Abstract
Background and aim
Accumulation of heavy metals in soil causes loss of cover vegetation and increases the production of reactive oxygen species (ROS). ROS accumulation induces the expression of genes encoding antioxidant enzymes and other proteins involved in redox homeostasis. This study aimed to evaluate the interaction between a saprophytic fungal consortium and mycorrhizal Rhizophagus irregularis with regard to the oxidative stress and molecular responses of Solanum lycopersicum L. grown in a soil contaminated with heavy metals.
Methods
We determined the effects of the saprophytic fungal consortium (Bjerkandera adusta and Mortierella sp) and the mycorrhizal fungus Rhizophagus irregularis on the plant antioxidant response and the expression levels of genes encoding metallothioneins (MT), phytochelatins (PC), the NRAMP transporter and heat shock protein (HSP) in Solanum lycopersicum cultivated in a heavy metal-contaminated soil.
Results
The fungal consortium increased plant growth, and the co-inoculation with R. irregularis synergistically improved soil biochemical activities. Superoxide dismutase activity decreased in all treatments. Peroxidase activity (ascorbate and guaiacol) increased in plants inoculated with R. irregularis and the fungal consortium. Dual inoculation decreased the malondialdehyde content in the leaves and increased transcription of the NRAMP, GR, MT2b, PCS and HSP90 genes.
Conclusions
Our results demonstrate that co-inoculation contributes to reduced plant stress by improving defence mechanisms and homeostasis
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal contamination is one of the most serious pollution issues caused by anthropogenic activities and can persist in soil for a long period of time. High concentrations of heavy metals in soil have a selective effect on plant populations and can bioaccumulate. Heavy metals such as copper, lead, mercury and zinc are continuously added to soil from toxic mining waste (Khan 2005) and pollute water and soil, producing changes in topography, hydrography and chemistry of terrestrial and aquatic systems (Coelho et al. 2011). Some heavy metals are essential nutrients required by plants for their normal development; however, excessive concentrations can cause several toxic effects. Toxicity can be caused by interfering with photosynthesis, respiratory processes, and by inactivating enzymes and protein synthesis (Chibuike and Obiora 2014). Tolerance is the capacity of plants or microorganisms to live and adapt to elevated heavy metal concentrations in soil. Soil microorganisms are important to plant development and growth because they produce growth-stimulating substances such as hormones and vitamins (Shetty et al. 1994). Fungi have great potential for use in bioremediation processes, and arbuscular mycorrhizal (AM) fungi are particularly attractive in this regard. AM fungi protect plant against heavy metal pollution (Andrade et al. 2010; Medina et al. 2010). Saprophytic fungi are also important components of the rhizosphere and can produce substances that promote the growth of microorganisms, as do AM fungi, by enhancing heavy metal tolerance in plants (Arriagada et al. 2009). The joint inoculation of plants with AM and saprophytic fungi enhance plant tolerance to high levels of heavy metals in soil. Saprophytic fungi such as Mortierella sp., in interaction with Glomus aggregatum and Glomus mosseae, improve plant growth due to their ability to solubilize phosphate, thus increasing plant nutrient uptake (Zhang et al. 2011).
In addition, heavy metals cause an imbalance in redox homeostasis by inducing reactive oxygen species (ROS) such as singlet oxygen (1O2), superoxide radicals (O2•¯), hydrogen peroxide (H2O2) and hydroxyl radicals (•OH) (Gill and Tuteja 2010). An increase in ROS can cause non-specific oxidation of proteins and membrane lipids, which results in an increase in the malondialdehyde (MDA) content (Chamseddine et al. 2009). Plants possess an antioxidant system to combat oxidative damage in response to high concentrations of heavy metals. These antioxidant defence systems are composed of several ROS-removing enzymes, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX), and glutathione reductase (GR). Plants also have non-enzyme defence mechanisms, such as ascorbic acid, glutathione, phenolic compounds, alkaloids and α-tocopherol (Schützendübel and Polle 2002). Moreover, plants have other mechanisms in place to resist heavy metal stress: they can reduce heavy metal concentrations in cells via extracellular precipitation, cell wall biosorption, uptake reduction or increased heavy metal efflux (Hossain et al. 2010), and binding of heavy metals by proteins/peptides such as metallothioneins (MTs) and phytochelatins (PCs) (Fosso-Kankeu and Mulaba-Bafubiandi 2014) inside the fungal or plant cells. Moreover, heavy metals induce the synthesis of proteins such as heat shock proteins (HSPs), which protect the membrane and repair the damage to proteins caused by metal stress (Neumann et al. 1994). In summary, the interaction of AM and saprophytic fungi improves plant growth in the presence of heavy metal oxidative stress because reactive oxygen species accumulate in the roots, thus inducing the expression of genes that are important to maintain redox homeostasis. We hypothesize that co-inoculation with an AM fungi and saprophytic fungal consortium can improve defence mechanisms and reduce plant stress, even in soil polluted with heavy metals. To address this hypothesis, we determined the effects of mixing a saprophytic fungal consortium (Bjerkandera adusta and Mortierella sp) and the AM fungi Rhizophagus irregularis on the oxidative stress and molecular response of Solanum lycopersicum L. cultivated in a soil contaminated with heavy metals.
Materials and methods
Study site
The soil samples were collected from the surface horizon (0–20 cm) in the Puchuncaví Valley in central Chile, 1.5 km southeast of the Ventanas copper smelter (32°46´30″S; 71° 28´17″W). The soil is classified as an Entisols Chilicauquén series (Cornejo et al. 2008) and has a pHw of 5.54, 2.41 % organic matter and N, P, K content (in mg kg−1) of 28.7, 40.3 and 210, respectively. The soil has been characterized as having higher contents of heavy metals due to air emissions from the copper smelter, which contain Cu, As, Pb and Zn (Chiang et al. 1985).
Microorganisms
The fungal consortium comprising Bjerkandera adusta and Mortierella sp. was obtained from the fungi collection of the Bioremediation Laboratory at the Universidad de La Frontera, Temuco, Chile. To assess their mutual compatibility, interaction studies were conducted on both strains. These were stored on potato dextrose agar (PDA) plates at 4 °C and periodically subcultured. The fungal consortium comprised B. adusta and Mortierella sp. (Almonacid et al. (2015)) and was grown on sterile wheat residue.
The arbuscular mycorrhizal (AM) fungus Rhizophagus irregularis (Krüger et al. 2012) was obtained from the culture collection at the Bioremediation Laboratory at the Universidad de La Frontera, Temuco, Chile. The AM inoculum was a root-and-soil inoculum consisting of rhizosphere soil containing spores and colonized root fragments of Medicago sativa L. in amounts of 8 g per pot, which were previously analysed and found to have a high potential to produce significant levels of root colonization.
Greenhouse experiments and plant growth conditions
Experiments were conducted using Solanum lycopersicum L. as test plants. Seeds were surface-sterilized with NaClO for 15 min, thoroughly rinsed with sterilized distilled water and sown in sterile vermiculite. Four weeks after germination, uniform seedlings were transferred to 1 L pots with a 1:4 (v:v) mixture of sand and soil (2 mm particle size). Plants were grown in a greenhouse with supplementary light provided by Sylvania incandescent cool white lamps (400 μmol m−2 s−1, 400–700 nm) with a 16/8 h day/night cycle at 24/16 °C and 50 % relative humidity. Plants were watered from below and fertilized every week with 10 mL of nutrient solution plus 50 mg L−1 of P (Hewitt and Bureaux 1966). Each pot was treated with 8 g of inoculum, an amount previously determined to achieve high levels of root colonization. Uninoculated plants were given a filtrate (Whatman no. 1 filter paper) of the inoculum containing the common soil microflora, but free of AM fungal propagules.
The treatments were as follows: (1) non-mycorrhizal control plants (NM) (2) plants inoculated with R. irregularis (M); each of these treatments were assayed with or without the saprophytic fungal consortium Bjerkandera adusta and Mortierella sp. Six replicate pots were used per treatment.
Measurements
Plants were harvested after 12 weeks of growth and the shoots and roots were separated and stored at −80 °C for antioxidant enzyme activity and relative expression assays. Plant samples were dried in an air-forced oven (70 °C, 48 h) and weighed to determine biomass production. N, P, K, Ca, Mg, Fe, As, Cd, Cu, Pb and Zn were quantified using inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Electron Corporation Model IRIS intrepid II XDL).
Saprobe fungal evaluation
Pot soil was sampled to evaluate the B. adusta and Mortierella sp. populations (García-Romera et al. 1998). Approximately 1.5 g of rhizosphere soil was taken from each of the experimental pots, and 10-fold aqueous dilution series (from 10−1 to 10−4) were prepared from each sample. The number of saprobe colony forming units (CFUs) in suitable dilutions of such samples, taken from the six replicate pots of each treatment, was counted on PDA medium and identified up to the genera (Domsch et al. 1980).
Mycorrhizal characterization
After the harvest, fresh samples of roots were taken from the entire root system at random. The samples were cleared in 10 % KOH (w/v) and stained with 0.05 % using trypan blue in lactic acid (Phillips and Hayman (1970)). The percentage of mycorrhizal root colonization was evaluated microscopically using 30 1-cm root fragments per sample and calculated as mycorrhizal frequency (F%), mycorrhizal intensity (M%) and arbuscule abundance (A%) (Trouvelot et al. (1986)), http://www.dijon.inra.fr/mychintec/Mycocal-prg/download.html).
Biochemical characterization of soil rhizosphere
β-glucosidase activity was determined by detection of p-nitrophenol (PNP) released from p-nitrophenyl-β-D-glucopyranoside (PNG). Acid phosphatase activity was determined using p-nitrophenyl phosphate (PNPP) as a substrate. In both assays, the amount of PNP formed was determined by spectrophotometry at 398 nm (Tabatabai and Bremner (1969)). Urease activity was determined using Kandeler and Gerber’s method (Kandeler and Gerber 1988) based on the colorimetric determination of ammonium. The absorbance of ammonium ions was measured at 490 nm. Fluorescein diacetate (FDA) hydrolysis was assessed as described by Adam and Duncan (2001) and expressed as μg fluorescein released per g of dry soil. The final concentration of FDA was measured as absorbance at 490 nm.
Antioxidant enzyme activity
The plant leaves were powdered in a cold mortar with liquid nitrogen and then homogenized in 2 ml of 0.1 M potassium phosphate buffer (pH 7.0). The homogenate was filtered and centrifuged at 17,000 g for 15 min at 4 °C. The supernatant was used to measure the enzymatic activities of superoxide dismutase (SOD), ascorbate peroxidase (APX) and guaiacol peroxidase (GPX).
Superoxide dismutase activity (SOD, EC 1.15.1.1) was determined based on the method of Beauchamp and Fridovich (1971) as described by Donahue et al. (1997), which measures the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT). One unit of SOD was defined as the amount of enzyme required to cause a 50 % inhibition of the photochemical reduction of NBT. Ascorbate peroxidase activity (APX, EC 1.11.1.11) was measured as a decrease in absorbance at 290 nm after 1 min. The molar extinction coefficient of 2.8 mM−1 cm−1 was used (Zhao and Blumwald 1998). Guaiacol peroxidase activity (GPX, EC 1.11.1.11) was determined following the change in absorbance at 470 nm due to oxidation of guaiacol according Pinhero et al. (1997). To calculate enzymatic activity, the molar extinction coefficient of 26.6 mM−1 cm−1 was used. Protein concentrations were estimated using the Bradford assay and known concentrations of bovine serum albumin as standards (Bradford 1976).
Lipid peroxidation
Lipid peroxidation was approximated as described by Heath and Packer (1968) and modified by Du and Bramlage (1992) based on the levels of malondialdehyde (MDA) as determined by the reaction given by thiobarbituric acid (TBA). The MDA concentration was calculated using a molar extinction coefficient of 155 mM−1 cm−1.
Real-time quantitative PCR
Total RNA was isolated from 100 mg of frozen leaf or root using RNA-Solv Reagent (EZNA) following the manufacturer’s instructions. Samples were treated with RNase-free DNase I (E.Z.N.A) and purified with HiBind RNA Mini Columns from the Total RNA Kit I (E.Z.N.A). RNA concentrations were spectrophotometrically measured using a MaestroNano spectrophotometer (Maestrogen®), and RNA quality was verified by visualization on an agarose denaturing gel stained with GelRed™ (Biotium). First-strand cDNA synthesis was obtained from 1 μg total RNA using the AffinityScript RT-qPCR cDNA Synthesis Kit (Stratagene, Cedar Creek, TX, USA) following the supplier’s instructions. Primer sets were designed using the AmplifiX 1.5.4 software. The sequence of target mRNA for S. lycopersicum was obtained from NCBI (National Center for Biotechnology Information). The names of the genes and primer sequences are shown in Table 1. Elongation factor 1 α (EF1) and phosphoglycerate kinase (PGK) were used for qPCR normalization in samples from leaves and ribosomal protein L2 (RPL2) and β-tubulin (TUB) were used for qPCR normalization in samples from roots. The cDNA samples were diluted tenfold and 2 μL of cDNA were mixed with 0.3 μM forward and reverse primer, 10 μL Fast SYBR Green Master Mix (2×) (Applied Biosystems) and 7.5 μL of nuclease-free water. Non-template controls contained 2 μL of nuclease-free water. Quantification PCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems) under the following thermal cycles: 10 min at 95 °C and 40 cycles of 15 s at 95 °C, 15 s at 60 °C, 15 s at 72 °C. A melting curve analysis was performed after the 40 cycles to verify the specificity of the reactions and the amplification of a single product for each primer pair.
Statistical analysis
The percentage values were arcsine-transformed before statistical analysis. The data were analysed using two-way analysis of variance with AM fungi treatment (control, R. irregularis), saprophytic fungal consortium treatment (control, B. adusta and Mortierella sp) and their interaction as sources of variation (Sokal and Rohlf 1981). Statistical procedures were carried out using the SPSS software, v. 11.0 (SPSS Inc., 1989–2001). Statistical significance was determined as P < 0.05.
Results
Growth parameters and mycorrhizal colonization
Figure 1 shows that the fungal consortium (B. adusta and Mortierella sp.) increased the shoot dry weight of S. lycopersicum in treatments with and without the inoculation with R. irregularis. Nevertheless, the means from these treatments did not differ significantly. The root dry weight was increased only in plants treated with the co-inoculation of the fungal consortium and R. irregularis (Fig. 1). The population of the different saprobe fungi in the rhizosphere of the tomato plants was not affected by the presence of the AM fungus (data not shown).
Table 2 shows the levels of mycorrhizal root colonization for each treatment. Plants inoculated with R. irregularis had low to moderate levels of S. lycopersicum root colonization, with maximum mycorrhizal frequencies (F) up to 37.6 %, a mycorrhizal intensity (M) up to 10.5 % and an arbuscule abundance up to 3.4 % (A). The same trend was observed for plants co-inoculated with both R. irregularis and the fungal consortium (they did not differ significantly).
Plant nutrients
The concentrations of the main nutrients in the biomass components are shown in Table 3. The N, P, K, Ca, and Fe levels increased in plants shoots of plants that were co-inoculated with R. irregularis and the fungal consortium (Table 3A). Furthermore, co-inoculation induced a decrease in the Mg content of the plant tissues, while metals such as Cd, Cu, Pb and As (metalloid) were significantly higher. The Zn levels decreased in co-inoculated plants compared to non-mycorrhizal plants. No significant differences were observed in N, P, K, Ca or Mg levels in plant roots, but the co-inoculation of R. irregularis and the fungal consortium induced a significant increase in the levels of Fe and Cd, Cu, Pb and As in the plant roots (Table 3b).
Soil enzyme activities
The β-glucosidase and acid phosphatase activities in the soil were significantly increased when plants were inoculated with R. irregularis (Table 4), whereas the urease activity and fluorescein diacetate was not significantly altered. Inoculation of the soil with the fungal consortium significantly increased the β-glucosidase, acid phosphatase, urease activities and the levels of FDA, but this effect was more evident in the plants co-inoculated with R. irregularis and the fungal consortium for all soil enzyme activities measured.
Antioxidant enzyme activities
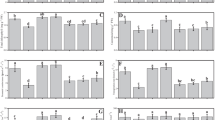
Table 5 shows that SOD activity decreased in plants treated with the fungal consortium, but a marked decrease in enzyme activity was observed in mycorrhizal plants. Increases in the peroxidase activity, APX and GPX were observed in plants treated with the fungal consortium; however, a higher antioxidant activity was observed when plants were colonized with R. irregularis.
MDA levels, often used as a marker of oxidative stress, were used to estimate the levels of lipid peroxidation. The MDA levels were higher in controls than in plants treated with the fungal consortium, whereas mycorrhizal-treated plants showed lower levels of MDA. The interaction of R. irregularis and the fungal consortium resulted in lower levels of MDA across all treatments.
Defence gene expression
In this study, we analysed the expression of genes involved in the stress response and in heavy metal homeostasis in leaves. Figure 2 shows that non-significant differences were observed between the relative expression of the root-specific metal transporter gene NRAMP 1 in mycorrhizal and non-mycorrhizal plant roots. There was a significant increase in NRAMP 1 gene expression in cells of plants inoculated with the fungal consortium compared to the control plants, and a greater relative level of NRAMP 1 gene expression was observed in AM plants co-inoculated with the fungal consortium.
The expression of the gene encoding glutathione reductase (Gr), an enzyme involved in ROS-scavenging often used as a marker for ROS, was also analysed. Higher expression levels of glutathione reductase (Gr) were observed in the leaves of the control AM plants in the AM plants with the fungal consortium (Fig. 3a). The fungal consortium also increased the expression of GR in non-mycorrhizal plants.
While a small increase in GR transcription was observed in non-mycorrhizal control plants, the transcription of the phytochelatin synthase gene (PCS) was increased significantly in the shoot when the plant was co-inoculated with R. irregularis and the fungal consortium (Fig. 3b). Similarly, the expression of the gene encoding a metallothionein (MT2b) was higher in plant leaves when the plant was co-inoculated with R. irregularis and the fungal consortium (Fig. 3c). However, mycorrhizal colonization increased the relative expression in control plants when compared to non-mycorrhizal plants.
HSPs have been characterized as markers of stress in toxic environments; in our analysis we found the highest induction of the HSP90 gene in leaves of plants inoculated with the AM fungus, which increased further with the addition of the saprophytic fungal consortium (Fig. 3d).
Discussion
The optimal condition for plant development is closely related to the soil-plant interactions and microbial processes in the rhizosphere. Soil near mining smelters commonly contains heavy metals that cause the loss of vegetation and microbial communities that live in the soil, producing an imbalance in this environment (Ginocchio 2000; Wang et al. 2007).
The incorporation of root free-living microorganisms and AM fungi into the soil, individually or in interacting consortia, benefits the establishment and development of plants even in soil contaminated with heavy metals (Arriagada et al. 2010). We found that the fungal consortium (B. adusta and Mortierella sp) inoculated individually or together with the AM fungus increased the dry matter of S. lycopersicum. However, the fungal consortium did not affect the levels of AM colonizing tomato plants. Saprophytic fungi do not always enhance the colonization, development and function of AM fungi because this process is highly dependent on the plant, fungi and soil characteristics (Fracchia et al. 1998; Gryndler 2000). On the other hand, as has been observed for several saprobe and AM fungal interactions (García-Romera et al. 1998; Fracchia et al. 2000), R. irregularis did not affect the number of saprobe fungi B. adusta or Mortierella sp. Despite this, it is known that interactions between saprophytic microorganisms and AM fungi in the so-called mycorrhizosphere can improve plant growth and metabolic function of soil microbial populations (Barea et al. 2013). Our results show that R. irregularis improved the beneficial effects of the fungal consortium on plant dry weight and shoot nutrient uptake. These microorganisms, AM and saprophytic fungi, increased the biochemical and biological activity of the soil, exerting an influence on plant growth and development, possibly due to their ability to decompose organic matter and cycle nutrients (Stark et al. 2007). In fact, there was greater biological activity in the soil, reflected in the high levels of FDA activity. Similarly, the highest values of β-glucosidase and urease enzymes are directly related to organic matter degradation and phosphatase involved in the mineralization of organic P (Ros et al. 2006). Moreover, the analysis of NRAMP 1 gene expression, which was higher in plants treated with R. irregularis co-inoculated with the fungal consortium, reinforces the idea that AM fungi and the saprophytic fungi enhance biological and biochemical activity of soil and are closely related to the increased availability of soil micronutrients and plant mineral nutrition. Previous studies have determined that the soil under study has a high content of zinc, cadmium, arsenic and copper until five times higher than normal (De Gregori et al. 2003). In fact, above average concentrations of As, Cu, Pb and Zn were found in plant roots. It is known that AM fungi are able to protect plants against heavy metals in the soil by concentrating them in the roots and preventing their access to plant shoots (Barea et al. 2013; Chibuike and Obiora 2014). However, non-significant differences between metal concentrations were observed in mycorrhizal- and non-mycorrhizal-treated plants (shoots and roots). It is possible that the low levels of AM root colonization achieved in our plants were not enough to accumulate and/or to avoid the transfer of heavy metals to the shoots. Nevertheless, saprophytic fungi were able to increase the accumulation of heavy metals in plant roots, contributing to their decrease in the soil. This increase was greater in plants co-inoculated with both AM and the saprophytic fungal consortium. The involvement of both AM and saprophytic fungi in the protection of plants against heavy metals by increasing their accumulation into plant roots has been previously observed (Arriagada et al. 2010). However, fungal inoculation cannot be declared as an inducer to reduce the phytotoxicity of heavy metal without considering the associated bacteria at the rhizosphere.
In our results, the antioxidant enzymes SOD decreased when plants were treated with the fungal consortium. This decrease was even more significant in plants co-inoculated with the fungal consortium and R. irregularis, most likely due to a direct effect of the accumulation of metals such as Cd, Cu and Pb in these treatments (Armada et al. 2014; Szőllősi 2014; Zhu et al. 2010).
The levels of MDA, commonly used as an indicator of lipid peroxidation and oxidative damage due to the formation and accumulation of ROS (Wang et al. 2009) by heavy metals, were decreased by the AM symbiosis; nevertheless, the co-inoculation showed a reduced MDA content in the leaves. Consistent with the antioxidant activities, AM colonization improved the antioxidant capacity of plants by reducing the formation of ROS.
Antioxidant enzyme activities are not the only mechanisms that contribute to stress tolerance to heavy metals; in this study, transcription of genes involved in the response to heavy metals was analysed. Expression of gene encoding glutathione reductase (GR), metallothioneins (MT) and heat shock proteins (HSP) was induced by an increase in the concentration of metals (Goupil et al. 2009). In the leaves of mycorrhizal plants, there was an increase in relative gene expression of GR, which protects against antioxidants by recycling glutathione (GSH) from its oxidized form to its reduced form. The application of the fungal consortium, however, increased GR expression in non-mycorrhizal-treated plants but did not alter the levels reached in mycorrhizal-treated plants. The increased expression of GR is associated with the greater concentration and availability of metals in the soil. Therefore, it is necessary to maintain a high ratio of GSH/GSSG, as GSH is needed to synthesize phytochelatin and other enzymes involved in ROS scavenging (Hossain et al. 2010). Moreover, this increased GR expression is closely linked to the amount of heavy metals absorbed by the plant as a result of presence of the fungal consortium. However, there were higher concentrations of heavy metals in the plants when the fungal consortium and AM fungus were co-inoculated.
Another strategy employed by plants to tolerate high concentrations of metals in the cell is to bind metals using proteins or peptides such as MT and phytochelatins. Co-inoculation with AM and the fungal consortium increased the relative transcription of the PCS gene. Moreover, the relative expression of the MT2b gene in the leaves of mycorrhizal plants was increased, and the addition of the fungal consortium led to a further increase in levels of MT transcripts. The increase in expression of PCS and MT transcription in leaves could be due to the increase in Pb, Cu and As levels. The levels of HSP90 expression increased in mycorrhizal plant leaves, with a higher level of transcripts found in plants co-inoculated with the fungal consortium. HSP90 was induced by the increased expression of PCS and MT and has an inverse relationship with lipid peroxidation, where an efficient repair was performed by HSPs resulting in reduced oxidative damage to lipids produced by ROS.
In conclusion, AM fungi affect MDA levels and the activity of enzymes involved in the oxidative stress response. Additionally, AM fungi result in changes in GR, MT2b, HSP90 gene expression in leaves. Consistent with what has been reported by other authors, arbuscular mycorrhizal colonization enhances chelation mechanisms by increasing transcription of chelation-related genes in plant leaves. Co-inoculation with the AM fungi and the fungal consortium improved plant antioxidant capacity and regulated the expression of several genes involved in the redox homeostasis, thereby improving redox balance and plant growth. The co-inoculation between mycorrhizal fungi and the fungal consortium (saprophytic fungi) synergistically improved plant growth, heavy metal uptake and enhanced plant tolerance to heavy metal stress. However, more studies are needed to reveal how rhizosphere microorganisms (bacterial/fungal interactions) contribute to the process of phytoremediation and reduce plant stress by improving defence mechanisms and homeostasis.
References
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951. doi:10.1016/S0038-0717(00)00244-3
Almonacid L, Fuentes A, Ortiz J, Salas C, Garcia-Romera I, Ocampo J, Arriagada C (2015) Effect of mixing soil saprophytic fungi with organic residues on the response of Solanum lycopersicum to arbuscular mycorrhizal fungi. Soil Use and Management: n/a-n/a doi:10.1111/sum.12160.
Andrade SAL, Gratão PL, Azevedo RA, Silveira APD, Schiavinato MA, Mazzafera P (2010) Biochemical and physiological changes in jack bean under mycorrhizal symbiosis growing in soil with increasing Cu concentrations. Environ Exp Bot 68:198–207. doi:10.1016/j.envexpbot.2009.11.009
Armada E, Portela G, Roldán A, Azcón R (2014) Combined use of beneficial soil microorganism and agrowaste residue to cope with plant water limitation under semiarid conditions. Geoderma 232–234:640–648. doi:10.1016/j.geoderma.2014.06.025
Arriagada C, Aranda E, Sampedro I, Garcia-Romera I, Ocampo JA (2009) Interactions of Trametes versicolor, Coriolopsis rigida and the arbuscular mycorrhizal fungus Glomus deserticola on the copper tolerance of Eucalyptus globulus. Chemosphere 77:273–278
Arriagada C, Pereira G, García-Romera I, Ocampo JA (2010) Improved zinc tolerance in Eucalyptus globulus inoculated with Glomus deserticola and Trametes versicolor or Coriolopsis rigida. Soil Biol Biochem 42:118–124. doi:10.1016/j.soilbio.2009.10.011
Barea J-M, Pozo M-J, Azcón R, Azcón-Aguilar C (2013) Microbial interactions in the rhizosphere. Molecular microbial ecology of the rhizosphere. John Wiley & Sons, Inc
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. doi:10.1016/0003-2697(71)90370-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Chamseddine M, Wided B, Guy H, Marie-Edith C, Fatma J (2009) Cadmium and copper induction of oxidative stress and antioxidative response in tomato (Solanum lycopersicon) leaves. Plant Growth Regul 57:89–99. doi:10.1007/s10725-008-9324-1
Chiang J, Cornejo P, López J, Romano S, Pascual J, Cea M (1985) Determinación de cadmio, cobre, manganeso, plomo, hierro, cinc y arsénico en sedimento atmosférico, en la zona de Quintero, V Región, Valparaíso, Chile. Chile Soc Chil Quim 30:139–158
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effect on plants and bioremediation methods. Applied and Environmental Soil Science 2014: 12. doi:10.1155/2014/752708.
Coelho PCS, Teixeira JPF, Gonçalves ONBSM (2011) Mining Activities: Health Impacts. In: ON Editor-in-Chief: Jerome (ed) Encyclopedia of Environmental Health. Elsevier, Burlington.
Cornejo P, Meier S, Borie G, Rillig MC, Borie F (2008) Glomalin-related soil protein in a Mediterranean ecosystem affected by a copper smelter and its contribution to Cu and Zn sequestration. Sci Total Environ 406:154–160
De Gregori I, Fuentes E, Rojas M, Pinochet H, Potin-Gautier M (2003) Monitoring of copper, arsenic and antimony levels in agricultural soils impacted and non-impacted by mining activities, from three regions in Chile. J Environ Monit 5:287–295. doi:10.1039/b211469k
Domsch KH, Gams W, Anderson T (1980) Compendium of soil fungi. Academic Press, London
Donahue JL, Okpodu CM, Cramer CL, Grabau EA, Alscher RG (1997) Responses of antioxidants to Paraquat in pea leaves (relationships to resistance). Plant Physiol 113:249–257. doi:10.1104/pp.113.1.249
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1566–1570. doi:10.1021/jf00021a018
Fosso-Kankeu E, Mulaba-Bafubiandi AF (2014) Implication of plants and microbial metalloproteins in the bioremediation of polluted waters: a review. Phys Chem Earth, Parts A/B/C 67–69:242–252. doi:10.1016/j.pce.2013.09.018
Fracchia S, Mujica MT, García-Romera I, García-Garrido JM, Martín J, Ocampo JA, Godeas A (1998) Interactions between Glomus mosseae and arbuscular mycorrhizal sporocarp-associated saprophytic fungi. Plant Soil 200:131–137. doi:10.1023/a:1004349426315
García-Romera I, García-Garrido JM, Martín J, Fracchia S, Mujica MT, Godeas A, Ocampo JA (1998) Interactions between saprotrophic Fusarium strains and arbuscular mycorrhizas of soybean plants. Symbiosis 24:235–246
Fracchia S, García-Romera I, Godeas A, Ocampo JA (2000) Effect of the saprophytic fungus Fusarium oxysporum on arbuscular mycorrhizal colonization and growth of plants in greenhouse and field trials. Plant Soil 223:175–184
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. doi:10.1016/j.plaphy.2010.08.016
Ginocchio R (2000) Effects of a copper smelter on a grassland community in the Puchuncavı́ valley, Chile. Chemosphere 41:15–23. doi:10.1016/S0045-6535(99)00385-9
Goupil P, Souguir D, Ferjani E, Faure O, Hitmi A, Ledoigt G (2009) Expression of stress-related genes in tomato plants exposed to arsenic and chromium in nutrient solution. J Plant Physiol 166:1446–1452. doi:10.1016/j.jplph.2009.01.015
Gryndler M (2000) Interactions of arbuscular mycorrhizal fungi with other soil organisms. In: Kapulnik Y, Douds Jr D (eds) Arbuscular mycorrhizas: physiology and function Springer Netherlands
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinet stoichiometry fat acid peroxidation Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Hewitt EJ, Bureaux CA (1966) Sand and water culture methods used in the study of plant nutrition. Cambridge Univ Press
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72. doi:10.1007/bf00257924
Khan AG (2005) Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J Trace Elem Med Biol 18:355–364. doi:10.1016/j.jtemb.2005.02.006
Krüger M, Krüger C, Walker C, Stockinger H, Schüßler A (2012) Phylogenetic reference data for systematics and phylotaxonomy of arbuscular mycorrhizal fungi from phylum to species level. New Phytol 193:970–984. doi:10.1111/j.1469-8137.2011.03962.x
Løvdal T, Lillo C (2009) Reference gene selection for quantitative real-time PCR normalization in tomato subjected to nitrogen, cold, and light stress. Anal Biochem 387:238–242. doi:10.1016/j.ab.2009.01.024
Medina A, Roldán A, Azcón R (2010) The effectiveness of arbuscular-mycorrhizal fungi and Aspergillus Niger or Phanerochaete chrysosporium treated organic amendments from olive residues upon plant growth in a semi-arid degraded soil. J Environ Manag 91:2547–2553. doi:10.1016/j.jenvman.2010.07.008
Neumann D, Lichtenberger O, Günther D, Tschiersch K, Nover L (1994) Heat-shock proteins induce heavy-metal tolerance in higher plants. Planta 194:360–367. doi:10.1007/bf00197536
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–IN118. doi:10.1016/S0007-1536(70)80110-3
Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA (1997) Changes in activities of antioxidant enzymes and their relationship to genetic and Paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol 114:695–704. doi:10.1104/pp.114.2.695
Ros M, Pascual JA, Garcia C, Hernandez MT, Insam H (2006) Hydrolase activities, microbial biomass and bacterial community in a soil after long-term amendment with different composts. Soil Biol Biochem 38:3443–3452
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365. doi:10.1093/jexbot/53.372.1351
Shetty KG, Hetrick BAD, Figge DAH, Schwab AP (1994) Effects of mycorrhizae and other soil microbes on revegetation of heavy metal contaminated mine spoil. Environ Pollut 86:181–188. doi:10.1016/0269-7491(94)90189-9
Sokal RR, Rohlf FJ (1981) Biometry: the principles and practice of statistics in biological research 2nd edition.
Stark C, Condron LM, Stewart A, Di HJ, O’Callaghan M (2007) Influence of organic and mineral amendments on microbial soil properties and processes. Appl Soil Ecol 35:79–93. doi:10.1016/j.apsoil.2006.05.001
Szőllősi R (2014) Chapter 3 - superoxide dismutase (SOD) and abiotic stress tolerance in plants: an overview. In: Ahmad P (ed) Oxidative damage to plants. Academic Press, San Diego
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307. doi:10.1016/0038-0717(69)90012-1
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Evaluation of VA infection levels in root systems. Research for estimation methods having a functional significance, in Physiological and Genetical Aspects of Mycorrhizae. France: INRA Press eds Gianinazzi-Pearson V., Gianinazzi S., editors: 217–221.
Wang C, Zhang SH, Wang PF, Qian J, Hou J, Zhang WJ, Lu J (2009) Excess Zn alters the nutrient uptake and induces the antioxidative responses in submerged plant Hydrilla verticillata (L.F.) Royle. Chemosphere 76:938–945. doi:10.1016/j.chemosphere.2009.04.038
Wang Y, Shi J, Wang H, Lin Q, Chen X, Chen Y (2007) The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol Environ Saf 67:75–81. doi:10.1016/j.ecoenv.2006.03.007
Zhang H, Wu X, Li G, Qin P (2011) Interactions between arbuscular mycorrhizal fungi and phosphate-solubilizing fungus (Mortierella sp.) and their effects on Kostelelzkya virginica growth and enzyme activities of rhizosphere and bulk soils at different salinities. Biol Fertil Soils 47:543–554. doi:10.1007/s00374-011-0563-3
Zhao S, Blumwald E (1998) Changes in oxidation-reduction state and antioxidant enzymes in the roots of jack pine seedlings during cold acclimation. Physiol Plant 104:134–142. doi:10.1034/j.1399-3054.1998.1040117.x
Zhu X, Song F, Xu H (2010) Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 20:325–332
Acknowledgments
This work was supported by FONDECYT Project 1130662 and 3150441; CONICYT Doctoral Fellowship and Universidad de La Frontera DIUFRO.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoav Bashan.
Rights and permissions
About this article
Cite this article
Fuentes, A., Almonacid, L., Ocampo, J.A. et al. Synergistic interactions between a saprophytic fungal consortium and Rhizophagus irregularis alleviate oxidative stress in plants grown in heavy metal contaminated soil. Plant Soil 407, 355–366 (2016). https://doi.org/10.1007/s11104-016-2893-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2893-2