Abstract
Background and aim
Mechanisms of drought tolerance based on root architecture and lipid composition in wheat are poorly understood. We quantified the differences in root morphological traits and phospholipids and galactolipids levels between winter and spring wheat genotypes at variable water supply amounts (drought stress).
Methods
Experiments were conducted using seven winter and four spring wheat (Triticum aestivum) genotypes. In the first experiment, solid agar medium was used to quantify seminal root angles. In the second experiment, the plants were grown in 150-cm columns in a greenhouse under full and deficit water supply for 65 days to record root architecture. The root tips (2-cm-long) were used for quantifying polar lipids.
Results
Drought stress at vegetative stage decreased plant height (14%), total dry matter production (48%), maximum root length (25%), root length:shoot length ratio (11%), and other root traits. Winter wheat genotypes had ~1.5 times higher maximum root length than spring wheat genotypes. Significant differences in molar percentages of root phospholipids and galactolipids, molecular species, and double bond index of galactolipids were observed among spring wheat but not winter wheat genotypes.
Conclusions
Based on the genotypes studied, the drought tolerant mechanism of winter wheat was associated with deep root system, and in spring wheat it was well branched (albeit shallow) root system with more unsaturated membrane lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is an important food crop for more than one third of the world population, and it is sensitive to drought stress, particularly during booting, flowering, and grain filling stages (Ihsan et al. 2016). Under rainfed conditions, wheat crop may suffer from drought stress due to unpredictable and infrequent rains, resulting in significant yield losses (Hossain et al. 2012). It is anticipated that the occurrence of drought stress in the major wheat-producing regions will increase in response to changing and variable climate (Reynolds and Ortiz 2010; Semenov and Shewry 2011). In general, breeding efforts to improve crop yields under drought stress are focused on aboveground plant parts (Wachsman et al. 2015), and the knowledge about genotypic differences between winter and spring wheat in root architecture influencing drought tolerance is limited.
Among the various plant organs, roots are severely affected by drought due to their direct contact with drying soil (Yoshimura et al. 2008). The plant capacity to alter the root system architecture (root number, length, positioning, and angle) under drought stress has been reported in many crops. Wasson et al. (2012) proposed that deep roots, greater root length density, decreased resistance to water movement from soil to root, and denser root hairs as the important traits associated with increased uptake of stored soil moisture from the deeper soil horizons. Indeed, well-branched and deeper root systems are often viewed as desirable traits for drought adaptation (Vadez 2014). Modelling studies have indicated that wheat genotypes with deep roots and greater root-length density could significantly improve water absorption under drought stress (Manschadi et al. 2006). In maize, the Steep, Cheap, and Deep (SCD) ideotype was found to be drought tolerant (Zhan et al. 2015), and many features of this ideotype may be relevant to wheat root systems.
Root structure and distribution (root biomass) determine the water extraction pattern from soil (Liu et al. 2004; Prasad et al. 2008, 2018). A wide genetic variability for root architectural traits were reported for spring wheat genotypes (Narayanan et al. 2014). Research on rice (Oryza sativa L.; Price et al. 2002), chickpea (Cicer arietinum L.; Zaman-Allah et al. 2011a), and peanut (Arachis hypogaea L.; Ratnakumar and Vadez 2011) indicated that drought tolerance was not associated with deep and profuse rooting systems. However, genotypes with greater root length density and extensive fibrous root systems in deeper layers of soil had improved water uptake in sorghum [Sorghum bicolor (L.) Moench; Masi and Maranville 1998] and soybean [Glycine max (L.) Merril; Pantalone et al. 1999]. However, in wheat the role of greater root length density to grain yield under drought is variable (Siddique et al. 1990). A study in maize revealed a close relationship between rooting depth, root length density, and water extraction pattern (Hund et al. 2009); whereas in chickpea (Zaman-Allah et al. 2011b) and peanut (Ratnakumar and Vadez 2011) there was no such relationship. In the field, winter wheat had twice the rooting depth of spring wheat, but only a single genotype of each was tested (Thorup-Kristensen et al. 2009). The spring wheat genotype Treasure had increased rooting depth and total root length, and ranked high for most of the other root traits (Narayanan and Prasad 2014). Hence, the relevance to drought resistance of different root traits in different winter and spring wheat genotypes remains unknown.
Tolerance to drought stress at the cellular level can be important. The membranes play an important role in sensing environmental change and signal transduction. Membranes are main targets of degradative processes induced by drought stress (Gigon et al. 2004). The two plant galactolipids, monogalactosyldiacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG) are the most abundant lipids in chloroplast membranes, with high levels of polyunsaturated fatty acids (Boudiere et al. 2014). The ratio between MGDG and DGDG appears to be stable under favourable growth conditions; however, under drought stress the ratio was decreased due to higher levels of DGDG than MGDG (Moellering and Benning 2011). In non-plastid membranes the proportion phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are crucial for membrane stability (Larsson et al. 2006; Norberg and Liljenberg 1991). Less abundant non-plastid phospholipids namely phosphatidylserine (PS) and phosphatidylinositol (PI) are involved in biosynthesis of other lipids and stress signaling (Larsson et al. 2006; Liu et al. 2013). The unsaturated fatty acids present in membrane lipids are highly vulnerable to peroxidation by reactive oxygen species and lipoxygenase (Farmer and Mueller 2013). Under abiotic stress, it is observed that oxidation of unsaturated fatty acids causes production of oxylipins (oxidized fatty acids), an important signal molecule related to stress responses (Andreou et al. 2009). Changes in the degree of unsaturation of glycerolipids, which affect membrane fluidity, are known and occur under abiotic stresses like drought, low and high temperatures (Djanaguiraman et al. 2018; Monteiro de Paula et al. 1993; Narayanan et al. 2016; Neale et al. 2000; Zheng et al. 2011). It is observed that drought tolerant plants had increased level of polyunsaturated fatty acids during drought, compared to the control (Gigon et al. 2004; Liu et al. 2013; Monteiro de Paula et al. 1993; Repellin et al. 1997; Torres-Franklin et al. 2007). Under drought, the membrane lipid content was decreased, correlating with inhibition of lipid synthesis or stimulation of lipolytic activities (Matos et al. 2001; Pham-Thi et al. 1985, 1987). However, most studies dealt with the change in phospholipids and galactolipids contents in leaves (Gigon et al. 2004; Pham-Thi et al. 1985, 1987), whereas comparable information on root phospholipids and galactolipids levels and lipid saturation is scarce. In this study, we quantified the differences in root morphological traits and phospholipids and galactolipids levels between winter and spring wheat genotypes at variable water supplies. We hypothesized that drought stress would influence the root architecture and root phospholipids and galactolipids levels in winter and spring wheat genotypes, with variation in these traits potentially associated with drought tolerance.
Materials and methods
Materials
Four spring wheat genotypes [Treasure (cultivar), MT1016 (elite line), MN08106–6 (elite line), and IDO686 (cultivar)] and seven winter wheat genotypes [TAM111 (cultivar), TAM112 (cultivar), Yumar (cultivar), Jerry83 (cultivar), BYRD (cultivar), CO07W245 (variety), and Gallagher (cultivar)] were used for this research. The spring wheat genotypes Treasure and IDO686 were ranked high, and MN08106–6 and MT1016 were ranked low, for most of the root traits (rooting depth, total root length, total surface area in 0- to 30-cm and 30- to 60-cm soil depths, fine root length, and fine root surface area) in the association panel, comprising 250 genotypes (Narayanan and Prasad 2014). In field studies, the winter wheat genotype TAM111 was found to be drought-tolerant by recording higher grain yield under dryland conditions (Xue et al. 2014) while BYRD and Jerry83 were drought-susceptible than other genotypes (Xue et al. 2014; Reddy et al. 2014; Shroyer 2016).
Experimental details
Experiment 1. Genetic variability for seedling root traits
The seeds of four spring and seven winter wheat genotypes were surface-sterilized using 10% v/v sodium hypochlorite for 5 min and then washed with deionized water for three times. The seeds were germinated in Petri plates using filter paper (Whatman no 42) moistened with 5 mL of deionized water for 2 days. Sterilized agar (Sigma Type A; 2% w/v) was poured into the square Petri plates (12 × 12 × 1.7 cm, L × W × H) up to the rim and allowed to solidify. All the sides of Petri plates were sealed using cellophane tape (Staples® Invisible Tape, 2 × 3200 cm, Staples, Manhattan, KS). On the third day, uniformly sized seedling (radicle emerged) were selected and placed one per vertically-positioned plate with the radicle facing downwards through the cuts in the side of the Petri plates containing agar. The Petri plates were incubated at 25 ± 1 °C for 5 days (Manschadi et al. 2008). After the stipulated time, the root angle of individual root axes of the first and second pair of seminal roots, counting upwards from the primary seminal root (or radicle), was measured at 3 cm distance from the seed relative to a vertical line passing through the stem base (Manschadi et al. 2006). The angles of the first and second root pairs were averaged. After measuring the root angle, the seedlings were removed from agar, and the coleoptile length, seedling root length and number of seminal roots were measured for each seedling. Graphical overview of the experiment is presented in Fig. 1.
Experiment 2. Genetic variability for morpho-physiological traits under drought
To understand the differences in the root system characteristics and phospholipids and galactolipids contents among winter and spring wheat genotypes, the experiment was conducted in the greenhouse facilities at the Department of Agronomy, Kansas State University, Manhattan, KS. Before starting experiments, the greenhouse was fumigated for 1 h by using an automated sulfur vaporizer (Rosemania, Franklin, TN) to avoid a powdery mildew attack. Plants were grown in polyvinyl chloride (PVC) columns with inside diameter of 7.5 cm and height of 150 cm. The bottom of the PVC columns had plastic caps with a central hole of 0.5 cm diameter for drainage. Turface MVP® (PROFILE Products LLC, Buffalo Grove, IL) with bulk density of 577 ± 32 kg m−3 was used as rooting medium. Turface is non-swelling illite and silica clay that allows easy separation of roots. Before sowing, each PVC column was filled with Turface and fertilized with 4 g of Osmocote, a slow-release fertilizer with 19:6:12 gravimetric percentages of N:P2O5:K2O (Scotts, Marysville, OH) and 1 g of Marathon 1% G (granular; a.i.: Imidacloprid:1-[(6-chloro-3-pyridinyl)methyl]-N-nitro-2-imidazolidinimine; OHP, Inc., Mainland, PA) and evenly mixed with Turface in the top 2 cm.
Two seeds of each genotype were sown at 4 cm depth in each PVC column. After emergence, columns were thinned to one plant per column. Plants were irrigated daily with water (electrical conductivity 0.77 dS m−1 and pH ~8) at 06:00 (300 mL), 12:00 (300 mL) and 18:00 h (300 mL) through an automated drip irrigation system. The emission from the drip tubes was examined on alternate days for proper water delivery. Plants were maintained at 24/14 °C (daytime maximum/night time minimum temperature) from sowing to harvest (65 days after sowing) at a photoperiod of 16 h (natural light and supplemental fluorescent lights). The fungicide, Bumper 41.8 EC (emulsifiable concentrate; a.i.: Propiconazole: 1- [[2-(2,4 dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4–triazole; 1.2 mL L−1; Makhteshim Agan of North America, Inc., Raleigh, NC) was applied on 20 days after sowing to prevent powdery mildew attack.
Drought stress imposition at vegetative stage
From sowing to final harvest (65 days after sowing), the control plants were maintained at 100% field capacity by drip irrigation. In the drought treatment, the water was withheld from 5 days after emergence to final harvest. The spring wheat at harvest was in booting stage (Feeks 10) and the winter wheat was at two nodes visible stage (Feeks 7.0). Under drought stress, all genotypes showed leaf rolling symptoms starting 45 days after sowing.
Measurements
Plant height, maximum root length, total root length, and root diameter
Plant height was measured one day before the harvest, as the distance from Turface level to the ligule of the youngest leaf. At harvest, the PVC columns were gently inverted at about 140° to let the content (Turface and plants with entire root system) of columns slip out. The roots were carefully separated from Turface without any breakage in the root system. The shoots were cut at the base of the stem; the roots were laid on a flat surface and straightened to measure the maximum root length (from base of the stem to the tip of the root system). The root system was carefully washed in water to remove any adhering Turface, placed between the moist paper towels, sealed in Ziploc bags (S.C. Johnson & Sons, Inc. Racine, WI), transported to the laboratory, and stored at 4 °C.
The root system of each plant was sliced into 30-cm-long portions; each portion was submerged in water in a tray (20 × 15 × 2 cm; L × W × H) and carefully spread to minimize root overlaps, and was scanned using an Epson flatbed photo scanner with built in transparency unit (Epson Perfection V700 with 6400 dpi resolution, Epson, Long Beach, CA). Images of scanned roots were analyzed using WinRHIZO Pro image system (Regent Instruments, Inc., Quebec City, QC) to estimate root length, and root diameter as explained by McPhee (2005). The shoots were dried in an oven at 60 °C for 7 d for determining biomass. Root length:shoot length ratio for each genotype was calculated as the ratio of maximum root length to plant height (Tomar et al. 2016).
Chlorophyll index and leaf temperature
Leaf chlorophyll index and leaf temperature were measured at 5, 10, and 15 days after the appearance of the leaf rolling symptom on the fully expanded topmost leaf in all genotypes. Chlorophyll index was measured using a self-calibrating soil plant analysis development (SPAD) chlorophyll meter (Spectrum Technologies, Plainfield, IL). Leaf temperature was measured using FLIR BCAM SD thermal imaging camera (FLIR Systems Inc., Wilsonville, OR). Chlorophyll index data and leaf temperature were taken three times from the middle portion of the fully expanded topmost leaf, and the readings were averaged.
Electrospray ionization with tandem mass spectrometry (ESI-MS/MS) lipid profiling in root tips
Lipid profiling was done by utilizing a direct-infusion ESI triple quadrupole MS strategy. At harvest, bottom roots (~2-cm-long and ~0.5 g in weight) were collected, chopped into pieces and transferred to a 50-mL glass tube with a Teflon-lined screw cap (Thermo Fisher Scientific, Inc., Waltham, MA) containing 6 mL of hot isopropanol containing 0.01% w/v butylated hydroxytoluene (BHT) maintained in dry bath (75 °C; Dry bath incubator, Thermo Fisher Scientific Inc., Waltham, MA) for 15 min to deactivate lipid-hydrolyzing enzymes. After cooling the samples to room temperature, 3 mL of chloroform and 1.2 mL of deionized water were added, and samples were stored at −80 °C until analysis.
The lipid extraction procedure described by Vu et al. (2012) is common and widely followed in many crops (Welti et al. 2002; Narayanan et al. 2016; Djanaguiraman et al. 2018). Briefly, lipids were extracted in isopropanol, BHT, chloroform, and water by shaking on an orbital shaker at room temperature for 1 h and transferred to a new glass tube using a Pasteur pipette, leaving the root pieces in the original tube. An aliquot of 4 mL of chloroform:methanol (2:1) was added to the root pieces, the samples were shaken on an orbital shaker at room temperature overnight, and the solvent was combined with the first extract. The addition of extractant, shaking, and transfer steps were performed 4 times. Then, the solvent was evaporated from the extract in an N-EVAP 112 nitrogen evaporator (Organomation Associates, Inc., Berlin, MA). Finally, the lipid extract was dissolved in 1 mL of chloroform and stored at −80 °C. The extracted root pieces were dried in an oven at 105 °C overnight, cooled, and weighed to express the lipid content on a dry weight basis. Dry weights were determined using a balance (Mettler Toledo AX, Mettler Toledo International, Inc., Columbus, OH), which had a detection limit of 2 μg. An automated electrospray ionization-tandem mass spectrometry approach was used to quantify the phospholipids and galactolipids contents. Data acquisition and lipid profiling were carried out as described previously (Xiao et al. 2010; Narayanan et al. 2016).
Detailed information about quality control and accuracy of lipid species quantification was reported previously (Narayanan et al. 2016). Briefly, from the chloroform extracts, a known volume of aliquot, corresponding to known dry weight of root was added to precise amounts of internal standards (representing most of the lipid molecular species). Sequential precursor and neutral loss scans of the extracts produce a series of spectra with each spectrum revealing a set of lipid species containing a common head group or acyl fragment. The background of each spectrum was subtracted, the data were smoothed and peak areas were integrated using a custom script and Applied Biosystems Analyst software. LipidomeDB Data Calculation Environment was used for isotopic deconvolution and quantification by comparison to the two internal standards of the same class (Welti et al. 2002). A response factor was applied to the galactolipids; data calculated in comparison to the saturated internal standards was divided by 2.8 to account for the greater response of the mass spectrometer to unsaturated lipids when analyzed as [M + NH4]+ adducts.
Data analyses
Statistical analyses were performed using SAS programs version 9.4 (SAS Institute 2003, Cary, NC). The first experiment (genetic variability for seedling root traits) was set in complete randomized design with five replications and the second experiment (genetic variability for morpho-physiological traits under drought) in split-plot design with three replications wherein the main plots were water regimes, and sub-plots were genotypes. Both experiments were repeated. Data from experiment 1 and 2 and their corresponding repeats were statistically analysed independently and found that there were no significant differences. Therefore, the data from both the experiments were pooled together for combined statistical analyses and the mean responses are presented. The data for root phospholipids and galactolipids were measured in experiment 2.
To assess the overall effect of wheat type (winter and spring), the data from control and drought-stressed winter and spring wheat genotypes were subjected to an analysis of variance with the GLM procedure, and Fisher’s least significant difference (LSD) at 5% significance level was used to test differences between mean values of winter and spring wheat genotypes. The classification of wheat genotypes for drought tolerance was performed using principal component analyses (PCA) as described by Kakani et al. (2005) by considering the percent change over control. Eigenvectors generated by PCA were used to identify parameters that differentiated wheat genotypes for drought tolerance. The factor loading values of variables and genotypes in PC1 and PC2 were used to classify the variables and genotypes.
Results
Experiment 1. Genetic variability for seedling root traits
Genotypes varied significantly (p ≤ 0.05) for coleoptile length, number of seminal roots, seedling root length, and root angle (Fig. 2a-d; Supplementary Table S1). Among the genotypes, the winter wheat genotypes, Yumar (4.0 cm) and CO07W245 (7.1 cm) had the shortest and longest coleoptile length, respectively (Fig. 2a). Overall, spring wheat genotypes had more seminal roots (4.3) compared to winter wheat genotypes (3.9) (Fig. 2b). The genotypes Gallagher and Jerry83 had the lowest seedling root length compared with other genotypes (Fig. 2c). Spring wheat genotype MN08106–6 (46°) and winter wheat genotype CO07W245 (66°) had the narrowest and widest root angles, respectively (Fig. 2d).
Experiment 2. Genetic variability for morpho-physiological traits under drought
Principal component analyses (PCA)
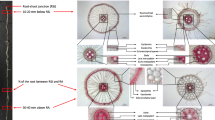
The PCA showed that the first two principal components represented 60% of the variability, with PC1 explaining 37% of the variance (Fig. 3). Along with the PC1, the major contributor to the variance was the molar percentage of MGDG (16%) followed by PG (16%). In PC2, the major contributors were root length:shoot length ratio (20%), followed by maximum root length (19%) (Fig. 3a). Among the genotypes, the highest variation along PC1 was caused by spring wheat genotypes IDO686 (33%) followed by MN08106-6 (27%), and in PC2 the highest variation was caused by winter wheat genotypes Gallagher (34%) followed by TAM111 (26%) (Fig. 3b).
First and second principal component scores (PC1 and PC2) for identifying traits conferring drought tolerance: a the factor loading values for variables were indicated by thick lines radiating from the center showing the direction (angle) and magnitude (length), and b classification of eleven wheat genotypes based on the factor scores of first and second principal components (experiment 2). Legend for (a): 1, plant height (cm); 2, total dry matter production (g plant−1); 3, maximum root length (cm); 4, root:shoot ratio; 5, total root length (cm); 6, root diameter (mm); 7, monogalactosyldiacylglycerol (MGDG, mol%); 8, digalactosyldiacylglycerol (DGDG, mol%); 9, phosphatidylglycerol (PG, mol%); 10, lysophosphatidylethanolamine (LPE, mol%); 11, lysophosphatidylcholine (LPC, mol%); 12, lysophosphatidylglycerol (LPG, mol%); 13, phosphatidic acid (PA, mol%); 14, phosphatidylethanolamine (PE, mol%); and 15, phosphatidylcholine (PC, mol%)
Plant height and total dry matter production
There were significant (P ≤ 0.05) effects of genotype, drought and interaction between genotype, and drought on plant height and total dry matter production (Fig. 4a, b; Supplementary Table S1). Overall, the spring wheat genotypes were taller (51 cm) than winter wheat genotypes (37 cm), and drought stress at vegetative stage decreased the plant height of all genotypes except the genotypes BYRD, TAM111, and IDO686 (Fig. 4a). Overall, the spring wheat genotypes accumulated less dry matter (0.9 g plant−1) than winter wheat genotypes (1.7 g plant−1) (Fig. 4b). Drought stress at vegetative stage decreased dry matter accumulation in all the genotypes and maximum decrease was observed in the genotypes BYRD (85%) followed by TAM112 (77%). The significant interaction for dry matter was caused by the drought effect being large in some genotypes and small in others.
Interaction effect of drought and genotype on (a) plant height (cm) and (b) total dry matter production (g plant−1) among winter and spring wheat genotypes (experiment 2). Vertical bars denote ± S.E. The control and drought treatments of each genotype were compared for significance at P ≤ 0.05 level, and the means with * were significantly different
Maximum root length and root length:Shoot length ratio
There were significant (P ≤ 0.05) effects of genotype, drought and interaction between genotype and drought on maximum root length, and root length:shoot length ratio (Fig. 5a, b; Supplementary Table S1). Overall, maximum root length (cm) was about ~1.5 times higher, and root length:shoot length ratio 2 times higher, in winter wheat than spring wheat genotypes (Fig. 5a, b). Drought stress at vegetative stage significantly (P ≤ 0.05) decreased maximum root length in BYRD, CO07W245, Jerry83, and TAM112. Similarly, in spring wheat the genotypes MT1016 and Treasure showed a decreased maximum root length (Fig. 5). Drought stress increased root length:shoot length ratio in the genotypes Gallagher, TAM111, and Yumar, with no change in the genotypes IDO686 and MN08106–6, whereas the other genotypes showed a decreased root length:shoot length ratio due to drought stress (Fig. 5b).
Interaction effect of drought and genotype on (a) maximum root length (cm) and (b) maximum root length:shoot length ratio among winter and spring wheat genotypes (experiment 2). Vertical bars denote ± S.E. The control and drought treatments of each genotype were compared for significance at P ≤ 0.05 level, and the means with * were significantly different
Total root length and average root diameter
Significant (P ≤ 0.05) effects of genotype, drought and interaction between genotype, and drought on total root length and average root diameter were observed (Fig. 6a, b; Supplementary Table S1). Across the genotypes, drought stress at vegetative stage decreased total root length by 59%. Among the genotypes maximum decrease was observed in Jerry83 (75%) followed by BYRD (73%), TAM112 (72%), CO07W245 (69%) and MT1016 (59%) (Fig. 6a). Across the genotypes, a subtle increase (3%) in root diameter was observed due to drought stress at vegetative stage (Fig. 6b), and the genotypes BYRD (22%), Jerry83 (15%), TAM111 (13%) and MT1016 (6%) had significant increase and genotype Treasure (7%) had significant decrease in root diameter under drought stress at vegetative stage.
Interaction effect of drought and genotype on (a) total root length (cm) and (b) average root diameter (mm) among winter and spring wheat genotypes (experiment 2). Vertical bars denote ± S.E. The control and drought treatments of each genotype were compared for significance at P ≤ 0.05 level, and the means with * were significantly different
Chlorophyll index and leaf temperature
Significant (P ≤ 0.05) differences among the genotypes were observed for leaf temperature and chlorophyll index (Supplementary Fig. S1a, b; Supplementary Table S1). The genotypes IDO686 (40 SPAD units) and Jerry83 (34 SPAD units) had the highest and lowest chlorophyll index, respectively (Supplementary Fig. S1a). Among the genotypes, Jerry83 and Yumar had the highest leaf temperature and genotypes MT1016 and Treasure had the lowest leaf temperature (Supplementary Fig. S1b).
The phospholipids and galactolipids profiles
There were significant (P ≤ 0.05) effects of genotype, drought and interaction between genotype and drought on the molar percentages of total MGDG, DGDG, PG (phosphatidylglycerol), PC) and PE (Fig. 7a-f; Supplementary Table S1). Across the genotypes, drought stress at vegetative stage has increased the molar percentage of total MGDG and DGDG by 23 and 32%, respectively (Fig. 7a, b). In contrast, the molar percentages of PG, PC and PE were decreased by 16, 11 and 14%, respectively (Fig. 7c-e). Overall, spring wheat genotypes had higher galactolipids (total MGDG and DGDG) molar percentages, compared to winter wheat genotypes. However, the phospholipids molar percentages (total PG and PC, and PA) were higher in winter wheat than spring wheat genotypes (Fig. 7a-f). On average, drought stress at vegetative stage increased the proportions of MGDG and DGDG in spring wheat genotypes by 60 and 96% respectively, relative to control. In contrast, no significant change was observed in winter wheat genotypes (Fig. 7a, b). Compared with control, the decreases in the proportions of PG, PC and PE due to drought were larger in spring wheat genotypes (38, 30, and 23%, respectively) than winter wheat genotypes (5, 1, and 8%, respectively) (Fig. 7c-e).
Interaction effect of drought and genotype on molar percentage of (a) total monogalactosyldiacylglycerol (MGDG), (b) total digalactosyldiacylglycerol (DGDG), (c) total phosphatidylglycerol (PG), (d) total phosphatidylcholine (PC), (e) total phosphatidylethanolamine (PE), (f) total phosphatidic acid (PA), (g) 34:2 MGDG, (h) 34:3 MGDG, (i) 36:4 MGDG, (j) 34:2 DGDG, (k) 34:3 DGDG, and (l) 36:4 DGDG molecular species (experiment 2). The double bond index (DBI) and total mol% of each lipid head group under control and drought stress along with its significance level was shown in their respective panel. Values shown are ± S.E. The control and drought treatments of each genotype were compared for significance at P ≤ 0.05 level, and the means with * were significantly different
Double bond index (DBI)
Across the genotypes, the DBI of DGDG and MGDG was significantly (P ≤ 0.05) decreased, and DBI of PG was significantly (P ≤ 0.05) increased by drought stress compared with the control (Fig. 7a-c). On average, in spring wheat genotypes drought stress at vegetative stage significantly (P ≤ 0.05) increased the DBI of PG, PC and PE, and decreased that of DGDG and MGDG in comparison with the control (data not shown). In contrast, in winter wheat genotypes no change in DBI of MGDG, DGDG, PC, PG, PE, and PA was caused by drought stress at vegetative stage (data not shown).
Lipid molecular species
There were significant (P ≤ 0.05) effects of genotype, drought and interaction between genotype, and drought on most of the molar percentages of 34:2, 34:3, and 36:4 molecular species of galactolipids (MGDG and DGDG; Fig. 7g-l; Supplementary Table S1) and 34:2, 34:3, 36:4 and 36:5 molecular species of phospholipids (PC, PG, PE, PI, and PA; Fig. 8a-l). Overall, the spring wheat genotypes had increased molar percentages of 34:2, 34:3 and 36:4 molecular species of MGDG and DGDG under drought stress compared to winter wheat genotypes (Fig. 7g-l). Similarly, taken as a whole, the molar percentages of 34:2, 34:3, 36:4, and 36:5 phospholipids were lower in spring than winter wheat genotypes due to drought stress at vegetative stage (Fig. 8a-l).
Interaction effect of drought and genotype on lipid molecular species (a) 34:2 PC, (b) 34:2 PG, (c) 34:2 PE, (d) 34:3 PC, (e) 34:3 PE, (f) 34:3 PA, (g) 36:4 PE, (h) 36:4 PG, (i) 36:4 PI, (j) 36:5 PC, (k) 36:5 PE, and (l) 36:5 PA (mol%) (experiment 2). Values shown are ± S.E. The control and drought treatments of each genotype were compared for significance at P ≤ 0.05 level, and the means with * were significantly different
Discussion
Principal component analysis has indicated that the major contributor for variability along PC1 was the levels of glycolipids and in PC2 it was root traits. Among the genotypes, the highest variation along PC1 was caused by spring wheat and in PC2 it was winter wheat. Hence, the overall effects of winter and spring wheat on root traits and lipid levels was considered to explain the mechanism of drought tolerance. The root architecture can influence the efficiency of water extraction from soil. Irrespective of the water regimes tested, winter wheat genotypes had ~1.5 times longer maximum root length and 2 times higher root length:shoot length ratio compared to spring wheat genotypes. The deep rooting system of winter wheat compared to spring wheat genotype may be due to its greater root penetration rate ability (Barraclough and Leigh 1984; Kirkegaard and Lilley 2007), longer duration of the crop (Kirkegaard and Lilley 2007) and presence of VERNALIZATION 1 gene, which is linked with narrow root angle (Voss-Fels et al. 2017). Deep rooting has been shown to be an important trait under drought stress because it underpins the potential to absorb soil moisture from deeper soil layers (Gowda et al. 2011; Uga et al. 2011; Comas et al. 2013; Vadez et al. 2013; Vadez 2014). A significant positive correlation between deep root system (having greater root length density and root mass in subsoil layers) with leaf relative water content, thousand grain weight and grain yield were observed in winter wheat under drought stress (Fang et al. 2017; Awad et al. 2018), indicating that abundant root length in deeper soil may enhance water acquisition. Similarly, under non-water limiting situation, a positive correlation among total root length, rooting depth and grain yield was observed in winter wheat (Xie et al. 2017), and the increased grain yield was associated with delayed maturity and longer grain filling period. Under drought stress, the tolerant plants tend to develop a deeper root system (high root length:shoot length ratio), resulting in roots that can reach into still-moist deeper soil layers (Rich and Watt 2013), to avoid the negative effects of drought stress (Manschadi et al. 2006; Kirkegaard et al. 2007). PCA analysis indicated that TAM111 is a drought tolerant genotype (Fig. 3b). Our result is in accordance with the finding of Xue et al. (2014), Reddy et al. (2014) and Thapa et al. (2018) who reported that TAM111 is a drought tolerant winter wheat genotype by increased water use efficiency.
The spring wheat genotype Treasure had a shallow rooting system but with high total root length compared to other spring wheat genotypes (Figs. 5a, b and 6a). High total root lengths for wheat were previously reported [Hu et al. 2018 > 30,000 cm; and Awad et al. 2018 > 11,500 cm]. As proposed by Lynch (2013), reduced lateral root branching in deep soil layers may be an adaptation to drought because it reduces the metabolic cost of soil exploration at depth. Shallow rooting systems, with strong branching, can occupy a greater topsoil volume than deep rooting systems, resulting in enhanced foraging within the topsoil (Valliyodan et al. 2017). He et al. (2017a, b) have observed that recently released soybean cultivar had higher yield than landraces and other cultivars under drought stress, and the increased yield is associated with lower root length densities and high specific root lengths. Spring wheat is mostly grown in areas where drought is episodic, which makes plant response to rewetting very important for maintaining yield. In this situation, shallow but highly branched root systems would take advantage of rewetting, essentially offering drought tolerance by avoidance (Price et al. 2002; Sanguineti et al. 2007; Zaman-Allah et al. 2011a; Vadez 2014).
The membrane lipid metabolism and remodeling, which modulate the lipid composition, fatty acyl group unsaturation, and membrane fluidity, is a way to adapt to abiotic stresses in plants (Repellin et al. 1997; Li et al. 2016). In the present study, drought stress increased the molar percentage of total galactolipid (MGDG and DGDG) in spring wheat genotypes, but no significant changes were observed in winter wheat genotypes (Fig. 7a, b). However, the levels of total PG in root was decreased by 16% due to drought stress. The increased molar percentage of galactolipid under drought stress in spring wheat genotypes may be due to direct or indirect trafficking of PC or PC-derived lipids to the plastids for galactolipid biosynthesis; alternatively, PA may be dephosphorylated to DAG to form galactolipids (Awai et al. 2001; Benning 2009; Kim et al. 2010; Moellering et al. 2010; Zheng et al. 2016; Lin et al. 2016). Earlier studies have shown that the total galactolipids level of leaves were decreased under drought stress (Repellin et al. 1997; Monteiro de Paula et al. 1993). However, in leaf the total PG level were increased under drought stress (Perlikowski et al. 2016). This opposite response of root and shoot under drought could be due to a shift in synthesis or allocation of metabolites to roots while decreasing allocation to shoots to sustain the root growth (Gargallo-Garriga et al. 2014). Modulation of the MGDG:DGDG ratio occurs in response to several abiotic stresses, including drought (Torres-Franklin et al. 2007).
In the present study the MGDG:DGDG ratio of spring wheat genotypes decreased by 18% under drought stress but there was no change in winter wheat genotypes. The MGDG:DGDG ratio is important for maintenance of lamellar bilayer structures (Hincha et al. 1998) because DGDG is a bilayer-forming lipid, whereas MGDG has a propensity to form non-lamellar hexagonal structures (Webb and Green 1991). Hence, a decrease in the MGDG:DGDG ratio under drought stress would have decreased the stability of the root-cell plasma membrane in spring wheat genotypes. The total PG and PC levels were decreased by drought stress in spring wheat genotypes, and no variation was observed in winter wheat genotypes (Fig. 7c, d). Similar decrease in PC level in roots of sugar beet roots exposed to low temperature, indicating the phospholipids in the membrane might have been hydrolyzed into fatty acids and other lipophilic substances by the activity of phospholipases (Guschina and Harwood 2006).
The ability to adjust membrane fluidity is often attributed to the regulation of membrane fatty acid desaturation and chain length (Orlova 2003). Under drought, there was no variation in the molar percentage of 34:2, 34:3 and 36:4 species of MGDG and DGDG in winter wheat genotypes than spring wheat genotypes (Fig. 7). This could be due to increased concentration of saturated 18:2 fatty acid and deactivation of desaturase under drought stress. The spring wheat genotypes had decreased total PC and increased total MGDG molar percentages under drought stress, indicating altered structure and fluidity of root-cell plasma membrane (Toumi et al. 2008). Earlier studies showed that the double bond index of phospholipids and glycolipid decreased in the drought-sensitive cultivar, whereas it remained unchanged in the drought-tolerant cultivars under drought stress (Repellin et al. 1997). Overall, lipid data indicates that under drought stress the membrane integrity is compromised under drought stress due to decreased levels of bilayer forming lipids and increased levels of saturated lipids.
Conclusions
Drought stress decreased plant height, total dry matter production, maximum root length, root length:shoot length ratio and other root traits. Winter wheat genotypes had ~1.5 times higher maximum root length than spring wheat genotypes. Significant differences in molar percentages of root phospholipids and galactolipid, molecular species and double bond index of galactolipid were observed among spring but not winter wheat genotypes. Based on the genotypes studied, the drought tolerant mechanism of winter wheat was associated with deep root system, and in spring wheat it was well branched (albeit shallow) root system with more unsaturated membrane lipids.
References
Andreou A, Brodhun F, Feussner I (2009) Biosynthesis of oxylipins in non-mammals. Progr Lipid Res 48:148–170
Awad W, Byrne PF, Reid SD, Comas LH, Haley SD (2018) Great plains winter wheat varies for root length and diameter under drought stress. Agron J 110:226–235
Awai K, Marechal E, Block MA, Brun D, Masuda T, Shimada H, Takamiya KI, Ohta H, Joyard J (2001) Two types of MGDG synthase genes, found widely in both 16:3 and 18:3 plants, differentially mediate galactolipid syntheses in photosynthetic and nonphotosynthetic tissues in Arabidopsis thaliana. Proc Natl Acad Sci 98:10960–10965
Barraclough PB, Leigh RA (1984) The growth and activity of winter-wheat roots in the field-the effect of sowing date and soil type on root-growth of high-yielding crops. J Agri Sci 103:59–74
Benning C (2009) Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol 25:71–91
Boudiere L, Michaud M, Petroutsos D, Rebeille F, Falconet D, Bastien O, Roy S, Finazzi G, Rolland N, Jouhet J et al (2014) Glycerolipids in photosynthesis: composition, synthesis and trafficking. Biochim Biophys Acta 1837:470–480
Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:442
Djanaguiraman M, Boyle DL, Welti R, Jagadish SVK, Prasad PVV (2018) Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol 18:55
Fang Y, Du Y, Wang J, Wu A, Qiao S, Xu B, Zhang S, Siddique KHM, Chen Y (2017) Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front Plant Sci 8:672
Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Ann Rev Plant Biol 64:429–450
Gargallo-Garriga A, Sardans J, Perez-Trujillo M, Rivas-Ubach A, Oravec M et al (2014) Opposite metabolic responses of shoots and roots to drought. Sci Rep 4:6829
Gigon A, Matos A, Laffray D, Zuily-fodil Y, Pham-Thi A (2004) Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann Bot 94:345–351
Gowda VRP, Henry A, Yamauchi A, Shashidhar HE, Serraj R (2011) Root biology and genetic improvement for drought avoidance in rice. Field Crops Res 122:1–13
Guschina IA, Harwood JL (2006) Mechanisms of temperature adaptation in poikilotherms. FEBS Lett 580:5477–5483
He J, Du Y-L, Wang T, Turner NC, Yang R-P, Jin Y et al (2017a) Conserved water use improves the yield performance of soybean (Glycine max (L.) Merr.) under drought. Agric Water Manag 179:236–245
He J, Jin Y, Du Y-L, Wang T, Turner NC, Yang R-P et al (2017b) Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front Plant Sci 8:1499
Hincha DK, Oliver AE, Crowe JH (1998) The effects of chloroplast lipids on the stability of liposomes during freezing and drying. Biochim Biophys Acta 1368:150–160
Hossain A, Teixeira da Silva JA, Lozovskaya MV, Zvolinsky VP (2012) High temperature combined with drought affect rainfed spring wheat and barley in south-eastern Russia: I. Phenology and growth Saudi J Biol Sci 19:473–487
Hu C, Tian Z, Gu S, Guo H, Fan Y, Abid M, Chen K, Jiang D, Cao W, Dai T (2018) Winter and spring night-warming improve root extension and soil nitrogen supply to increase nitrogen uptake and utilization of winter wheat (Triticum aestivum L.). Eur J Agron 96:96–107
Hund A, Ruta N, Liedgens M (2009) Rooting depth and water use efficiency of tropical maize inbred lines. differing in drought tolerance Plant Soil 318:311–325
Ihsan M, El-Nakhlawy FS, Ismail SM, Fahad S, Daur I (2016) Wheat phenological development and growth studies as affected by drought and late season high temperature stress under arid environment. Front Plant Sci 7:795
Kakani VG, Reddy KR, Koti S, Wallace TP, Prasad PVV, Reddy VR, Zhao D (2005) Differences in in-vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann Bot 96:59–67
Kim KU, Vijayan P, Carlsson AS, Barkan L, Browse J (2010) A mutation in the LPAT1 gene suppresses the sensitivity of fab1 plants to low temperature. Plant Physiol 153:1135–1143
Kirkegaard JA, Lilley JM (2007) Root penetration rate-a benchmark to identify soil and plant limitations to rooting depth in wheat. Aust J Exp Agric 47:590–602
Kirkegaard JA, Lilley JM, Howe GN, Graham JM (2007) Impact of subsoil water use on wheat yield. Aust J Agric Res 58:303–315
Larsson KE, Nystrom B, Liljenberg C (2006) A phosphatidylserine decarboxylase activity in root cells of oat (Avena sativa) is involved in altering membrane phospholipid composition during drought stress acclimation. Plant Physiol Biochem 44:211–219
Li N, Xu C, Li-Beisson Y, Philippar K (2016) Fatty acid and lipid transport in plant cells. Trends Plant Sci 21:145–158
Lin YT, Chen LJ, Herrfurth C, Feussner I, Li HM (2016) Reduced biosynthesis of digalactosyldiacylglycerol, a major chloroplast membrane lipid, leads to oxylipin overproduction and phloem cap lignification in Arabidopsis. Plant Cell 28:219–232
Liu HS, Li FM, Xu H (2004) Deficiency of water can enhance root respiration rate of drought-sensitive but not drought-tolerant spring wheat. Agr Water Manage 64:41–48
Liu X, Zhai S, Zhao Y, Sun B, Liu C, Yang A et al (2013) Overexpression of the phosphatidylinositol synthase gene (ZmPIS) conferring drought stress tolerance by altering membrane lipid composition and increasing ABA synthesis in maize. Plant Cell Environ 36:1037–1055
Lynch JP (2013) Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112:347–357
Manschadi AM, Christopher J, DeVoil P, Hammer GL (2006) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33:823–837
Manschadi AM, Hammer GL, Christopher JT, de Voil P (2008) Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 303:115–129
Masi CEA, Maranville JW (1998) Evaluation of sorghum root branching using fractals. J Agr Sci (Cambridge) 131:259–265
Matos AR, d'Arcy-Lameta A, Franca M, Petres S, Edelman L, Kader J, Zuily-Fodil Y, Pham-Thi AT (2001) A novel patatin-like gene stimulated by drought stress encodes a galactolipid acyl hydrolase. FEBS Lett 491:188–192
McPhee K (2005) Variation for seedling root architecture in the core collection of pea germplasm. Crop Sci 45:1758–1763
Moellering ER, Benning C (2011) Galactoglycerolipid metabolism under stress: a time for remodeling. Trends Plant Sci 16:98–107
Moellering ER, Muthan B, Benning C (2010) Freezing tolerance in plants requires lipid remodeling at the outer chloroplast membrane. Science 330:226–228
Monteiro de Paula F, Pham-Thi AT, Zuily-Fodil Y, Ferrari-Iliou R, Vieira da Silva J, Mazliak P (1993) Effect of water stress on the biosynthesis and degradation of polyunsaturated lipid molecular species in leaves of Vigna unguiculata. Plant Physiol Biochem 31:707–715
Narayanan S, Prasad PVV (2014) Characterization of a spring wheat association mapping panel for root traits. Agron J 106:1593–1604
Narayanan S, Mohan A, Gill KS, Prasad PVV (2014) Variability of root traits in spring wheat germplasm. PLOSOne 9:e100317
Narayanan S, Tamura PJ, Roth MR, Prasad PV, Welti R (2016) Wheat leaf lipids during heat stress: I. High day and night temperatures result in major lipid alterations. Plant Cell Environ 39:787–803
Neale AD, Blomstedt CK, Bronson P, Le TN, Guthridge K et al (2000) The isolation of genes from the resurrection grass Sporobolus stapfianus which are induced during severe drought stress. Plant Cell Environ 23:265–277
Norberg P, Liljenberg P (1991) Lipids of plasma membranes prepared from oat root cells. Plant Physiol 96:1136–1141
Orlova IV (2003) Transformation of tobacco with a gene for the thermophilic acyl-lipid desaturase enhances the chilling tolerance of plants. Plant Cell Physiol 44:447–450
Pantalone VR, Rebetzke GJ, Burton JW, Carter TE, Israel DW (1999) Soybean PI 416937 root system contributes to biomass accumulation in reciprocal grafts. Agron J 91:840–844
Perlikowski D, Kierszniowska S, Sawikowska A, Krajewski P, Rapacz M, Eckhardt A, Kosmala A (2016) Remodeling of leaf cellular glycerolipid composition under drought and re-hydration conditions in grasses from the Lolium-Festuca complex. Front Plant Sci 7:1027
Pham-Thi AT, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P (1985) Effects of water stress on lipid metabolism in cotton leaves. Phytochemistry 24:723–727
Pham-Thi AT, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P (1987) Effects of drought on [1-14C]-oleic and [1-14C]-linoleic acid desaturation in cotton leaves. Physiol Plant 69:147–150
Prasad PVV, Staggenborg SA, Ristic Z (2008) Impact of drought and heat stress on physiological, growth and yield process. In: modeling water stress effects on plant growth processes. L.H. Ahuja and S.A. Saseendran (Eds). Advances in Agricultural Systems Modeling 1:301–355
Prasad PVV, Djanaguiraman M, Jagadish SVK, Ciampitti IA (2018) Drought and high temperature stress and traits associated with tolerance. In: Ciampitti I, Prasad PVV (eds) Sorghum: state of the art and future perspectives, agronomy monograph 58. ASA and CSSA, Madison, WI, USA
Price AH, Cairns JE, Horton P, Jones HG, Griffiths H (2002) Linking drought-resistance mechanisms to drought avoidance in upland rice using a QTL approach: progress and new opportunities to integrate stomatal and mesophyll responses. J Exp Bot 53:989–1004
Ratnakumar P, Vadez V (2011) Groundnut (Arachis hypogaea) genotypes tolerant to intermittent drought maintain a high harvest index and have small leaf canopy under stress. Funct Plant Biol 38:1016–1023
Reddy SK, Liu S, Rudd JC, Xue Q, Payton P, Finlayson SA, Mahan J, Akhunova A, Holalu SV, Lu N (2014) Physiology and transcriptomics of water deficit stress responses in wheat cultivars TAM 111 and TAM 112. J Plant Physiol 171:1289–1298
Repellin A, Pham-Thi AT, Tashakorie A, Sahsah Y, Daniel C, Zuily-Fodil Y (1997) Leaf membrane lipids and drought tolerance in young coconut palms (Cocos nucifera L.). Eur J Agron 6:25–33
Reynolds MP, Ortiz R (2010) Adapting crops to climate change: a summary. In: Reynolds MP (eds) Climate change and crop production, CAB international, pp 1–8
Rich SM, Watt M (2013) Soil conditions and cereal root system architecture: review and considerations for linking Darwin and weaver. J Exp Bot 64:1193–1208
Sanguineti MC, Li S, Maccaferri M, Corneti S, Rotondo F, Chiari T, Tuberosa R (2007) Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann Appl Biol 151:291–305
SAS Institute (2003) SAS Users Guide. Version 9.1. SAS Institute, Cary, NC
Semenov MA, Shewry PR (2011) Modelling predicts that heat stress and not drought will limit wheat yield in Europe. Sci Rep 1:1–5
Shroyer KJ (2016) The effects of drought and high temperature stress on reproduction, physiology, and yield of spring and winter wheat. Ph. D thesis submitted to Department of Agronomy, Kansas State University, Manhattan, Kansas 66502
Siddique KHM, Belford RK, Tennant D (1990) Root:shoot ratios of old and modern, tall and semi-dwarf wheats in a Mediterranean environment. Plant Soil 121:89–98
Thapa S, Reddy SK, Fuentealba MP, Xue Q, Rudd JC, Jessup KE, Devkota RN, Liu S (2018) Physiological responses to water stress and yield of winter wheat cultivars differing in drought tolerance. J Agro Crop Sci 00:1–12. https://doi.org/10.1111/jac.12263
Thorup-Kristensen K, Salmeron Cortasa M, Loges R (2009) Winter wheat roots grow twice as deep as spring wheat roots, is this important for N uptake and N leaching losses? Plant Soil 322:101–114
Tomar RSS, Tiwari S, Vinod NBK, Chand S, Deshmukh R et al (2016) Molecular and morpho-agronomical characterization of root architecture at seedling and reproductive stages for drought tolerance in wheat. PLoS One 11(6):e0156528
Torres-Franklin M-L, Gigon A, de Melo DF, Zuily-Fodil Y, Pham-Thi A-T (2007) Drought stress and rehydration affect the balance between MGDG and DGDG synthesis in cowpea leaves. Physiol Plant 131:201–210
Toumi I, Gargouri M, Nouairi I, Moschou PN, Ben Salem-Fnayou A, Mliki A, Zarrouk M, Ghorbel A (2008) Water stress induced changes in the leaf lipid composition of four grapevine genotypes with different drought tolerance. Biol Plant 52:161–164
Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot 62:2485–2494
Vadez V (2014) Root hydraulics: the forgotten side of roots in drought adaptation. Field Crop Res 165:15–24
Vadez V, Rao JS, Bhatnagar-Mathur P, Sharma KK (2013) DREB1A promotes root development in deep soil layers and increases water extraction under water stress in groundnut. Plant Biol 15:45–52
Valliyodan B, Ye H, Song L, Murphy M, Shannon JG, Nguyen HT (2017) Genetic diversity and genomic strategies for improving drought and waterlogging tolerance in soybeans. J Exp Bot 68:1835–1849
Voss-Fels KP, Robinson H, Mudge SR, Richard C et al (2017) VERNALIZATION1 modulates root system architecture in wheat and barley. Mol Plant 11:226–229
Vu HS, Tamura P, Galeva NA, Chaturvedi R, Roth MR, Williams TD, Wang X, Shah J, Welti R (2012) Direct infusion mass spectrometry of oxylipin-containing Arabidopsis membrane lipids reveals varied patterns in different stress responses. Plant Physiol 158:324–339
Wachsman G, Sparks EE, Benfey PN (2015) Genes and networks regulating root anatomy and architecture. New Phytol 208:26–38
Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SV, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M (2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63:3485–3498
Webb MS, Green BR (1991) Biochemical and physical properties of thylacoid acyl lipids. Biochim Biophys Acta 1060:133–158
Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277:31994–32002
Xiao S, Gao W, Chen QF, Chan SW, Zheng SX, Ma J, Wang M, Welti R, Chye ML (2010) Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell 22:1463–1482
Xie Q, Fernando KMC, Mayes S, Sparkes DL (2017) Identifying seedling root architectural traits associated with yield and yield components in wheat. Ann Bot 119:1115–1129
Xue Q, Rudd JC, Liu S, Jesup E, Devkota RN, Mahan JR (2014) Yield determination and water-use efficiency of wheat under water-limited conditions in the U.S southern high plains. Crop Sci 54:34–47
Yoshimura K, Masuda A, Kuwano M, Yokota A, Akashi K (2008) Programmed proteome response for drought avoidance/tolerance in the root of a C3 xerophyte (wild watermelon) under water deficits. Plant Cell Physiol 49:226–241
Zaman-Allah M, Jenkinson DM, Vadez V (2011a) Chickpea genotypes contrasting for seed yield under terminal drought stress in the field differ for traits related to the control of water use. Funct Plant Biol 38:270–281
Zaman-Allah M, Jenkinson DM, Vadez V (2011b) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J Exp Bot 62:4239–4252
Zhan A, Schneider H, Lynch JP (2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168:1603–1615
Zheng G, Tian B, Zhang F, Tao F, Li W (2011) Plant adaptation to frequent alterations between high and low temperatures: remodelling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ 34:1431–1442
Zheng G, Li L, Li W (2016) Glycerolipidome responses to freezing- and chilling-induced injuries: examples in Arabidopsis and rice. BMC Plant Biol 16:70
Acknowledgements
We thank the Triticeae Coordinated Agricultural Project (Triticeae-CAP, Grant no. 2011-68002-30029 from the USDA NIFA), and United States Agency for International Development (USAID) Feed the Future Innovation Lab for Climate Resilient Wheat (Grant no. AID-0AA-A-13-00008); and Feed the Future Innovation Lab for Collaborative Research on Sustainable Intensification (Grant no. AID-OAA-L-14-00006). The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory; instrument acquisition and lipidomics method development was supported by the National Science Foundation (EPS 0236913, MCB 0920663, MCB 1413036, DBI 0521587, DBI 1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of the National Institute of Health (P20GM103418), and Kansas State University. The senior author (MD) thanks Tamil Nadu Agricultural University, India, for permitting him to perform post-doctoral research at Kansas State University. Author ZR is supported by Australian Research Council (DP160104434). Mention of trademark or proprietary product does not constitute a guarantee or warranty of the product by Kansas State University and does not imply its approval to the exclusion of other products, which may also be suitable. This publication is Contribution no. 18-137-J from the Kansas Agricultural Experiment Station.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jairo A. Palta.
Electronic supplementary material
ESM 1
(DOCX 448 kb)
Rights and permissions
About this article
Cite this article
Djanaguiraman, M., Prasad, P.V.V., Kumari, J. et al. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 439, 57–73 (2019). https://doi.org/10.1007/s11104-018-3794-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3794-3