Abstract
Backgrounds
There are growing concerns regarding the restoration of karst rocky desertification (KRD) areas. However, the soil conditions and its residing microorganisms, which are essential for the plants, remain largely unclear.
Methods
We studied soil characteristics and microbial communities in natural forests (non-KRD) and shrubs with eroded soil and surface soil run-off, using Illumina Miseq sequencing.
Results
Our results showed that despite KRD reduced soil fertility and altered microbial community structures, microbial diversity did not diminish. Interestingly, bacterial OTU richness and diversity were greater in the KRD areas than in the non-KRD areas, which had relatively greater plant density and diversity. Fungal OTU richness and diversity remained unchanged by KRD. Although the KRD areas had been clear-cut and trees were mostly absent, ectomycorrhizal fungi did not differ in diversity and relative abundance between the two land types, indicating that the KRD shrubs hosted surprisingly diverse and abundant ectomycorrhizal fungi.
Conclusions
Our results highlight the highly diverse microbes under environmental and anthropogenic stresses in KRD areas. Despite the fact that degraded soil properties and an altered microbial community structure remain, KRD did not erode ectomycorrhizal fungal species richness, which is crucial in the revegetation of trees in KRD areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil degradation harms many areas of the planet, particularly in Mediterranean area and south-west China, where the anthropogenic activities and climate changes are responsible for the increasing desertification (Bastida et al. 2010; Zhang et al. 2017). The severity of soil degradation has been evaluated by comparing changes in selected soil properties. For example, soil fertility decreases along with processes of urbanization (Wang et al. 2018), conversions of primary forest to agricultural field (Owuor et al. 2018) and aggravations of rocky desertification (Li et al. 2018; Xie et al. 2015). Karst landscapes, representing approximately 13% of the world’s land surface, are mostly formed from the dissolution of soluble rocks such as limestone, dolomite, and gypsum (Chen et al. 2017). Rocky desertification, a process of land degradation, poses a potential threat to the environment and ecosystem in karst areas (Yuan 1997). Besides lithological and climatological conditions, karst rocky desertification (KRD) often results from intensive and irrational land use (e.g. clear-cutting of natural forest) that causes geo-ecological destruction in fragile karst environments (Wang et al. 2004; Yan and Cai 2015). KRD is one of the most critical forms of land degradation, covering over 1,071,600 km2 in the world (Zhang et al. 2016), presenting a great threat to sustainable development and human survival.
Recent studies on KRD have shown that rocky desertification reduced soil quality and plant diversity (Dai et al. 2018; He et al. 2008; Qi et al. 2017; Sheng et al. 2018). However, much less attention has been paid to the soil microorganisms in KRD areas, which are essential in vegetation maintenance and environmental restoration. Scattered evidence has reported that KRD and non-KRD soils are different in bacterial community structures (Qi et al. 2018; Xue et al. 2017). Yet, fungal communities of KRD area have received much less attention. Despite a significant impact of KRD on soil fungal community profile has been determined using PCR-DGGE (Wang et al. 2016), detailed fungal community changes remain largely unknown, particularly the changes of tree growth promoter - ectomycorrhizal fungi (ECM).
Soil pH, total carbon (TC) and total nitrogen (TN) are major factors correlating with microbial communities in various ecosystems (Bahram et al. 2018; Fierer and Jackson 2006; Peay et al. 2017; Van Der Linde et al. 2018), including KRD areas (Qi et al. 2018). The accumulation of carbon was the core factor contributing to phylogenetic and catabolic diversity of soil microbial communities in karst ecosystems (Zhu et al. 2012). Soil-inhabiting microbes are certainly important to the KRD area as they mediate many essential soil ecosystem services and functions e.g. nutrient cycling, maintaining plant health and biomass production, and pedogenesis (Harris 2009; Jonsson et al. 2001; Nannipieri et al. 2003; Van Der Heijden et al. 2008). Interactions between soil microbial communities and soil conditions determine the functions, resilience and stability of ecosystems (Francini et al. 2018; Hui et al. 2017a). However, these interactions are largely unclear in the fragile KRD ecosystems.
Besides the effect of soil properties on microorganisms, plants can directly shape soil microbial community structure and influence its diversity (Hui et al. 2017a; Tedersoo et al. 2008). Plant diversity is usually positively correlated with soil microbial diversity in various environments (Prober et al. 2015), including natural karst areas (He et al. 2008). KRD significantly reduces plant diversity, with different plant traits being found there (Jiang et al. 2014). KRD may thus adversely influence microbial communities. Those microbes that are closely associated with plants, e.g. ECM fungi, are likely diminished, as ECM-associated trees do not grow well in KRD areas (Jiang et al. 2014). Indeed, restoration of KRD areas mainly relies on the return of trees, and therefore it is important to understand the in situ tree boosting microorganisms which facilitate host nutrient acquisition (Velmala et al. 2014) and protect them against soil pathogens (Laliberté et al. 2015).
In south-western China, the KRD area of Yunnan Province covers 33,300 km2, approximately 8.6% of the total land area of the province (Jiang et al. 2014). In Mengzi, a typical karst area located in Yunnan, anthropogenic disturbances, e.g. forest clear-cutting, grazing and tilling, have pushed primary forests to the KRD shrubs during 1960s to 1990s. To collect information for the future KRD area restoration, we studied edaphic conditions and screened soil microbial communities in primary forests and rocky desertified secondary shrubs in Mengzi. We also focused on the tree growth promoters – ECM fungal communities and discussed their potentials for reforestation of the KRD area. The aim of this work was to determine the effects of KRD on soil characteristics and microbial communities. We hypothesized that (i) soil microbial diversity is greater in non-KRD area (natural karst forest) than in KRD area (degraded karst shrubs), because there are more plant species/functional groups that produce divergent substrates/resources in forests than in shrubs; (ii) soil microbial community composition differs between non-KRD and KRD areas, because soil properties and vegetation, which affect soil microorganisms, are often different between the two land types (Peng and Wang 2012); (iii) KRD poses a potential threat to ECM fungi commonly associated with trees, because KRD results in shifts in vegetation types (Jiang et al. 2014).
Materials and methods
Study area
Our study area, a typical karst zone, is located in the vicinities of two villages: Xibeile (23° 48′ N, 103° 45′ E) and Zhicun (23° 34′ N, 103° 53′ E) near the city of Mengzi in south Yunnan, China. The area experiences a subtropical monsoon climate with an annual mean temperature of 18.6 °C (https://en.climate-data.org). April through August accounts for approximately 70% of the annual precipitation of 815.8 mm. Severe droughts occur frequently during other periods of the year. In this area, soil is classified as mollic inceptisols, with a depth between 0 and 20 cm. The karst geomorphology and harsh climate result in surface soil run-off in the area. Together with anthropogenic activities, e.g. timber harvesting (clear-cutting), grazing and tilling, KRD is intensively used. Currently the natural undisturbed primary forests are often scattered and still decreasing.
In September 2017, we sampled 28 sites (Fig. 1) in the vicinity of Xibeile and Zhicun (14 sites per village), representing two land types (14 sites per type): (i) the mixed evergreen broadleaf forests (oldest trees >100 years old) with natural undisturbed soil and vegetation (non-KRD), and (ii) the shrubs with heavily eroded soil and surface soil run-off (KRD), which were clear-cut from forests. The sampling sites were selected in discrete forest/shrub patches, with at least 1 km between them. The non-KRD forest mainly consists of trees (Craigia yunnanensis, Lithocarpus confinis and Toxicodendron vernicifluum as dominant trees), shrubs (Coriaria nepalensis, Rosa spp.) and herbs (Heteropogon contortus and Rubia cordifolia). Among the trees, Craigia and Lithocarpus are ECM fungi host plants (Tedersoo and Brundrett 2017), while Toxicodendron has solely arbuscular mycorrhizas (Cornwell et al. 2001). The KRD shrubs also comprised the same plant species, e.g. C. yunnanensis and L. confinis, as those in the non-KRD sites, but they were mostly in the form of shrubs. Other plant species in the KRD sites were the shrubs Spiraea salicifolia, Lonicera japonica and Smilax china and the herbs Artemisia annua, H. contortus and Inula cappa.
Soil sampling and analyses
The sampling plots in the two land type categories (non-KRD and KRD) were chosen randomly close to a L. confinis tree/shrub. In each of these plots, one soil sample (pooled by three subsampled soil cores) was collected using a soil corer (2.5 cm diameter, depth 0–10 cm excluding Oi-layer) 1–2 m from the trunk of the tree/shrub. This made 7 samples per land type per village (28 samples across the whole study). To avoid age effects, we selected young trees/shrubs, with age range from 5 to 11 years (estimated by a local forest expert according to tree/shrub size and land management history) in both the non-KRD and KRD areas. We kept soil samples in polyethylene bags on dry ice in the field until being frozen at −20 °C in the laboratory. The samples were thawed at room temperature (for chemical analyses) and at +4 °C (for DNA analyses), and sieved, using 2 mm mesh size, to remove stones, roots and large particles. Edaphic conditions were determined for all samples. Soil pH was measured by adding 2 g of soil into 20 ml of 0.01 M CaCl2. TC and TN were determined by dry combustion at 1350 °C using a LECO CNS-2000 Elemental Analyzer (0.07% C and 0.09% N detection limits) (Nelson and Sommers 1996). After wet digestion with H2SO4 and HClO4, total phosphorus (TP) and total potassium (TK) were determined colorimetrically by Lachat QuikChem AE Flow-injection Autoanalyzer, respectively (Parkinson and Allen 1975).
DNA extraction, amplification and sequencing
Total DNA was extracted from approximately 0.5 g of soil using the Fast DNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA), following the manufacturer’s instructions. The DNA yield was visualized in agarose gel electrophoresis (1.0% 1 × TAE buffer agarose gel run at 120 V for 1 h) with ethidium bromide. The quantity and quality of extracted DNA were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The extracted DNA was stored at −20 °C before polymerase chain reaction (PCR) amplification.
PCR amplification of the V3–V4 region of the bacterial 16S rRNA genes was performed using the forward primer 338F 5′-ACTCCTACGGGAGGCAGCA-3′ and the reverse primer 806R 5′-GGACTACHVGGGTWTCTAAT-3′(Caporaso et al. 2012; Suzuki and Giovannoni 1996). For fungal internal transcribed spacer (ITS) amplification, a similar approach was employed using the forward primers fITS7 5′-GTGARTCATCGAATCTTTG-3′ and the reverse primer ITS4 5′-TCCTCCGCTTATTGATATGC-3′ (Ihrmark et al. 2012; White et al. 1990). Sample-specific 7 bp barcodes were incorporated into the primers for multiplex sequencing. We amplified the bacterial and fungal rRNA genes using a two-step PCR protocol following “16S Metagenomic Sequencing Library Preparation” (Protocol 15044223B, Illumina). PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN, USA) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After the individual quantification step, amplicons were pooled in equal amounts, and paired-end 2 × 300 bp sequencing was performed using the Illlumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). Negative controls were included throughout the PCR and sequencing steps. The paired fastq files are available in the Sequence Read Archive at the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov) under accession numbers SRR8383030-SRR8383057 for bacteria and SRR8393149-SRR8393176 for fungi, respectively.
Bioinformatics
Paired-end sequence data (.fastq) were processed using mothur version 1.38.1 (Schloss et al. 2009). Bacterial and fungal .fastq files were combined, respectively. Any sequences with ambiguous bases, with more than one mismatch to the primers, with homopolymers longer than 8 bp for bacteria and 13 bp for fungi (Soonvald et al. 2019), and any without a minimum overlap of 50 bp were removed.
Bacterial sequences were aligned against a SILVA reference, pre-clustered to remove erroneous reads (Huse et al. 2010) and screened for chimeras with the Vsearch algorithm (Rognes et al. 2016), and non-chimeric sequences were assigned to taxa using the naive Bayesian classifier (Wang et al. 2007) against the RDP training set. Non-target sequences (mitochondria, chloroplast, archaea) were removed. The final bacterial data consisted of 556,540 sequences (19,876 ± 696 per sample, mean ± se) prior to calculating a pairwise distance matrix. Sequences were clustered to OTUs at 97% similarity using nearest neighbour (single linkage) joining.
Fungal sequences were screened with the Vsearch algorithm and putative chimeras removed. These fungal sequences were assigned to taxa using the naive Bayesian classifier and the UNITE-curated International Nucleotide Sequence Database (Abarenkov et al. 2010). Any sequences not assigned to kingdom Fungi were removed. After quality control, the fungal data consisted of 532,331 sequences in total, 19,011 ± 833 sequences per sample (mean ± se). Unique sequences were pairwise aligned and the resultant distance matrix clustered to OTUs at a 97% threshold using nearest neighbour joining as described for bacteria. Fungal OTUs were assigned to functional guild using the FUNGuild database with “highly probable” confidence (Nguyen et al. 2016). Multifunctional guilds, e.g. Ectomycorrhizal-Saprotroph, were considered as independent guilds.
In both bacterial and fungal datasets, low abundance OTUs (≤ 10 sequences across all 28 samples; 8.8% of bacterial and 1.1% of fungal total reads) were removed, as they may be PCR or sequencing artefacts (Brown et al. 2015; Oliver et al. 2015; Tedersoo et al. 2010). We estimated richness and diversity metrics for bacterial, fungal and ECM fungal communities in mothur. Observed OTU richness (Sobs), the complement of Simpson’s diversity (1/D: 1/∑pi2), and Simpson’s evenness (ED: 1/∑pi2/S), with pi representing frequency of each OTU within a sample, were iteratively calculated and rarefied at 8157 sequences per sample for bacteria and 2653 sequences per sample for fungi.
Statistical analyses
All statistical analyses were performed in R (version 3.2.1, R Development Core Team, 2015) using various packages.
The response of the soil properties and the major bacterial and fungal groups to land types (non-KRD and KRD) was tested using a generalized linear mixed model (GLMM) with the lmer function in the lme4 package in R. The response variables were ln transformed to approximate normality when necessary. Since our samples were taken in the vicinities of two villages, sampling village was added as a random term to the models.
Non-metric multidimensional scaling (NMDS) analyses were performed for bacterial, fungal and ECM fungal data sets using the vegan package to visualize the bacterial and fungal communities. Community structure responds to land types and villages were determined using permutation test (envfit function in vegan, permutations = 99,999) (Oksanen et al. 2013). Bray–Curtis coefficient was used as the dissimilarity measure. TC, TN, TP, TK and pH were correlated with the community structure as the vector fitting procedure in the same envfit analyses.
To identify OTUs and genera that were over-represented in specific land type, we conducted indicator taxon analyses using the indicspecies package in R (De Caceres et al. 2016). The genus level analyses are distinct as they could be considered as summary data that are cohesive taxonomic and often even functional units. False detection rate (FDR) corrections were used for post-hoc multiple comparisons of statistical significance in the indicator species analysis.
Results
Soil properties
We observed that rocky desertification significantly influenced edaphic conditions in the karst zone (Table 1). Soil pH was higher in KRD soils than in non-KRD soils, whereas TC, TN and TP showed the opposite trend, with higher values observed in non-KRD soils than in KRD soils, indicating an adverse effect of KRD on soil fertility. Soil TK remained unchanged between the two land types. None of the above soil properties differed between the two villages.
Microbial richness and diversity
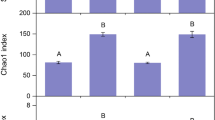
Despite losses in soil TC, TN and TP, rocky desertification did not erode microbial community diversity. Surprisingly, soil bacterial OTU richness was approximately 7.9% higher (t = 2.126, p = 0.014) in the KRD areas than in the non-KRD areas (Fig. 2, Table S1). Bacterial diversity showed the same trend with a higher value in the KRD areas than in the non-KRD areas. Soil bacterial evenness did not differ between the two land types. Bacterial diversity and evenness were correlated negatively with TK in the soil (Table S1). Fungal OTU richness, diversity and evenness did not show any clear pattern between the non-KRD and KRD soils. In addition, ECM fungal communities did not respond to the KRD effect in terms of OTU richness, diversity and evenness.
Bacterial (upper panels), fungal (middle panels) and ectomycorrhizal (ECM) fungal (lower panels) OTU richness, diversity and evenness (generalized linear mixed model results) in non-KRD and KRD areas. Error bars represent standard error (SE). KRD = karst rocky desertification. Asterisk indicates non-KRD was significantly different from KRD land type (p < 0.05)
Microbial community compositional shifts
Multivariate analysis of bacterial community structures of the non-KRD areas and those associated with soils in the KRD areas indicated differences between those communities (r2 = 0.561, p < 0.001; Fig. 3). Soil properties of TC, TN, TP and pH were associated with the bacterial community composition (Fig. 3). We observed 28 phyla across the bacterial data set, where Proteobacteria were the most abundant phylum (24.7% sequences), followed by Actinobacteria (21.7%) and Acidobacteria (17.6%). The relative abundance of Acidobacteria (phylum), Planctomycetes (phylum), Verrucomicrobia (phylum), Alphaproteobacteria (class) and Acidobacteria subgroup 2 (class) was greater in non-KRD soils than in KRD soils, whereas Actinobacteria, Gemmatimonadetes and Acidobacteria subgroup 6 (class) showed the opposite trend, with higher relative abundance in KRD soils than non-KRD soils (Fig. 4, Table S2). We also considered the village effect on bacterial community structures. However, we did not observe any significant difference between the two villages (r2 = 0.262, p = 0.432).
Non-metric multidimensional scaling plots (based on Bray–Curtis dissimilarity matrix) for bacterial, fungal and ectomycorrhizal fungal communities. Statistically significant (p < 0.05) environmental variable are shown as arrows. All three analyses (permutation tests by envfit function in vegan package R) suggest significant differences (p < 0.05) between non-KRD and KRD areas. KRD = karst rocky desertification. C = total carbon; P = total phosphorous; N = total nitrogen. Ellipses (black for KRD; gray for non-KRD) indicate mean ± standard deviation of samples
Relative abundance of bacterial phyla (a–h), bacterial class (i–n), fungal phyla (o and p) and fungal functional guilds (q–t) between non-KRD and KRD (generalized linear mixed model results). KRD = karst rocky desertification; AMF = arbuscular mycorrhizal; ECM = ectomycorrhizal; SAP = saprotroph. Error bars represent standard error (SE). Asterisk indicates non-KRD was significantly different from KRD land type (p < 0.05)
Similarly to bacteria, fungal communities differed between non-KRD soils and KRD soils (r2 = 0.441, p < 0.001; Fig. 3), indicating again that microbial communities associated with the undisturbed karst forest soil do not approximate those in the rocky desertified shrub soil. Two edaphic parameters (TC and pH) were correlated with the fungal community composition (Fig. 3). Fungal OTUs were classified into six phyla. Basidiomycota (89.7%) dominated the fungal data set, followed by Ascomycota (5.8%) and Glomeromycota (0.7%) (Table S3). The relative abundance of Ascomycota and Glomeromycota was greater in KRD soils than in non-KRD soils (Fig. 4, Table S3). To study fungal trophic mode, we assigned fungal OTUs into functional guilds. We observed that ECM fungi (65.7%) were the most abundant guild and there was no significant difference in relative abundance of ECM fungi between land types (Fig. 4, Table S3). The relative abundance of AMF (arbuscular mycorrhizal) fungi (taxonomically as Glomeromycota) and saprotroph (SAP) fungi was greater in KRD soils than in non-KRD soils (Fig. 4, Table S3). Lichenized fungi contrasted this trend, with greater relative abundance in non-KRD soils than in KRD soils. The relative abundance of SAP fungi was positively associated with pH. In addition to the general fungal community, ECM fungal composition also differed between non-KRD and KRD soils (r2 = 0.331, p < 0.001; Fig. 3). TC was associated with ECM fungal composition. We considered village effect on fungal community structures. However, neither general fungal (r2 = 0.177, p = 0.331) nor ECM fungal (r2 = 0.422, p = 0.106) community composition differed between the two villages.
Indicator taxa
We identified 104 bacterial indicator OTUs (Table S4), 57 OTUs for non-KRD soils and 47 OTUs for KRD soils. At genus level, Acidobacterium, Actinospica, Paraburkholderia and Rhizomicrobium were enriched in non-KRD soils, while Crossiella was more common in KRD soils (Table 2). In the fungal data set, we detected 61 indicator OTUs (Table S5): 29 OTUs for non-KRD soils and 32 OTUs for KRD soils. There were four ECM fungal genera (Amanita, Clavulina, Inocybe and Lactarius) enriched in non-KRD soils, while KRD soils had diversified indicator genera in terms of fungal functional guilds: one AMF genus (Rhizophagus), two ECM genera (Astraeus and Boletus) and four SAP genera (Geminibasidium, Micropsalliota, Skeletocutis and Tricholosporum).
Discussion
Microbial diversity
KRD can influence soil microbial communities in several ways, via degradations in plant diversity or through changes in soil properties (Cartwright et al. 2016; He et al. 2008; Li et al. 2018; Qi et al. 2018; Xue et al. 2017). Despite the adverse effects of KRD on the chemistry of soils was confirmed by this and a previous study (Peng and Wang 2012), vegetation in the KRD area can be an important reservoir for bacterial and fungal abundance (Li et al. 2018). Our data indicated that KRD did not pose a threat to the soil microbial diversity, which led us to reject our first hypothesis. Surprisingly, the rocky desertified shrub soil, with less diverse vegetation (Jiang et al. 2014) and less TC, TN and TP, was richer in bacterial OTU richness and diversity. This finding agrees with a previous study, which demonstrates a higher soil bacterial diversity in KRD shrubs than in secondary forests (Xue et al. 2017). Rich-resource non-KRD soils likely create less microbial niche differentiation, whereas poor-resource KRD soils are more inclined to unique niches, which have a crucial effect on bacterial diversity (Bakker et al. 2013; Hui et al. 2018; Schlatter et al. 2015).
Bacterial community structure
We observed that bacterial community compositions in non-KRD soils were different from those in KRD soils. These findings support our second hypothesis and are in agreement with previous studies. For example, soil bacterial community structures differ between non-KRD and KRD areas using 16S rRNA gene sequencing in southwest China (Qi et al. 2018). The different community structures are likely attributed to the effect of litter on soil physical–chemical parameters. Non-KRD forests are typified by mollic inceptisol soils with forest litter on top of the soil, whereas KRD soils are heavily eroded and mostly uncovered by plant litter. The shortage of litter can cause changes in soil carbon and nutrient dynamics (Bardgett and Wardle 2010; Wardle et al. 2004), thus alters microbial community structure (Hui et al. 2017a).
The soil bacteria compositional differences between non-KRD and KRD areas were largely attributable to the different edaphic conditions, which affected the distribution of major bacterial taxa. In the current study, many microbial taxa correlated with various edaphic parameters. However, we did not observe any universally influential parameter, but different drivers affected different groups, potentially suggesting that the environmental tolerances of major community members were largely different. Soil pH is often documented to correlate with bacterial communities (Fierer and Jackson 2006), including karst regions (Qi et al. 2018; Tripathi et al. 2012; Yun et al. 2016), which may impose a selection pressure on the community structure. Here we showed that soil pH drove bacterial community structure and was correlated positively with Gemmatimonadetes, Acidobacteria subgroup 6 and Deltaproteobacteria and negatively with Acidobacteria subgroup 2. Other soil conditions, e.g. TC and TN, are also reported to correlate with microbial communities in various environments (Hui et al. 2017a; Liu et al. 2016), including karst soils (He et al. 2008; Li et al. 2018). In this study, KRD promoted the relative abundance of Actinobacteria and Gemmatimonadetes, which was likely explained by the low levels of TC, TN and TP in KRD soils. The two phyla are known as oligotrophic K-strategists in the degraded soil, thus tend to be enriched in nutrient poor environments (Bastida et al. 2015; Zhang et al. 2003). On the other hand, Alphaproteobacteria showed the opposite trend, with a greater relative abundance in non-KRD soils than in KRD soils. Indeed, this bacterial phylum is classified as a copiotrophic r-strategist that prefers apparent optimal conditions (Bastida et al. 2015). Although Acidobacteria occupied a major portion of the bacterial community, the high pH in KRD soils inhibited their increase (Yun et al. 2016). However, as a class of Acidobacteria, Acidobacteria subgroup 6 contradicted the general trend by being greater in relative abundance in KRD than in non-KRD soils. This bacterial class has been documented to be more related to pasture soils than forest soils in forest-to-pasture conversions resulting from forest clear-cutting and burning (Navarrete et al. 2015).
Fungal community structure
The responses of the bacterial community to KRD are relatively well documented, whereas the status of the fungal community in KRD areas has received little attention. In the fungal data set, the KRD effect was more pronounced on the fungal functional guilds than fungal phyla, as Basidiomycota, which dominated fungal communities, did not respond to land type. ECM fungal communities were the most abundant fungal guild in both non-KRD and KRD soils. To our knowledge, this is the first study to characterize ECM fungal communities in KRD regions. In boreal, temperate and subtropical forests, ECM fungi drastically enhance plant growth and performance, especially in low nutrient environments (Jonsson et al. 2001; Phillips et al. 2013; Sousa et al. 2011). However, ECM fungi are sensitive to environmental change, e.g. land-use change, urban anthropogenic disturbance, and pollution (Hui et al. 2011; Hui et al. 2017b; Schmidt et al. 2017). Our data showed that KRD did not modify ECM fungal OTU richness or diversity between the two types of habitat, which led us to reject our third hypothesis. Among the 102 ECM fungal OTUs in this study, we determined 13 indicator OTUs in non-KRD area and 6 indicator OTUs in KRD area (Table S5), suggesting a minor species replacement rather than a major diversity loss. Although the non-KRD stands have interacted with the local fungal communities over an extended period, the short revegetation period after clear-cutting in KRD shrubs seems not to restrict ECM fungi from dominating the soil, indicating ubiquitous ECM inoculum presence in rocky desertified environment. Consequently, soils under KRD shrubs recruit as much diverse ECM fungi as those in non-KRD forest, suggesting a favourable ecological potential for the restoration of KRD area.
Despite possible resistance and resilience, the ECM fungal community composition differed between non-KRD and KRD areas. Clear-cutting alters the relative abundance of ECM taxa in secondary forest compared with primary forests (Kyaschenko et al. 2017). In karst zones, soil in primary forest has greater soil moisture than soil in secondary shrubs that has been converted from primary forest by clear-cutting for 3 years (Zhang et al. 2016). In this study, four ECM genera – Amanita, Clavulina, Inocybe and Lactarius – showed a preponderant correlation to non-KRD soils. Indeed, these ECM fungal genera are often more frequent in mature stands than in revegetated stands (Holste and Kobe 2017; Hui et al. 2018; Sousa et al. 2011). The low relative abundance of the four genera in KRD soils was likely due to the lower moisture and thinner depth (0–20 cm) than in non-KRD soils (Qi et al. 2018). Amanita, Inocybe and Lactarius form hydrophilic ectomycorrhizas that focus on soluble forms of soil nutrients (Lilleskov et al. 2011; Trudell et al. 2004). Amanita and Inocybe ectomycorrhizas acquire nitrogen from the deep (15–30 cm) soil layer (Hobbie et al. 2014). On the other hand, we found that Astraeus and Boletus were enriched in the KRD soils. The two ECM fungal genera are often observed in dry environments (Morgado et al. 2015; Ruankaew Disyatat et al. 2016). The mycelium of immature Astraeus specimens is fibrous, with a strong hygroscopic character (Miller and Miller 1988).
Conclusions
Our results showed that rocky desertification reduced soil fertility and altered microbial community structures in karst areas. It is widely understood that soil microbial community diversity is positively correlated with plant diversity, because copious plant species/traits provide diverse substrates and resources. However, our results suggested that bacterial OTU richness and diversity were greater in the KRD areas than in the non-KRD areas, the latter having relatively greater plant density and diversity. Fungal OTU richness and diversity remained unchanged by KRD. We also determined that although the KRD areas had been clear-cut and trees were mostly absent, ECM fungi, the most abundant functional guild in the fungal data set, did not differ in diversity and relative abundance between the two land types, indicating that KRD shrubs hosted surprisingly diverse and abundant ECM fungi. Despite the fact that degraded soil properties and an altered microbial community structure remain in KRD areas, we observed diverse ECM fungi in the rhizosphere of L. confinis, the dominant local tree species in Mengzi. Such information is particularly important for restoration of the degraded ecosystem, as the ECM fungal pool could largely benefit tree seedlings and promote their growth. Finally, continuing studies on soil microbiomes in planted secondary forest in KRD areas are needed, as tree health and growth depend on soil microbial communities.
References
Abarenkov K, Henrik Nilsson R, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T (2010) The UNITE database for molecular identification of fungi–recent updates and future perspectives. New Phytol 186:281–285
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H (2018) Structure and function of the global topsoil microbiome. Nature 560:233
Bakker MG, Otto-Hanson L, Lange A, Bradeen JM, Kinkel LL (2013) Plant monocultures produce more antagonistic soil Streptomyces communities than high-diversity plant communities. Soil Biol Biochem 65:304–312
Bardgett RD, Wardle DA (2010) Aboveground-belowground linkages: biotic interactions, ecosystem processes, and global change. Oxford University Press, Oxford
Bastida F, Hernández T, Garcia C (2010) Soil degradation and rehabilitation: microorganisms and functionality. Microbes at Work. Springer, Heidelberg
Bastida F, Selevsek N, Torres IF, Hernández T, García C (2015) Soil restoration with organic amendments: linking cellular functionality and ecosystem processes. Sci Rep 5:15550–15561
Brown SP, Veach AM, Rigdon-Huss AR, Grond K, Lickteig SK, Lothamer K, Oliver AK, Jumpponen A (2015) Scraping the bottom of the barrel: are rare high throughput sequences artifacts? Fungal Ecol 13:221–225
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621
Cartwright J, Dzantor EK, Momen B (2016) Soil microbial community profiles and functional diversity in limestone cedar glades. Catena 147:216–224
Chen Z, Auler AS, Bakalowicz M, Drew D, Griger F, Hartmann J, Jiang G, Moosdorf N, Richts A, Stevanovic Z (2017) The world karst aquifer mapping project: concept, mapping procedure and map of Europe. Hydrogeol J 25:771–785
Cornwell WK, Bedford BL, Chapin CT (2001) Occurrence of arbuscular mycorrhizal fungi in a phosphorus-poor wetland and mycorrhizal response to phosphorus fertilization. Am J Bot 88:1824–1829
Dai QH, Peng XD, Wang PJ, Li CL, Shao HB (2018) Surface erosion and underground leakage of yellow soil on slopes in karst regions of Southwest China. Land Degrad Dev 29:2438–2448
De Caceres M, Jansen F, De Caceres MM (2016) Package ‘indicspecies’. Relationship between species and groups of sites R package version 1
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci 103:626–631
Francini G, Hui N, Jumpponen A, Kotze DJ, Romantschuk M, Allen JA, Setälä H (2018) Soil biota in boreal urban greenspace: responses to plant type and age. Soil Biol Biochem 118:145–155
Harris J (2009) Soil microbial communities and restoration ecology: facilitators or followers? Science 325:573–574
He XY, Wang KL, Zhang W, Chen ZH, Zhu YG, Chen HS (2008) Positive correlation between soil bacterial metabolic and plant species diversity and bacterial and fungal diversity in a vegetation succession on Karst. Plant Soil 307:123–134
Hobbie EA, Diepen LT, Lilleskov EA, Ouimette AP, Finzi AC, Hofmockel KS (2014) Fungal functioning in a pine forest: evidence from a 15N-labeled global change experiment. New Phytol 201:1431–1439
Holste EK, Kobe RK (2017) Tree species and soil nutrients drive tropical reforestation more than associations with mycorrhizal fungi. Plant Soil 410:283–297
Hui N, Jumpponen A, Niskanen T, Liimatainen K, Jones KL, Koivula T, Romantschuk M, Strömmer R (2011) EcM fungal community structure, but not diversity, altered in a Pb-contaminated shooting range in a boreal coniferous forest site in Southern Finland. FEMS Microbiol Ecol 76:121–132
Hui N, Jumpponen A, Francini G, Kotze DJ, Liu X, Romantschuk M, Strömmer R, Setälä H (2017a) Soil microbial communities are shaped by vegetation type and park age in cities under cold climate. Environ Microbiol 19:1281–1295
Hui N, Liu XX, Kotze DJ, Jumpponen A, Francini G, Setälä H (2017b) Ectomycorrhizal fungal communities in urban parks are similar to those in natural forests but shaped by vegetation and park age. Appl Environ Microbiol 83:e01797
Hui N, Liu XX, Jumpponen A, Setälä H, Kotze DJ, Biktasheva L, Romantschuk M (2018) Over twenty years farmland reforestation decreases fungal diversity of soils, but stimulates the return of ectomycorrhizal fungal communities. Plant Soil 427:231–244
Huse SM, Welch DM, Morrison HG, Sogin ML (2010) Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol 12:1889–1898
Ihrmark K, Bödeker I, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE (2012) New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677
Jiang Z, Lian Y, Qin X (2014) Rocky desertification in Southwest China: impacts, causes, and restoration. Earth Sci Rev 132:1–12
Jonsson LM, Nilsson MC, Wardle DA, Zackrisson O (2001) Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93:353–364
Kyaschenko J, Clemmensen KE, Hagenbo A, Karltun E, Lindahl BD (2017) Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. The ISME Journal 11:863–878
Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soil-borne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206:507–521
Li D, Liu J, Chen H, Zheng L, Wang K (2018) Soil microbial community responses to forage grass cultivation in degraded karst soils, Southwest China. Land Degrad Dev 29:4262–4270
Lilleskov E, Hobbie EA, Horton T (2011) Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4:174–183
Liu XX, Hui C, Bi L, Romantschuk M, Kontro M, Strömmer R, Hui N (2016) Bacterial community structure in atrazine treated reforested farmland in Wuying China. Appl Soil Ecol 98:39–46
Miller OK, Miller H (1988) Gasteromycetes: morphological and developmental features, with keys to the orders, families, and genera. Mad River Press, Eureka
Morgado LN, Semenova TA, Welker JM, Walker MD, Smets E, Geml J (2015) Summer temperature increase has distinct effects on the ectomycorrhizal fungal communities of moist tussock and dry tundra in Arctic Alaska. Glob Chang Biol 21:959–972
Nannipieri P, Ascher J, Ceccherini M, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670
Navarrete AA, Venturini AM, Meyer KM, Klein AM, Tiedje JM, Bohannan BJ, Nüsslein K, Tsai SM, Rodrigues JL (2015) Differential response of Acidobacteria subgroups to forest-to-pasture conversion and their biogeographic patterns in the western Brazilian Amazon. Front Microbiol 6:1443
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis Part 3 SSSA Book Ser 5. SSSA, Madison, pp 961–1010
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’hara R, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Package ‘vegan’. Community ecology package, version 2:1–295
Oliver AK, Brown SP, Callaham MA, Jumpponen A (2015) Polymerase matters: non-proofreading enzymes inflate fungal community richness estimates by up to 15%. Fungal Ecol 15:86–89
Owuor SO, Butterbach-Bahl K, Guzha A, Jacobs S, Merbold L, Rufino MC, Pelster DE, Díaz-Pinés E, Breuer L (2018) Conversion of natural forest results in a significant degradation of soil hydraulic properties in the highlands of Kenya. Soil Tillage Res 176:36–44
Parkinson J, Allen S (1975) A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun Soil Sci Plant Anal 6:1–11
Peay KG, von Sperber C, Cardarelli E, Toju H, Francis CA, Chadwick OA, Vitousek PM (2017) Convergence and contrast in the community structure of Bacteria, Fungi and Archaea along a tropical elevation–climate gradient. FEMS Microbiol Ecol 93:fix045
Peng T, Wang SJ (2012) Effects of land use, land cover and rainfall regimes on the surface runoff and soil loss on karst slopes in Southwest China. Catena 90:53–62
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51
Prober SM, Leff JW, Bates ST, Borer ET, Firn J, Harpole WS, Lind EM, Seabloom EW, Adler PB, Bakker JD (2015) Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol Lett 18:85–95
Qi D, Wieneke X, Zhou X, Jiang X, Xue P (2017) Succession of plant community composition and leaf functional traits in responding to karst rocky desertification in the Wushan County in Chongqing, China. Community Ecol 18:157–168
Qi D, Wieneke X, Tao J, Zhou X, Desilva U (2018) Soil pH is the primary factor correlating with soil microbiome in karst rocky desertification regions in the Wushan County, Chongqing, China. Front Microbiol 9:1027–1039
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Ruankaew Disyatat N, Yomyart S, Sihanonth P, Piapukiew J (2016) Community structure and dynamics of ectomycorrhizal fungi in a dipterocarp forest fragment and plantation in Thailand. Plant Ecol & Divers 9:577–588
Schlatter DC, Bakker MG, Bradeen JM, Kinkel LL (2015) Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 96:134–142
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Schmidt DJE, Pouyat R, Szlavecz K, Setälä H, Kotze DJ, Yesilonis I, Cilliers S, Hornung E, Dombos M, Yarwood SA (2017) Urbanization erodes ectomycorrhizal fungal diversity and may cause microbial communities to converge. Nat Ecol Evol 1:0123
Sheng M, Xiong K, Wang L, Li X, Li R, Tian X (2018) Response of soil physical and chemical properties to rocky desertification succession in South China karst. Carbonates Evaporites 33:15–28
Soonvald L, Loit K, Runno-Paurson E, Astover A, Tedersoo L (2019) The role of long-term mineral and organic fertilisation treatment in changing pathogen and symbiont community composition in soil. Appl Soil Ecol 141:45–53
Sousa NR, Franco AR, Ramos MA, Oliveira RS, Castro PM (2011) Reforestation of burned stands: the effect of ectomycorrhizal fungi on Pinus pinaster establishment. Soil Biol Biochem 43:2115–2120
Suzuki MT, Giovannoni SJ (1996) Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol 62:625–630
Tedersoo L, Brundrett MC (2017) Evolution of ectomycorrhizal symbiosis in plants. Biogeography of mycorrhizal symbiosis. Springer, Cham
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Sadam A, Saar I, Bahram M, Bechem E, Chuyong G, Kõljalg U (2010) 454 pyrosequencing and sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301
Tripathi BM, Kim M, Singh D, Lee-Cruz L, Lai-Hoe A, Ainuddin A, Go R, Rahim RA, Husni M, Chun J (2012) Tropical soil bacterial communities in Malaysia: pH dominates in the equatorial tropics too. Microb Ecol 64:474–484
Trudell SA, Rygiewicz PT, Edmonds RL (2004) Patterns of nitrogen and carbon stable isotope ratios in macrofungi, plants and soils in two old-growth conifer forests. New Phytol 164:317–335
Van Der Heijden MG, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Van Der Linde S, Suz LM, Orme CDL, Cox F, Andreae H, Asi E, Atkinson B, Benham S, Carroll C, Cools N (2018) Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 558:243
Velmala SM, Rajala T, Heinonsalo J, Taylor AF, Pennanen T (2014) Profiling functions of ectomycorrhizal diversity and root structuring in seedlings of Norway spruce (Picea abies) with fast-and slow-growing phenotypes. New Phytol 201:610–622
Wang SJ, Liu QM, Zhang DF (2004) Karst rocky desertification in southwestern China: geomorphology, landuse, impact and rehabilitation. Land Degrad Dev 15:115–121. https://doi.org/10.1002/ldr.592
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Wang P, Mo B, Chen Y, Zeng Q, Wang LJG (2016) Effect of karst rocky desertification on soil fungal communities in Southwest China. Genet Mol Res: GMR 15. https://doi.org/10.4238/gmr.15038460
Wang W, Wang Q, Zhou W, Xiao L, Wang H, He X (2018) Glomalin changes in urban-rural gradients and their possible associations with forest characteristics and soil properties in Harbin City, northeastern China. J Environ Manag 224:225–234
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van Der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
White T, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA et al (eds) PCR protocols: A guide to methods and applications. Academic Press, San Diego, pp 315–322
Xie L, Zhong J, Chen F, Cao F, Li J, Wu L (2015) Evaluation of soil fertility in the succession of karst rocky desertification using principal component analysis. Solid Earth 6:515–524
Xue L, Ren H, Li S, Leng X, Yao X (2017) Soil bacterial community structure and co-occurrence pattern during vegetation restoration in karst rocky desertification area. Front Microbiol 8:2377
Yan X, Cai YL (2015) Multi-scale anthropogenic driving forces of karst rocky desertification in Southwest China. Land Degrad Dev 26:193–200
Yuan D (1997) Rock desertification in the subtropical karst of South China. Z Geomorphol 108:81–90
Yun Y, Wang H, Man B, Xiang X, Zhou J, Qiu X, Duan Y, Engel AS (2016) The relationship between pH and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front Microbiol 7:1955
Zhang H, Sekiguchi Y, Hanada S, Hugenholtz P, Kim H, Kamagata Y, Nakamura K (2003) Gemmatimonas aurantiaca gen. nov., sp. nov., a Gram-negative, aerobic, polyphosphate-accumulating micro-organism, the first cultured representative of the new bacterial phylum Gemmatimonadetes phyl. nov. Int J Syst Evol Microbiol 53:1155–1163
Zhang X, Liu S, Li X, Wang J, Ding Q, Wang H, Tian C, Yao M, An J, Huang Y (2016) Changes of soil prokaryotic communities after clear-cutting in a karst forest: evidences for cutting-based disturbance promoting deterministic processes. FEMS Microbiol Ecol 92:26–39
Zhang H, Wang R, Chen S, Qi G, He Z, Zhao X (2017) Microbial taxa and functional genes shift in degraded soil with bacterial wilt. Sci Rep 7:39911
Zhu H, He X, Wang K, Su Y, Wu J (2012) Interactions of vegetation succession, soil bio-chemical properties and microbial communities in a Karst ecosystem. Eur J Soil Biol 51:1–7
Acknowledgements
This work was financially supported by the National Key R & D Programme of China (2016YFC0502501). The chemical analyses were carried out at the Instrumental Analysis Center of Shanghai Jiao Tong University. We thank Huimin Tao, Jun Yuan and numerous station workers and interns for their outstanding help during the field work.
Author information
Authors and Affiliations
Contributions
NH, NS, HD, MR and CL planned and designed the research. NH, NS, HD, MU, HK, XL and MR performed experiments, conducted fieldwork, analysed data etc. NH, NS, XL and CL wrote the manuscript. NH and NS contributed equally.
Corresponding author
Additional information
Responsible Editor: Thom W. Kuyper.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Hui, N., Sun, N., Du, H. et al. Karst rocky desertification does not erode ectomycorrhizal fungal species richness but alters microbial community structure. Plant Soil 445, 383–396 (2019). https://doi.org/10.1007/s11104-019-04319-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04319-z