversity and Distributions 18:493–503. https://doi.org/10.1111/j.1472-4642.2012.00882.x
Pellegrini AFA (2016) Nutrient limitation in tropical savannas across multiple scales and mechanisms. Ecology 97:313–324. https://doi.org/10.1890/15-0869.1
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2020). _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1–147, URL: https://CRAN.R-project.org/package=nlme
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Ratter JA, Ribeiro JF, Bridgewater S (1997) The Brazilian Cerrado vegetation and threats to its biodiversity. Ann Bot 80:223–230. https://doi.org/10.1006/anbo.1997.0469
Reich PB, Oleksyn J (2004) Global patterns of plant leaf N and P in relation to temperature and latitude. Proc Natl Acad Sci 101:11001–11006. https://doi.org/10.1073/pnas.0403588101
Resende JCF, Markewitz D, Klink CA et al (2011) Phosphorus cycling in a small watershed in the Brazilian Cerrado: impacts of frequent burning. Biogeochemistry 105:105–118. https://doi.org/10.1007/s10533-010-9531-5
Ribeiro LF, Tabarelli M (2002) A structural gradient in cerrado vegetation of Brazil: changes in woody plant density, species richness, life history and plant composition. J Trop Ecol 18:775–794. https://doi.org/10.1017/S026646740200250X
Sano SM, Almeida SP, Ribeiro JF (2008). Cerrado: Ecologia e flora (vol. 2). Brasilia: Embrapa.
Sarker TC, Maisto G, Marco AD et al (2020) Species-specific root proliferation of tree seedlings in tropical litter: do nutrients matter? Oikos 129:598–606. https://doi.org/10.1111/oik.06786
Scalon MC, Rossatto DR, Franco AC (2014) Do litter manipulations affect leaf functional traits of savanna woody plants? Plant Ecol 215:111–120. https://doi.org/10.1007/s11258-013-0282-y
Schimel JP, Bennett J (2004) Nitrogen mineralization: Challenges of a changing paradigm. Ecology 85:591–602. https://doi.org/10.1890/03-8002
Silva LCR, Hoffmann WA, Rossatto DR et al (2013) Can savannas become forests? A coupled analysis of nutrient stocks and fire thresholds in central Brazil. Plant Soil 373:829–842. https://doi.org/10.1007/s11104-013-1822-x
Silva LCR, Sternberg L, Haridasan M et al (2008) Expansion of gallery forests into central Brazilian savannas. Glob Change Biol 14:2108–2118. https://doi.org/10.1111/j.1365-2486.2008.01637.x
Singh JS, Gupta SR (1977) Plant decomposition and soil respiration in terrestrial ecosystems. Botanical Reviews 43:449–528. https://doi.org/10.1007/BF02860844
Staver AC, Archibald S, Levin SA (2011) The global extent and determinants of savanna and forest as alternative biome states. Science 334:230–232. https://doi.org/10.1126/science.1210465
Stevens N, Lehmann CER, Murphy BP, Durigan G (2017) Savanna woody encroachment is widespread across three continents. Global Change Biology 23:235–244. https://doi.org/10.1111/gcb.13409
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707. https://doi.org/10.1016/0038-0717(87)90052-6
Veen GF, Fry EL, ten Hooven FC, et al (2019) The role of plant litter in driving plant-soil feedbacks. Front Environ Sci 7. https://doi.org/10.3389/fenvs.2019.00168
Viani RAG, Rodrigues RR, Dawson TE, Oliveira RS (2011) Savanna soil fertility limits growth but not survival of tropical forest tree seedlings. Plant Soil 349:341–353. https://doi.org/10.1007/s11104-011-0879-7
Villalobos-Vega R, Goldstein G, Haridasan M, et al (2011) Leaf litter manipulations alter soil physicochemical properties and tree growth in a Neotropical savanna. Plant Soil 346:385. https://doi.org/10.1007/s11104-011-0860-5
Warman L, Moles AT (2009) Alternative stable states in Australia’s Wet Tropics: a theoretical framework for the field data and a field-case for the theory. Landscape Ecol 24:1–13. https://doi.org/10.1007/s10980-008-9285-9
Wei T, Simko V (2017). R package "corrplot": Visualization of a Correlation Matrix (Version 0.84). Available from https://github.com/taiyun/corrplot
Wickham H (2007). Reshaping data with the reshape package. J Statistic Softw, 21(12), 1–20. URL http://www.jstatsoft.org/v21/i12/.
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Whitaker J, Ostle N, Nottingham AT et al (2014) Microbial community composition explains soil respiration responses to changing carbon inputs along an Andes-to-Amazon elevation gradient. J Ecol 102:1058–1071. https://doi.org/10.1111/1365-2745.12247
Xiong S, Nilsson C (1999) The effects of plant litter on vegetation: a meta-analysis. J Ecol 87:984–994. https://doi.org/10.1046/j.1365-2745.1999.00414.x
Zechmeister-Boltenstern S, Keiblinger KM, Mooshammer M et al (2015) The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol Monogr 85:133–155. https://doi.org/10.1890/14-0777.1
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93. https://doi.org/10.1093/jpe/rtn002
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. We are grateful for AL Mansur, R Pereira and L Quimbayo for the help in the fieldwork and in the preparation of the glasshouse experiment. We also thanks D Mescolotti and F Picollo for the help in the laboratory analysis. RSO acknowledges funding from CNPq (productivity grant) and NERC-FAPESP 19/07773-1 research grant.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
André D’Angioli, Vinicius Dantas and Rafael Oliveira contributed to the study conception and design. Experiment preparation and data collection were performed by André D’Angioli, with substantial support in methodological procedure by all authors. The data analysis, interpretation and manuscript writing were led by André D’Angioli, with substantial contribution of all authors. All authors read and approved the final manuscript.
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Responsible Editor: Alfonso Escudero.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
D’Angioli, A.M., Dantas, V.L., Lambais, M. et al. No evidence of positive feedback between litter deposition and seedling growth rate in Neotropical savannas. Plant Soil 469, 305–320 (2021). https://doi.org/10.1007/s11104-021-05163-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05163-w