Abstract
Aims
We investigated N2O emissions from stems of Fraxinus angustifolia and Fagus sylvatica, hypothesizing that trees emit N2O through the stem via diffusion out of the transpiration stream.
Methods
We used static chambers fixed at different heights of the stem to estimate N2O stem effluxes. Chambers were also used for monitoring soil N2O emissions. To stimulate soil N2O production and stem N2O emissions we fertilized the soil.
Results
Before soil fertilization, stem N2O emissions were at most 2 μg N2O-N m−2 bark h−1. After fertilization, stem and soil emissions were linearly correlated; stem emissions decreased linearly with increasing height. Stems of Fagus sylvatica emitted up to 80 μg N2O-N m−2 bark h−1 at 20 cm above soil level; at 200 cm, stem N2O emissions were below detection limit. Fraxinus angustifolia stem N2O emissions reached 35 μg N2O-N m−2 bark h−1 after soil fertilization.
Conclusions
Stem N2O emissions in upland trees occur even without aerenchyma, associated with xylem water transport. However, stem N2O emissions represented only 1–3 % of total (soil + stem) N2O emissions at the forest level. If this holds for other forest ecosystems, stem N2O emissions would be a minor pathway of N2O loss from terrestrial ecosystems into the atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial processes in the soil are considered to be the most dominant source of nitrous oxide (N2O) in terrestrial ecosystems. Specifically, denitrification and nitrification are the key processes for N2O production in soils (e.g., Butterbach-Bahl et al. 2013). Once N2O is produced within the soil profile, its most likely fate is the further reduction to N2 (Vieten et al. 2009); or the diffusion through the soil profile and the further release into the atmosphere (Shcherbak and Robertson 2014). To a lesser extent, N2O can also dissolve in the soil solution, be transported via percolation and potentially degas elsewhere (Haas et al. 2013). It is therefore the soil-atmosphere interface (i.e., the soil surface) which has devoted the most scientific attention to investigate the contribution of terrestrial ecosystems to the N2O efflux to the atmosphere.
The vast majority of experiments studying the N2O source strength of forest ecosystems apply soil chambers (e.g., Butterbach-Bahl et al. 2002b; Luo et al. 2012; Pilegaard et al. 2006), despite some studies used the eddy covariance flux measurement technique within and above the forest canopy (Eugster et al. 2007; Pihlatie et al. 2005a). Measurement chambers –either static or dynamic– are of reduced dimensions and do not allow enclosing trees in the chamber headspace. Thus, only the tree-free soil surface is usually monitored for N2O effluxes to the atmosphere.
However, several authors have already showed that herbaceous (Chang et al. 1998; Chen et al. 1999) as well as various tree species (Pihlatie et al. 2005b; Rusch and Rennenberg 1998) may directly emit N2O to the atmosphere. The uptake mechanism is believed to be via dissolved N2O which is absorbed by roots and transported via the xylem to the aboveground organs of the plants. Some authors argue that N2O is ultimately emitted by the stomata, after being transported by the transpiration stream (Chang et al. 1998; Pihlatie et al. 2005b). There are experimental indications that N2O may be released through the aerenchyma (Rusch and Rennenberg 1998), a tissue containing enlarged gap spaces for downward transport of oxygen to the rhizosphere (Evans 2003), which could also act as a upward escape conduct for gases.
Still, a potentially overlooked N2O loss pathway is the direct emission from the stem of N2O dissolved in transpiration water, specially for trees lacking aerenchyma (Machacova et al. 2013). So far, there are no empirical evidences that adult trees may show this capability of emitting N2O under field conditions, despite indications from studies with saplings (Machacova et al. 2013; Pihlatie et al. 2005b). If tree stems emit N2O, the N2O source strength of forests ecosystems could have been underestimated and would need to be revised by incorporating this missing pathway.
In the present study, we investigated the effects of tree species and age on the amount of N2O released by tree stems and its relative contribution compared to soil N2O emissions. For this purpose, we conducted simultaneous field measurements of stem and soil N2O emissions from mature ash (Fraxinus angustifolia Vahl) and both mature and young European beech (Fagus sylvatica L.). We applied a series of fertilization experiments, differing in fertilization type and application rates, to test whether stem N2O emissions were occurring under varying conditions. We hypothesized that N2O produced by soil microorganisms dissolves in the soil water, is absorbed by tree roots and transported upwards via xylem sap flow from where it diffuses into the atmosphere.
Material and methods
Site description & experimental design
Stem and soil N2O emissions of ash trees
Ash trees were investigated directly adjacent to the Institute of Meteorology and Climate Research, Atmospheric-Environmental Research (IMK-IFU), Garmisch-Partenkirchen, Southern Germany (47°30′ N; 11° 6′ E). Garmisch-Partenkirchen is located about 720 m a.s.l., and is characterized by a mean annual temperature of 7.5 °C and a mean precipitation of 1360 mm (period 1993–2013). Total N deposition in the area is around 12 N kg ha−1 a−1 (Kirchner et al. 2014). Topsoil samples were analyzed in a commercial laboratory (Dr. Janssen, Gillersheim, Germany); total soil carbon and soil carbonate contents were estimated following DIN ISO 10694 and Hoffman (1991), respectively. Total soil nitrogen was determined after DIN ISO 13878. Main soils characteristics are depicted in Table 1.
Stem and soil N2O emissions were monitored by using manual static chambers from the 2nd to the 20th of August 2012; measurements were conducted every 1–2 days. In order to stimulate soil N2O production and emission, ammonium-nitrate (NH4NO3) dissolved in distilled water was applied to the field two times during the course of the experiment (2nd and 6th of August, 75 kg N ha−1 each time, mimicking 20 mm precipitation events) to an area of about 100 m2.
Static chambers were used for measurements of stem N2O emissions. Chambers were made out of opaque PVC boxes (152 × 104 × 102 mm3), equipped with a rubber septum for gas collection and a thick rubber sealing (PTFE, 30 mm broad, 24 mm high) to assure air-tightness between the chamber and the tree bark. Tight fixation of the chamber onto the stem was achieved with elastic adjustable rubber bands. Chambers were placed at 20 cm and 130 cm above the soil surface (N = 2 for each height).
For the investigation of soil N2O emissions, similar but larger (355 × 255 × 120 mm3) static PVC chambers than those used for stem emissions were used. PVC frames (355 × 255 mm2) were inserted about 25 mm into the soil before the start of the measurements; the frames remained in the soil during the whole duration of the experiment. At the beginning of each manual measurement cycle, PVC chambers (N = 4) were placed onto the frames with metal clamps and a rubber sealing to assure gas tightness between the frame and the chamber. Each chamber was equipped with a non-forced pressure equilibrator port and a rubber septum for the collection of gas samples. At 15 min intervals, gas samples were collected from stem and soil chamber headspace with a plastic syringe equipped with a luer-lock stopcock valve at 0, 15, 30 and 45 min after chamber closure.
Stem and soil N2O emissions of mature European beech
European beech individuals (diameter at breast height, dbh = 50 cm approximately) were investigated at IMK-IFU for stem and soil N2O effluxes from the 27th of August to the 19th of September 2012. Soil characteristics are shown in Table 1. Potassium nitrate (KNO3) was applied two times to the tree surrounding (1st and 12th of September, at a rate of 50 kg N ha−1 each time) to increase available substrate for microbial N turnover and consecutive soil N2O production, covering approximately 150 m2. Both soil and stem N2O effluxes at 20, 130 and 200 cm height (N = 3) were monitored one to three times per day, following the same methodology as for the ash trees site. Soil temperature and soil volumetric water content was measured at 10 cm depth with ECH2O 5TM sensors (DECAGON, Pullman, Washington, U.S.A.) at 10 min intervals.
In addition to stem and soil N2O measurements, investigations of the N2O concentration at different soil depths (N = 3) were conducted from the 5th September until the end of the experiment. For this purpose, a modification of the gas probes used by Butterbach-Bahl and Papen (2002) was applied. Briefly, the probe consists on a stainless steel column inserted into the soil (6 cm diameter). Small headspaces at different depths (7.5, 15, 23, 33, 43 and 63 cm) are in contact with the soil atmosphere by a PTFE filter; the headspace is connected to a rubber septum located on the surface by 1/16″ stainless steel tubing. Through the septum, the headspace is sampled with a plastic syringe and concentration of N2O at different soil depths is determined by gas chromatography.
Stem and soil N2O emissions of young European beech
Young European beech individuals were monitored at the Höglwald forest, South Germany (48°50′ N, 11°17′ E). The area is located about 540 m a.s.l., with a mean annual precipitation of 932 mm and a mean annual temperature of 8.6 °C (period 2004–2010). The soil is a dystric Cambisol (FAO 2006) and it is very acidic in the topsoil (Kreutzer 1995). The area has a N deposition rate of about 30 kg N ha−1 a−1 and it can be considered as N saturated (Butterbach-Bahl et al. 2002a). Comprehensive site descriptions can be found Table 1 as well as in several articles (e.g., Butterbach-Bahl et al. 2002a; Kreutzer and Weiss 1998; Kreutzer 1995; Luo et al. 2012; Matejek et al. 2010). The area under investigation is a former Norway spruce stand, which underwent a clear cut followed by plantation of European beech in February 2000. At the time of the experiment (September to November 2012), beech trees had a dbh of 6–8 cm. Fifty kg N ha−1 was applied in form of KNO3 dissolved in water on October 21st 2012 in the tree surrounding area, equaling a precipitation event of 20 mm. The area under investigation was about 500 m2. Soil temperature and soil moisture was monitored at 10 cm depth by PT100 probes (UMS, Germany) every minute and results were aggregated for obtaining hourly values.
Due to the low stem size, a cylindrical transparent chamber (N = 3) was made out of PVC (27 cm height, 10 cm diameter) for monitoring stem-atmosphere N2O exchange rates. The chamber permitted the measurement of stem N2O fluxes corresponding to a height from 7 to 34 cm. The chamber opened in its longitudinal axis and both the top and the base of the chamber had a circular orifice to allow the enclosure of the whole circumference of the tree stem in the headspace of the chamber. Since tree stems are not perfectly circular and bark shows some irregularities, there was usually a small air space between the tree stem and the top and base of the chamber. This space was carefully filled with sealing material to ensure gas tightness of the headspace of the chamber. Likewise, sealing tape was used in the unions between the two longitudinal halves of the chamber, assuring the air tightness of the headspace. The chamber was equipped with a gas sampling port via a PTFE septum and fixed to the trees by means of elastic rubber bands firmly placed around the tree stem. Five ml gas samples were collected at 15 min intervals (0, 15, 30 and 45 min after chamber closure) with a plastic syringe equipped with a luer-lock stopcock valve. For investigation of soil N2O emissions, an automatic chamber system was used, allowing flux measurements in two-hourly time resolution. Specific details on the chamber characteristic, configuration aspects and analytical determination can be found in Butterbach-Bahl et al. (1997) and Butterbach-Bahl and Papen (2002). Stem N2O emissions rates were determined three times a day at two occasions prior to the application of fertilizers (27th of September and 2nd of October). After the fertilization event, stem N2O emissions were monitored between one and three times a day, one week long.
Determination of N2O concentrations and calculation of N2O efflux rates
On the same day of sample collection, gas samples were analyzed by gas chromatography with a Shimadzu GC-14B equipped with a 63Ni Electron capture detector. The gas samples were manually injected from syringes to the gas chromatograph. Reference gas (360 ppb N2O in synthetic air, Air Liquide) was periodically injected into the gas chromatograph for calibration purposes. For further details on analytical conditions see Butterbach-Bahl et al. (1997).
Stem and soil N2O emissions were calculated by using the increase in N2O concentration within the headspace along closing time. Since no saturation effect was observed with time, a linear regression was used for calculation of the N2O concentration vs. time slope. Relatively small headspace volume together with long deployment chamber time allowed for a detection limit below 5 μg N2O-N m−2 h−1 for soil emissions (Parkin et al. 2012). Given the different volume and area of the stem chambers for the different sites, detection limits for stem N2O emissions were slightly different and amounted to 4 and 1.5 μg N2O-N m−2 bark h−1 for IMK-IFU and Höglwald, respectively (Parkin et al. 2012).
Up-scaling of stem N2O emissions to the forest level
The contribution of stem N2O emissions to the total forest N2O emissions (soil + stem emissions) depends on the N2O emission rate of the bark and the area of the bark per unit of land; the latter depends on the forest structure i.e., number of tree stems per ha and distribution of tree diameter classes (Eq 1).
where N 2 O forest is the total N2O efflux at the forest level in μg N2O-N ha−1 h−1; N 2 O soil is the N2O efflux coming from the soil surface in μg N2O-N ha−1 h−1; N 2 O stem is the mean N2O efflux coming from the effective emitting stem surface in μg N2O-N m−2 bark h−1; N is the number of tree stems per ha; dbh is the mean diameter at breast height in m; and height is the effective N2O emitting height of the tree stem in m.
By applying Eq. 1, we estimated the significance of stem N2O emissions at the forest level under three contrasting forest scenarios: 1) a dense young forest, with 6.000 stems ha−1 and a dbh of 8 cm; 2) a mature open forest with 200 stems ha−1 and a dbh of 65 cm and 3) an uneven forest with a reverse J-shaped diameter distribution (von Oheimb et al. 2005). For the uneven forest, contribution of stem N2O efflux from each diameter class was calculated separately and then summed up.
Results
Ash trees experiment
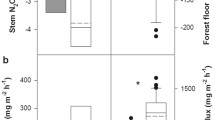
Addition of fertilizers to the soil surface of the ash stand resulted in peak soil N2O emissions of nearly 300 μg N2O-N m−2 h−1; which occurred at the same time as the maximum stem N2O efflux of 36 μg N2O-N m−2 bark h−1 (Fig. 1). Across the 20 days observation period, N2O emissions at 20 cm stem height were 14.5 ± 3.4 μg N2O-N m−2 bark h−1 and were linearly correlated to soil N2O emissions (R 2 = 0.69, p < 0.001, N = 16, Fig. 2), which were 94.8 ± 19.0 μg N2O-N m−2 h−1 during the monitoring period. Stem N2O effluxes at 130 cm high were three-fold lower than at 20 cm high (4.7 ± 1.4 μg N2O-N m−2 bark h−1, Fig. 1) and were not correlated to soil N2O effluxes anymore (p = 0.593, N = 16, Table 2).
Adult beech trees experiment
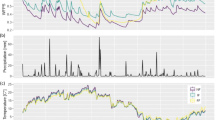
Nitrous oxide emissions from adult beech stems at the IMK-IFU site remained below the detection limit prior to the addition of fertilizers. Soil temperature averaged 16.3 °C (5 cm soil depth), with a maximum of 19.0 °C and values of approximately 13.0 °C towards the end of the experiment (Fig. 3). Soil moisture was high throughout the experiment and influenced by application of fertilizers. Thus, volumetric soil water content was about 60 % before fertilization and reached values up to 75 % afterwards. The application of KNO3 to the soil led to a sudden increase in soil N2O emissions up to 150 and 130 μg N2O-N m−2 h−1 after the first and second fertilization event, respectively. Both fertilization events also stimulated stem N2O emissions 20 cm above the ground (80 and 40 μg N2O-N m−2 bark h−1). The maximum efflux rate from stems was observed one day after soil N2O peak emissions (Fig. 3). Nitrous oxide emission rates from soil and stems at 20 cm were linearly correlated (Fig. 4). Roughly, emission of 100 μg N2O-N m−2 soil h−1 corresponded to a stem emission of 36 ± 7 μg N2O-N m−2 bark h−1 (R 2 = 0.48, p < 0.001, Table 1, Fig. 4). At 130 cm stem height, mean N2O emissions were already about 50 % lower than at 20 cm (16 vs. 7.7 μg N2O-N m−2 bark h−1 respectively) but still correlated to soil N2O emissions (R 2 = 0.40, p < 0.001, Table 1, Fig. 4). At two meters stem height, N2O effluxes were always below the detection limit (data not shown).
Upper panel: Soil and stem N2O emissions; middle panel: N2O concentrations at different soil depths; lower panel: soil temperature (black line) and soil moisture (blue line) measured at 10 cm depth for mature European beech at the IMK-IFU, Garmisch-Partenkirchen, Germany. Dark red arrows indicate fertilization (KNO3) events
Soil N2O concentrations in 7.5 cm depth reached values up to 1600 ppb and increased with increasing soil depth to values up to 9000 ppb N2O in 63 cm depth (Fig. 3). Soil N2O emissions were correlated to soil air N2O concentrations at any soil depth, with higher correlation coefficients and statistical significance in upper soil layers (8 cm, R 2 = 0.60, p < 0.001) than in lower soil layers (63 cm, R 2 = 0.21, p = 0.05). Stem N2O emissions were detectable only when N2O concentration in the uppermost soil layer was above 1400 ppb N2O, but no significant correlation was found between N2O emitted from stems and N2O concentration at any soil depth.
Young beech trees experiment
Prior to the application of fertilizers, soil and stem N2O effluxes at the Höglwald forest were low (around 2 μg N2O-N m−2 h−1) but above detection limit (Fig. 5). Soil temperature was 10.7 °C and the volumetric soil moisture content was about 20 % prior to the application of fertilizer, which took place on the 21st of October. After fertilization, soil N2O emission rates increased from previous levels of about 13.1 to 25.6 μg N2O-N m−2 h−1. Ten days after fertilization, soil N2O effluxes returned to initial levels (13.6 μg N2O-N m−2 h−1; Fig. 5). The temporal evolution of N2O emitted from the tree stems followed the same pattern as N2O emitted from the soil. Thus, baseline stem N2O emissions were about 2.2 μg N2O-N m−2 bark h−1 before fertilization and increased to 7.3 μg N2O-N m−2 bark h−1 during the week after fertilizer application. Stem N2O emissions decreased with time, and 10 days after fertilization they showed similar values as before fertilization (2.3 μg N2O-N m−2 bark h−1). Soil and stem N2O emissions were significantly correlated (R 2 = 0.30, p < 0.01).
Upscaling to the tree and forest scale
We inferred the distribution of the N2O emissions along the whole stem using the observed decrease in N2O emission rates with increasing stem height. According to the linear, negative relationship between stem N2O emission rates and stem height, we calculated that stem N2O emissions peaked at the soil level; then, stem N2O emissions decreased linearly until the height of 2 m, where they equaled zero. With this information we estimated an effective emitting stem length of 2 m for beech trees, and a mean N2O emission rate along the emitting stem height, which resulted in a ratio of 1:5 (μg N2O-N m−2 bark: μg N2O-N m−2 soil) if stem emissions are directly compared to soil emissions. In other words, 5 μg N2O-N emitted per unit of soil area equaled 1 μg N2O-N emitted per unit of bark area. For ash trees, no up-scaling was used due to the lack of correlation between stem and soil N2O emissions for stem heights above 20 cm (Table 2, Fig. 2).
Results of single stem measurements from beech were further used for up-scaling stem N2O emissions to the forest scale (Eq. 1). Depending on the forest structure and the ratio of N2O emitting bark to soil area, stem N2O emissions from beech forests represented 1 to 3 % of the N2O emitted by the soil surface. Here, young dense beech forests showed a higher contribution of stem N2O emissions to total N2O efflux than adult and un-even aged forests (Table 3).
Discussion
We show here for the first time that young (Fagus sylvatica) and adult (Fagus sylvatica and Fraxinus angustifolia) trees can emit significant amounts of N2O via the stem surface under field conditions. The experiments illustrate that N2O can be directly released from the stem of trees under field conditions if soil N2O production and concentration are sufficiently high, as evidenced by peak emission from ash (30–40 μg N2O-N m−2 bark h−1) and beech trees (80 and 12 μg N2O-N m−2 bark h−1, for mature and young individuals, respectively) in response to fertilization. For the young beech stand, modest but significant stem N2O emissions (2.2 μg N2O-N m−2 bark h−1) were detected even without soil fertilization, likely due to high N deposition rates in the area –Höglwald forest-, which have been shown to promote soil N2O production (Butterbach-Bahl et al. 2002a). Results from European beech, a tree species lacking aerenchyma, are especially relevant. So far, direct plant-mediated N2O release was observed only for plants having aerenchymous tissues (Chang et al. 1998; Rusch and Rennenberg 1998) or in tree saplings and seedlings under laboratory conditions (Machacova et al. 2013; Pihlatie et al. 2005b).
For both tree species, stem N2O effluxes were significantly correlated with soil fluxes, and, for the adult beech experiment, stem N2O effluxes were correlated with soil N2O concentrations, strongly indicating that the N2O produced in the soil by microbes was taken up by the trees and released to the atmosphere through their stems. In previous studies (Chang et al. 1998; Jørgensen et al. 2012) it was argued that the likely mechanism of N2O release is diffusion through the stem from the transpiration stream. Apparently, the N2O molecule, high soluble in water (Weiss and Price 1980), is taken up by the root system, transported through the xylem and finally released by the stomata. Gas diffusion through the bark in the absence of aerenchyma was thought to be only of minor importance (Pihlatie et al. 2005b). Our observations contradict this statement and indicate that dissolved N2O from the sap flow is released through the bark to the atmosphere, even in beech trees, which do not develop aerenchymous structures, but still possess lenticels. Given the lack of aerenchyma within the stem, it is likely that the N2O diffuses from the sap flow across the cambium and meristem to the bark where it is then further released into the atmosphere most likely via lenticels. In spite of the fact that the diffusion coefficient of N2O in water is several orders of magnitude lower than in air (Heincke and Kaupenjohann 1999), our results show that this process takes place and leads to detectable N2O flux rates through the stem. Some dissolved N2O might be released into the atmosphere from transpiration water outside the stem, due to the increase of the water-air partial pressure difference in the atmosphere.
The decrease of stem N2O emission rates with increasing stem height can be explained by two factors affecting the degassing of N2O. First, higher parts of the stem usually have lower rates of transpiration (Perämäki et al. 2001) and; second, the N2O concentration in the plant tissues reduces with height (Pihlatie et al. 2005b), thus diminishing N2O diffusion, according to First Fick’s law, by which diffusion is proportional to concentration differences. Whereas in beech seedlings and saplings N2O might be directly emitted by the stomata (Machacova et al. 2013; Pihlatie et al. 2005b), for larger trees it seems that most of the plant-mediated N2O emission takes place at the bark surface of the trunk near the soil level, since we were not able to detect emissions from the bark at stem heights > 2.0 m. However, since we did not conduct N2O exchange measurements at the canopy level, we cannot exclude that leaves are an effective source of N2O through their stomata.
Differences in stem N2O effluxes were larger between tree species than between tree ages. Ash, a typical riparian tree species, has the capacity to develop hypertrophied lenticels (Jaeger et al. 2009). On the contrary, European beech is an upland tree and the development of porous spaces in stem tissues has not been documented. Still, beech yielded higher stem N2O efflux peaks; however, the decrease in N2O emissions with increasing height was not as sharp as in the case of ash, and the ratio between stem and soil N2O emissions was also higher at beech stands. Enlarged gas spaces within the ash tissues likely allowed for rapid release of N2O –even from belowground organs, i.e., roots - so that N2O might be no longer present in the sap flow stream at 1.30 above the soil surface. As a consequence, differences in stem:soil N2O emissions between adult and young trees were of minor importance, and likely due to different transpiration rates, whereas the outgasing mechanism for N2O was the same for young and adult individuals. Still, even if the mechanism releasing N2O seems to be the same independently of tree age, it is possible that the N2O emission rates of trees of the same species but at different development stages are different.
It has already been shown that trees and tree crowns create different conditions in the soil near to them as opposed to more distant areas, and that these effects have implications for the dynamics and magnitude of soil N2O fluxes (e.g., Butterbach-Bahl et al. 2002b). Therefore, distribution of trees within the forest needs to be taken into account for obtaining reliable N2O budgets in forest ecosystems. We now show that there is a potential risk of underestimation of N2O emissions from forested ecosystems due to the so far overlooked stem N2O releases. However, our up-scaling calculation reveals that stem N2O emission may contribute up to 3 % to total forest N2O emissions (Table 3). This number holds for a young dense forest, where the proportion of stem surface effectively emitting is large (about 1500 m2 ha−1). In an adult stand with lower relative stem surface area, stem N2O emissions may be as little as 1 % of total forest N2O efflux. Therefore, despite the ecological importance of the proof of a new pathway for N2O release in vascular plants, the implications of direct stem N2O emissions for the accurate estimation of N2O budgets of forest ecosystems seem to be of minor importance, at least for the type of forest investigated.
References
Butterbach-Bahl K, Papen H (2002) Four years continuous record of CH4-exchange between the atmosphere and untreated and limed soil of a N-saturated spruce and beech forest ecosystem in Germany. Plant Soil 240:77–90
Butterbach-Bahl K, Gasche R, Breuer L, Papen H (1997) Fluxes of NO and N2O from temperate forest soils: impact of forest type, N deposition and of liming on the NO and N2O emissions. Nutr Cycl Agroecosyst 48:79–90
Butterbach-Bahl K, Gasche R, Willibald G, Papen H (2002a) Exchange of N-gases at the Höglwald forest: a summary. Plant Soil 240:117–123
Butterbach-Bahl K, Rothe A, Papen H (2002b) Effect of tree distance on N2O and CH4-fluxes from soils in temperate forest ecosystems. Plant Soil 240:91–103
Butterbach-Bahl K, Baggs EM, Dannenmann M, Kiese R, Zechmeister-Boltenstern S (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos T Roy Soc B 368
Chang C, Janzen HH, Cho CM, Nakonechny EM (1998) Nitrous oxide emission through plants. Soil Sci Soc Am J 62:35–38
Chen X, Boeckx P, Shen S, Van Cleemput O (1999) Emission of N2O from rye grass (Lolium perenne L.). Biol Fertil Soils 28:393–396
Eugster W, Zeyer K, Zeeman M, Michna P, Zingg A, Buchmann N, Emmenegger L (2007) Methodical study of nitrous oxide eddy covariance measurements using quantum cascade laser spectrometery over a Swiss forest. Biogeosciences 4:927–939
Evans DE (2003) Aerenchyma formation. New Phytol 161:35–49
FAO (2006) World reference base for soil resourcces 2006, first update 2007. World Soil Resources Reports, n° 103. Food and Agriculture Organization of the United Nations, Rome
Haas E, Klatt S, Fröhlich A, Kraft P, Werner C, Kiese R, Grote R, Breuer L, Butterbach-Bahl K (2013) LandscapeDNDC: a process model for simulation of biosphere-atmosphere-hydrosphere exchange processes at site and regional scale. Landsc Ecol 28:615–636
Heincke M, Kaupenjohann M (1999) Effects of soil solution on the dynamics of N2O emissions: a review. Nutr Cycl Agroecosyst 55:133–157
Hoffman G (1991) Methodenbuch Band I. Die Untersuchung von Böden. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten e.V., Speyer
Jaeger C, Gessler A, Biller S, Rennenberg H, Kreuzwieser J (2009) Differences in C metabolism of ash species and provenances as a consequence of root oxygen deprivation by waterlogging. J Exp Bot 60:4335–4345
Jørgensen CJ, Struwe S, Elberling B (2012) Temporal trends in N2O flux dynamics in a Danish wetland: effects of plant-mediated gas transport of N2O and O2 following changes in water level and soil mineral-N availability. Glob Chang Biol 18:210–222
Kirchner M, Fegg W, Römmelt H, Leuchner M, Ries L, Zimmermann R, Michalke B, Wallasch M, Maguhn J, Faus-Kessler T, Jakobi G (2014) Nitrogen deposition along differently exposed slopes in the Bavarian Alps. Sci Total Environ 470–471:895–906
Kreutzer K (1995) Effects of forest liming on soil processes. Plant Soil 168–169:447–470
Kreutzer K, Weiss T (1998) The Höglwald field experiments—aims, concept and basic data. Plant Soil 199:1–10
Luo GJ, Brüggemann N, Wolf B, Gasche R, Grote R, Butterbach-Bahl K (2012) Decadal variability of soil CO2, NO, N2O, and CH4 fluxes at the Höglwald Forest, Germany. Biogeosciences 9:1741–1763
Machacova K, Papen H, Kreuzwieser J, Rennenberg H (2013) Inundation strongly stimulates nitrous oxide emissions from stems of the upland tree Fagus sylvatica and the riparian tree Alnus glutinosa. Plant Soil 364:287–301
Matejek B, Huber C, Dannenmann M, Kohlpaintner M, Gasche R, Papen H (2010) Microbial N turnover processes in three forest soil layers following clear cutting of an N saturated mature spruce stand. 337:93–110
Parkin T, Venterea R, Hargreaves S (2012) Calculating the detection limits of chamber-based soil greenhouse gas flux measurements. J Environ Qual 41:705–715
Perämäki M, Nikinmaa E, Sevanto S, Ilvesniemi H, Siivola E, Hari P, Vesala T (2001) Tree stem diameter variations and transpiration in Scots pine: an analysis using a dynamic sap flow model. Tree Physiol 21:889–897
Pihlatie M, Rinne J, Ambus P, Pilegaard K, Dorsey JR, Rannik Ü, Markkanen T, Launiainen S, Vesala T (2005a) Nitrous oxide emissions from a beech forest floor measured by eddy covariance and soil enclosure techniques. Biogeosciences 2:377–387
Pihlatie M, Ambus P, Rinne J, Pilegaard K, Vesala T (2005b) Plant-mediated nitrous oxide emissions from beech (Fagus sylvatica) leaves. New Phytol 168:93–98
Pilegaard K, Skiba U, Ambus P, Beier C, Brüggemann N, Butterbach-Bahl K, Dick J, Dos Santos J, Eash NS, Gallardo A, Gasche R, Horvath L, Kitzler B, Leip A, Pihlatie M, Rosenkranz P, Seufert G, Vesala T, Westrate H, Zechmeister-Boltenstern S (2006) Factors controlling regional differences in forest soil emissions of nitrogen oxides (NO and N2O). Biogeosciences 3:651–661
Rusch H, Rennenberg H (1998) Black alder (Alnus Glutinosa (L.) Gaertn.) trees mediate methane and nitrous oxide emission from the soil to the atmosphere. Plant Soil 201:1–7
Shcherbak I, Robertson GP (2014) Determining the diffusivity of nitrous oxide in soil using in situ tracers. Soil Sci Soc Am J 78:79–88
Vieten B, Conen F, Neftel A, Alewell C (2009) Respiration of nitrous oxide in suboxic soil. Eur J Soil Sci 60:332–337
von Oheimb G, Westphal C, Tempel H, Härdtle W (2005) Structural pattern of a near-natural beech forest (Fagus sylvatica) (Serrahn, North-east Germany). For Ecol Manag 212:253–263
Weiss R, Price B (1980) Nitrous oxide solubility in water and seawater. Mar Chem 8:347–358
Acknowledgments
This work has received funding from the European Community’s Seventh Framework Program, under project number 262060 (ExpeER) within its framework of Transnational Access Activity (subproject FLUSTTREE).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ute Skiba.
Rights and permissions
About this article
Cite this article
Díaz-Pinés, E., Heras, P., Gasche, R. et al. Nitrous oxide emissions from stems of ash (Fraxinus angustifolia Vahl) and European beech (Fagus sylvatica L.). Plant Soil 398, 35–45 (2016). https://doi.org/10.1007/s11104-015-2629-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2629-8