Abstract
Aims
Stomata can close to avoid cavitation under decreased soil water availability. This closure can be triggered by hydraulic (‘H’) and/or chemical signals (‘C’, ‘H + C’). By combining plant hydraulic relations with a model for stomatal conductance, including chemical signalling, our aim was to derive direct relations that link soil water availability, expressed as fraction of roots in dry soil (fdry), to transpiration reduction.
Methods
We used the mechanistic soil-root water flow model R-SWMS to verify this relation. Virtual split root experiments were simulated, comparing horizontal and vertical splits with varying fdry and different strengths of stomatal regulation by chemical and hydraulic signals.
Results
Transpiration reduction predicted by the direct relations was in good agreement with numerical simulations. For small enough potential transpiration and large enough root hydraulic conductivity and stomatal sensitivity to chemical signalling isohydric plant behaviour originates from H + C control whereas anisohydric behaviour emerges from C control. For C control the relation between transpiration reduction and fdry becomes independent of transpiration rate whereas H + C control results in stronger reduction for higher transpiration rates.
Conclusion
Direct relations that link effective soil water potential and leaf water potential can describe different stomatal control resulting in contrasting behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants subjected to low soil water availability have developed different strategies to cope with drought. One of these is stomatal closure to avoid cavitation in the xylem vessels (Choat et al. 2012). Experimentalists observed two types of plant stomatal reaction to low water availability: isohydric, when leaf water potential was kept constant at a certain threshold, or anisohydric, when large fluctuations in leaf water potential were observed under different water statuses (Tardieu and Simonneau 1998). The control of leaf water potential on stomatal closure has been known for decades (Gardner and Ehlig 1963). However, by decreasing transpiration, stomatal closure can directly increase leaf water potential, thereby making it difficult to draw conclusions on whether leaf water potential controls stomatal conduction (Dodd et al. 2009). Moreover, chemical signals such as plant hormones (e.g., abscisic acid - ABA), originating from plant roots or other plant tissues, can induce stomatal closure (Gowing et al. 1990; Stoll et al. 2000).
Although there are many of models that describe stomatal conductance in relation to environmental factors (see review by Damour et al. 2010), there still remains a need to understand whether the current approaches that link stomatal conductance to soil water availability are valid if transpiration is regulated by chemical signalling (Javaux et al. 2013). Furthermore, the observed stomatal behaviour (anisohydric or isohydric) does not necessarily imply a priori a specific control mechanism. For instance, depending on hormone production and corresponding stomatal sensitivity, a chemical signalling could either result in iso- or anisohydric behaviour (Brodribb et al. 2014; Tardieu and Simonneau 1998).
Two types of models were proposed to simulate stomatal regulation induced by drought. On one hand, empirical models try to relate stomatal closure directly to soil water potential and/or root zone soil moisture content. These relations were obtained from experiments, which have for decades proven the link between transpiration reduction and low soil water content. Eco-hydrological models compute stomatal regulation often as a function of lumped soil water content (Rodriguez-Iturbe and Porporato 2005). In hydrological models stomatal regulation is implicitly accounted for through stress functions, which relate the reduction of the potential transpiration rate to bulk soil water status. There are functions that link the reduction factor linearly to the soil water potential (Feddes et al. 1978) or to the matric flux potential (van Lier et al. 2006). In addition to soil water potential, these stress functions also depend on the transpiration rate with a stronger reduction in transpiration rate for the same soil water potential when the transpiration rate is higher.
On the other hand, mechanistic models aim at linking stomatal regulation to plant variables triggering or controlling the stomatal aperture in a more direct way. These models use functional relationships between hormone concentrations, plant hydraulic variables like leaf water potential and stomatal resistance (Tardieu et al. 1993). In order to further link plant variables (e.g., leaf water potential and leaf hormone concentrations) with soil environmental variables (e.g., soil water potentials in the root zone), meteorological conditions (e.g., potential transpiration rate or the transpiration rate when stomata are fully opened), but also leaf and root properties (e.g., root hydraulic architecture, hydraulic conductivities of roots, production of hormones as a function of root water potentials), eco-physiological models have been developed (Rodriguez-Iturbe et al. 2001).
For the case that stomatal regulation is only a function of the leaf water potential (i.e., fully hydraulic control) and that this function is a step function (i.e., stomata are not closed when the leaf water potential is above a critical threshold value and adjust their aperture to keep a constant leaf water potential), a hydraulic model of the soil-plant system can be used to describe the transpiration fluxes (Doussan et al. 2006; Javaux et al. 2008). Direct relations between hydraulic properties of the root system, the spatial distribution of the soil water potentials, the maximal possible or potential transpiration rate, T pot,, and the distribution of the water uptake in the root zone emerged from a theoretical analysis of such a system (Couvreur et al. 2012; Javaux et al. 2013).
However, such relations have not been established when chemical signalling by plant hormones also plays a role. In order to bridge the gap between models and observations we included a module, which explicitly accounts for chemical signalling, to a numerical mechanistic root and soil water flow model. Recent attempts to model both hydraulic and chemical control of stomata adequately reproduced experimental observations (Huber et al. 2014). Therefore, in this manuscript, we use simulations with this model to investigate the relation between transpiration, water potential distribution in the root zone, and potential transpiration rate for different signalling mechanisms: hydraulic versus chemical signalling.
Another goal is to examine the effect of heterogeneous soil moisture distribution within the root zone on transpiration. Horizontal or vertical gradients of soil water content are usually induced by dividing the root zone in different compartments that receive different treatments. The compartments can also be hydraulically isolated by split layers. These types of experiments have been conducted widely for vertical as well as horizontal split setups (Gowing et al. 1990; Herkelrath et al. 1977). A pot experiment, which mimicked field conditions by varying the location of irrigation (either top or bottom), could not find a good correlation between gradients of soil water potential and concentration of ABA in the roots (Puértolas et al. 2013). When the rootzone was vertically divided and different parts were exposed to wet and dry soil, the root fraction in dry soil did not clearly affect evapotranspiration but more roots in drying soil increased leaf ABA concentration independently of any change in leaf water potential (Martin-Vertedor and Dodd 2011). Drawing conclusions from experimental studies may be difficult when the plant water potentials in the root zone are not known or the relation between plant water potential and transpiration rate is not accounted for. The relation between soil water potential, root and plant water potential can be expected to depend on the distribution of the soil water potentials in the root zone. For vertical variations in soil water potential, individual roots transverse through regions with different water potentials so that these regions are ‘connected’ by the root system. For lateral variations in soil water potential, these connections can be considerably smaller, or even absent during transpiration in vertical split root experiments with a hydraulically isolating barrier dividing the soil compartments. The effect of vertical versus horizontal variations in soil water potential on plant transpiration may therefore be different. Another process complicating the interpretation of experimental data is the redistribution of soil water between wet and dry regions during night with very low or zero transpiration, usually termed hydraulic lift. This may have confounded the interpretations of the results of Puértolas et al. (2013). A modelling study that describes soil, root and shoot water potentials and fluxes, and includes the transport of root zone produced plant hormone could provide additional insights.
We set up a series of virtual split root experiments with vertical and horizontal splits to further evaluate the influence of soil water heterogeneity on transpiration regulation. It should be noted that vertical splits induce a horizontal gradient in soil moisture content and vice versa. In addition, similar to what was done by Couvreur et al. (2012) for the hydraulically controlled soil-plant system, we did a theoretical system analysis of the chemically controlled and chemically-hydraulically controlled systems from which novel direct relations emerged. These relations, which might be useful in larger scale models to describe stomatal closure in a quantitative and mechanistic but simple way, were evaluated by simulations with the numerical model. In the following text we will refer to signalling by chemicals as ‘C’, and by both hydraulics and chemicals as ‘H + C’. We hypothesise that H and H + C controlled plants result in apparent isohydric behaviour, while C controlled plants lead to apparent anisohydric behaviour.
Methods
Numerical mechanistic model, R-SWMS
Soil-root water flow model and water potentials
R-SWMS (Root-Soil Water Movement and Solute transport modelling) is a coupled soil and root water flow model that computes water fluxes through the soil-plant-atmosphere continuum as a function of the hydraulic gradients in the system (Javaux et al. 2008). The Richards equation (Richards 1931) is solved for a three-dimensional regular soil grid. The root architecture is explicitly represented in three dimensions. The water flow into the root xylem is governed by the pressure gradient between the soil root interface and the xylem pressure. The resulting radial flow is directly linked to the sink term of the Richard’s equation. Flow through the roots towards the shoot is explicitly modelled and is driven by pressure gradients within the root system (Doussan et al. 2006).
The total water potential is expressed as the energy per volume of water, which is equivalent to a pressure. The total water potential consists of different partial potentials. In the R-SWMS simulations, the partial potentials related to the elevation and to the water pressure (or matric potential) are considered. For the water pressures, the difference to the atmospheric pressure is considered so that negative water pressures refer to pressures that are smaller than the atmospheric pressure. Partial potentials related to the osmotic potential can also be included (Schröder et al. 2013), but were not considered in this study.
Chemical signalling
In R-SWMS an additional chemical signalling was implemented (Huber et al. 2014) using a particle tracking algorithm that generates during each time step and in each root segment a particle with a mass that is proportional to the duration of the time step and that depends on local xylem water pressure. If the xylem water potential is lower (more negative) than a threshold water pressure, ψ lim , particles are created.
The production rate of chemical in a root segment, M i , [N T-1] is given by Simonneau et al. (1998):
where a [N P−1 T-1 M−1] is the production rate per root mass, m i is the mass of segment i and ψ x,i is the xylem water pressure in segment i. The particles are further transported via the xylem water flow calculated by the root water flow model upwards towards the shoot, which is represented by a single segment with a volume, V buffer [L3]. This shoot segment acts as a mixing bucket or buffer and was introduced to suppress oscillations in stomatal regulation (Huber et al. 2014). The total mass of chemical signal in the shoot volume is calculated by adding the newly arrived chemical mass to and subtracting the mass that flows out from the prevalent mass in the shoot. The concentration is obtained by dividing the total mass by the volume of the shoot. The resulting concentration affects stomatal closure.
There are many mechanistic models based on the gas balance within the stomata and the atmosphere that link stomatal conductance to meteorological variables (e.g., Ball et al. 1987; Jarvis et al. 1999) to estimate Tpot. For this study the model proposed by Tardieu and Davies (1993), describing the following relation between stomatal aperture, signal concentration, and leaf water pressure (Eq. 2) was chosen to estimate Tact.
where T act [L3 T-1] is the actual transpiration, T pot [L3 T-1] is the potential transpiration, i.e., the transpiration when stomata are fully open, αr (−) is the residual stomatal aperture, c leaf [N L-3] is the chemical concentration in the leaves, |ψ leaf | [P] is the absolute value of the leaf water potential, and s c [L3 N-1] and s p [P-1] are fitting parameters.
In R-SWMS, an H + C scenario is modelled using Eq. 2 with s p ≠ 0 and Eq. 1 ψ lim < 0, and the C scenario using Eq. 2 with s p = 0 and Eq. 1 with ψ lim = 0. For an H scenario, Eq. 2 is not used directly but a critical water pressure ψ lim,H is defined in the shoot/leaves. As long as ψ leaf > ψ lim,H , T act is equal to T pot . When ψ leaf = ψ lim,H , the water potential in the leaves is kept constant and the water flow from the soil to the root and the shoot is calculated from the difference between the soil water pressure and ψ lim,H considering the soil and root resistances to flow in the system. For this study an H scenario is not computed using the numerical model. It is, however, a commonly used approach and results can be found e.g., in Couvreur et al. (2014b), Javaux et al. (2008), or Huber et al. (2014). Thus the numerical model is capable of simulating different stomatal control mechanisms.
Although mechanistic models like R-SWMS can be used to simulate the behaviour of single plants in which transpiration is controlled by chemical signalling, simpler models are needed to get insight into fundamental differences of the plant’s response to drying out of the root zone when stomata are regulated by chemical or by hydraulic signalling. Such models are also potentially useful for large scale system models (crop models), in which stress prediction is crucial but still poorly estimated. A direct approach will be described in the following paragraphs.
Direct relations linking transpiration and root zone water distribution including chemical signalling
In order to assess the regulation of transpiration by chemical signalling directly, a simple relation based on the following assumptions was developed (Fig. 1): (i) soil is constituted of two isolated compartments, (ii) the wet part is constantly irrigated and the water pressure at the root-soil interface in the wet part, ψ root,wet , remains close to zero, (iii) soil water pressure and root xylem water pressure in the dry parts of the root zone ψ root,dry is equal to the leaf water pressure ψ leaf and differences in total water potential due to differences in elevation are neglected, (iv) chemical signal is only produced in the dry part of the root zone and is a linear function of the water pressure in the root, (v) the produced chemical signal is instantaneously effective in the stomata (no transport limitations considered), and (vi) the fraction of the water uptake from a compartment to the total water uptake by the entire root system when water pressure in both compartments is the same, is equal to the root mass fraction in the compartment. The last assumption implies that the distribution of fine and coarser roots and roots segments with different hydraulic conductivities is the same in the different compartments. The direct relations in the following paragraphs rely on these assumptions.
According to the third, fourth, and fifth assumption, dividing the production of signal by the actual transpiration results in the signal concentration in the leaves c leaf , Eq. 3:
where a* [N P−1 T-1] is the signal production rate of the total root system which is multiplied by f dry , representing the mass fraction of the roots in the dry compartment, to account for the fact that only roots in the dry compartment are producing the signal.
With the assumption that stomata close completely during night (α r = 0) and that the pressure difference between the dry and the wet soil compartment is large enough (ψ root,wet ≈ 0), combining Eqs. 2 and 3 becomes (H + C) for ψ leaf < ψ lim :
This equation relates T act to ψ leaf and T pot but does not include the effect of the water flow in the soil root-plant system on the water pressure differences between the soil and the leaves. Based on the second and third assumption, it can be stated that the plant-soil system is in a steady state and that the actual transpiration is maintained only from the wet part of the soil and thus driven by the water pressure gradient between the leaf ψ leaf [P] and the root-soil interface in the wet soil compartment ψ root,wet , Eq. 5:
or
where K root [L3 P-1 T-1] is the effective hydraulic conductivity of the entire root system. According to assumption (vi), f dry can be either the mass fraction of roots in the dry compartment or the fraction of water that would be taken up from the dry compartment when it is equally wet as the wet compartment. Based on this assumption and since water uptake only occurs from the wet part, the effective conductivity of the root system in the wet part is a fraction (1-f dry ) of the conductivity of the entire root system, K root . Combining Eqs. 4 and 5 and assuming ψ root,wet ≈ 0 leads to:
This relation can be further rewritten as:
Equation 7 provides an indirect relation between T act , T pot , plant parameters (s c , s p , a*, ψ lim , K root ), and the fraction of roots in dry soil which can be solved for T act iteratively.
Once T act is known, ψ leaf can be calculated from Eq. 5 so that the effective soil water pressure ψ soil,eff [P] can be described according to Couvreur et al. (2012):
Where according to assumption (vi) (1-f dry ) and f dry take up the role of the standard sink term fractions in the wet and dry parts of the root zone respectively. Plugging Eq. 8 into Eq. 5 leads to:
which is exactly the same equation as derived by Couvreur et al. (2012) to assess the effect of the soil water pressure and its spatial distribution on the transpiration rate. However, in contrast to Couvreur et al. (2012), who consider a isohydric plant behaviour with a constant ψ leaf = ψ lim,H for T act < T pot , according to Eq. 6, ψ leaf changes with T act .
Apparent isohydric behaviour
Through inspection of Eq. 6, conditions or plant properties or parameters that lead to an isohydric plant behaviour can be inferred. In isohydric plants, leaf water potential is kept larger than or equal to a fixed value: ψ leaf ≥ ψ lim,H . In case of declining soil water potential the leaf water potential declines as well until a certain threshold value, ψ lim,H , is reached, where it remains constant during further soil drying. According to Eq. 6, such a behaviour can be obtained for s p > 0 since stomatal conductance decreases sharply with |ψ leaf | when |ψ leaf | > 1/s p so that ψ lim,H ≈ 1/s p . Also for s p = 0, when only chemical signalling is considered or active, apparent isohydric behaviour may emerge. When the sensitivity of the stomatal conductance to the chemical signal is sufficiently large, i.e., for a relatively large \( \frac{s_c{f}_{dry}{a}^{*}}{\left(1-{f}_{dry}\right){K}_{root}} \), the actual transpiration rate decreases sharply within a relatively narrow range of ψ leaf ≈ ψ root,dry < ψ lim so that ψ lim,H ≈ ψ lim.
Apparent anisohydric behaviour
When s p is close to zero, i.e., |ψ leaf | must be very large before the stomatal conductance starts to decrease drastically with increasing |ψ leaf |, the leaf water potentials can vary over a large range before a pronounced effect on the stomatal closure arises. For C controlled plants with ψ lim = 0 and s p = 0, and for |ψ root,wet | < < |ψ leaf | (so that transpiration is non zero by Eq. 5), Eq. 6 simplifies to:
This equation illustrates that the relation between T act /T pot and f dry neither depends on ψ leaf nor on ψ soil,eff .
Further the three parameters s c , a*, and K root can be grouped in a dimensionless factor so that a change in one of these parameters can be compensated by a change in the other parameter without any effect on the relation between T act and f dry . Eq. 10 implies that for chemically controlled plants, the relation between the water pressure in the root zone and the transpiration rate is fundamentally different from the relation for hydraulically controlled plants. For a given plant characterized by one sc , one a* and one Kroot, for a given f dry , the ratio of T act /T pot is constant and therefore independent of T pot . According to Eq. 9, this implies that for a given f dry , the difference between effective soil water and leaf water pressure will be larger for a larger T pot which implies in turn that for larger T pot , ψ leaf will be smaller, even when T act < T pot . In other words, the control exerted on stomatal closure in C plants cannot maintain a critical threshold leaf water pressure that is independent of the atmospheric demand for water or T pot leading to apparent anisohydric behaviour. For H or H + C plants, the leaf water pressure varies within a small range when T act < T pot . According to Eq. 9, this implies that for a given ψ soil,eff , T act is independent of T pot (when T act < T pot ).
In the following, relations between ψ leaf , ψ soil,eff and T act for chemically controlled plants are derived. With assumption (ii) \( {\psi}_{soil, eff}\approx {f}_{dry}{\psi}_{leaf} \) and for s p = 0 and ψ lim = 0, T act can be written for C plants in terms of \( {\psi}_{soil, eff} \) as:
Using Eq. 8 with assumption (ii) |ψ root,wet | < < |ψ leaf | and Eq. 5, ψ leaf can be related to T pot and f dry for C plants as:
Equation 12 shows that ψ leaf does not remain constant when T act (for a constant T pot ) decreases due to an increasing f dry . Note that the same effect would occur if plants changed their K root in time (Caldeira et al. 2014). The direct relations given above hinge on several assumptions. By comparing relations obtained from numerical simulations of 3-D flow and transport in the soil-plant system with the relations given above the validity of these assumptions and approximations for isohydric and anisohydric behaviour will be assessed. Further the comparison between the H (Eq. 9 with ψ leaf = ψ lim,H ) and the H + C model (Eq. 6) will indicate if the simple hydraulic approach is valid to describe stomatal regulation or in which cases an additional chemical signal is needed. A sensitivity analysis will show to which extent variations of s c and K root influence the stomatal regulation mechanism.
Model setup
Numerical model
Soil
We set up a cubical domain with 20 cm side lengths, z tot , and a uniform discretisation of 0.5 cm and either one horizontal or two vertical, hydraulically impermeable, split layers (Table 1) with a width of two voxels. Soil hydraulic parameters for the bulk soil were set to those of a clay loam (Table 2, #1) (Carsel and Parrish 1988) and for the impermeable layers to a non-conductive material (Table 2, #2).
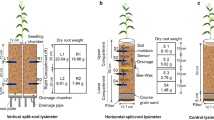
For the horizontal setup one thin impermeable layer was inserted at one of three soil depths (0.25, 0.5, or 0.75*z tot ) to divide the domain in two compartments. Boundary conditions were set to a constant water pressure either at the bottom (0 hPa) or at the top (-20 hPa). Irrigation from either top or bottom was introduced to obtain a greater variance in fractions of roots in dry soil. The remaining boundaries were defined as no flow. The vertical split was arranged by two thin impermeable perpendicular vertical layers that divided the simulation domain into four equal compartments (insets in Fig. 2). The top boundary conditions of each of these compartments could be set individually to a flow condition. Either 25, 50, 75, or 100 % of the top surface was irrigated but the amount of water that was applied, Q irr [L3 T-1], was always larger than the pre-defined transpiration demand, T pot . The bottom boundary was set to free drainage.
Root length densities over depth of the total domain for (a) the vertical and (b) the horizontal split setup. Root length densities for the individual compartments in (a) are overlapping. The horizontal lines in (b) represent the individual height of the split layer. The insets show the root architectures within the soil domains
As initial conditions the water pressure for the horizontal split was in a hydrostatic equilibrium with a bottom water pressure of 0 hPa. The vertical split setup started initially with steady state flow conditions in the entire domain, which were obtained in a separate, preliminary run under uniform irrigation.
Root
Two static (non-growing), fibrous root systems: one for the vertical and one for the horizontal splits were generated with the R-SWMS root growth model (Clausnitzer and Hopmans 1994) and used for the simulations (Fig. 2). The root systems varied slightly in their root length density distributions. Root fractions in the vertical split setup were equally distributed over the four compartments, while for the horizontal split the fraction of roots did not equal the fraction of the soil domain that was irrigated. The fraction of roots, f dry , was defined equal to the relative root length per compartment. For the vertical split the resulting maximum difference in water uptake from each compartment was lower than 0.4 % under uniform irrigation. The uptake from the horizontal split roots followed the root length density distribution in case of hydrostatic equilibrium.
Root hydraulic parameters for radial conductivity and axial hydraulic conductance were chosen from a data set for maize plants (Doussan et al. 1998) and kept uniform and constant for all root segments.
According to Couvreur et al. (2012), the system hydraulic conductivity, K root , can be derived directly from the simulated water uptake by the system for a uniform soil water pressure distribution and a given leaf water pressure. The water uptake that is simulated for this case in a single voxel and divided by the total water uptake represents the so-called standard sink fraction, SSF. The SSF distribution in the soil is subsequently used to calculate the effective soil water pressure ψ soil,eff as:
where i refers to the i th soil voxel. It should be noted that when ψ soil is uniform in a soil compartment and when the sum of the SSFs in the dry and wet compartments equal f dry and (1-f dry ), respectively, Eq. 8 is obtained.
Root water pressure in the dry and wet compartments respectively were calculated from the simulated water potentials in the dry and wet compartments using:
and analogous for the wet compartment.
In Table 3, the parameters used for the simulations and the different types of control (H + C, C) are given.
Scenarios
The relations between transpiration, root zone water potential and fraction of roots in dry soil obtained from numerical simulations and from approximate direct relations (Eqs. 6 and 10) were compared for H + C and C plants and for three potential transpiration rates. Each numerical setup consisted of 12 individual runs (4 vertical + 8 horizontal splits).
The parameters for the direct relations were equal to the parameters used in the numerical model. As the value for the sensitivity to leaf pressure s p was chosen in a way that 1/s p > ψ lim , the chemical production and stomatal closure due to chemical concentration starts at higher pressures than the amplification of the chemical signal by the leaf pressure. The only parameter that could not be directly transferred to the direct model was the signal production a. The numerical model uses a production rate at each segment, a, while in the direct model a* refers to a global signal production rate (Table 3). In theory a* = m a. With a total root mass of 14.2 g this would lead to a value of a* = 3.85 * 10-11 [mol hPa-1 d-1]. However, using this value led to a too strong reduction in transpiration predicted by the direct relations. A first reason is that due to transport limitations part of the produced mass was trapped in the root system. A second reason is that the simulated ψ x,dry were still considerably larger than the ψ leaf so that the production of chemical signal was overestimated when ψ x,dry was assumed to be equal to ψ leaf . Especially for the H + C case, in which signal is only produced when ψ xdry < ψ lim , this had an important impact. Therefore, a* was manually adjusted to match the transpiration reduction. It must be noted though that the same a* was used for all scenarios with different f dry and T pot
Results and discussion
Comparison of the direct and the numerical relations
The numerical simulations indicated that responses for the vertical and the horizontal splits were comparable so that all simulation results were combined and compared with the direct relations (Fig. 3) between T act /T pot , leaf and soil water pressure, and the fraction of roots in dry soil. The results from the numerical model refer to the states after a maximum of 100 days simulation time. Most of the runs established a steady state; however, 17 of the 72 runs did not reach steady state.
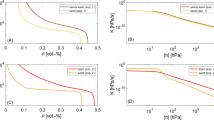
Relative transpiration rates at steady state for hydraulic + chemical (a–c) and chemical control (d–f) as a function of leaf water potential (a, d), effective soil water potential (b, e), and fractions of roots in dry soil (c, f). The numerical results (R-SWMS) are depicted as symbols and the corresponding theoretical (direct) results that were derived either by Eq. 6 or Eq. 10 are shown as lines. It should be noted that the axes ranges differ for (a), (b), (d), and (e)
For illustration, distributions of water potential in the root xylem and soil water contents at steady state and the time course of root water uptake from each soil compartment are given for the 50 % splits (2 × horizontal, 1 × vertical) in Supplementary Material 1.
For H + C control the direct relations (Eq. 6) match the numerical results qualitatively (Fig. 3a–c). When plotted versus ψ leaf , the relative transpiration rate T act /T pot remains equal to one with decreasing ψ leaf until a critical leaf pressure head is reached. Since ψ leaf is approximately equal to the root water potential in the dry zone, ψ root,dry , no signal is produced and no reduction in transpiration takes place as long as ψ root,dry and ψ leaf are larger than ψ lim . In this range, ψ leaf decreases due to a decreasing fraction of roots in the wetted zone and therefore a lower effective hydraulic conductivity of the root system (1-f dry ) K root so that a larger gradient between the wetted root zone and the shoot is required to sustain the potential transpiration. When ψ root,dry and ψ leaf become smaller than ψ lim , chemical production in the dry root zone is triggered, stomatal conductance decreases and T act becomes smaller than T pot . According to Eq. 2, the reduction depends on the chemical concentration which in turn depends on the chemical production rate and the transpiration rate. For a higher transpiration rate, the dilution of the produced chemicals is larger and ψ lim is reached for a smaller fraction of roots in the dry zone. Consequently ψ leaf must be smaller for a high than for a low potential transpiration to achieve the same chemical concentration and consequently the same transpiration reduction (Fig. 3a).
The relation between T act /T pot and ψ leaf is non-monotonous. For high T act /T pot , T act /T pot decreases with decreasing ψ leaf until a minimal ψ leaf is reached after which T act /T pot decreases with increasing ψ leaf . In our model setup, T act decreases since a larger part of the root zone is in the dry soil compartment. When f dry increases, the effective hydraulic conductivity of the root system (1-f dry )K root decreases and the fraction of the root system in which chemical signals are produced increases, which leads to a reduction in stomatal conductance. When f dry is small, i.e., for high T act /T pot , besides an increase in the fraction of roots that produce chemicals, also increase in production rate due to a decrease in ψ root,dry or ψ leaf is required to reduce Tact/Tpot. For larger f dry , the increase in chemical production and decrease of the dilution when T act /T pot decreases is large enough to decrease the stomatal conductance so that the production rate may decrease and hence ψ root,dry and ψ leaf may increase with decreasing T act /T pot . Although the non-monotonic behaviour may be observed for the different transpiration rates, except for the highest one, the difference between the minimally reached ψ leaf and ψ lim is not so large. This implies that a quasi isohydric behaviour of the H + C plant emerges with a more or less constant ψ leaf = ψ lim,H when T act < T pot . Figure 3 illustrates that, for the scenarios considered, this constant ψ leaf or ψ lim,H was almost equal to the threshold root water pressure ψ lim that triggered chemical production (-6000 hPa, Table 3). The relation between T act /T pot and ψ soil,eff that is derived from the numerical simulations and the approximate direct relations for the H + C control is monotonous. Opposite to the relation between T act /T pot and ψ leaf , ψ soil,eff is for the same T act /T pot lower for a low than a high T pot . This is in line with relations that were derived for isohydric plants (see e.g., Couvreur et al. 2012; Javaux et al. 2013). T act /T pot remains equal to one with decreasing ψ soil,eff until a critical ψ soil,eff is reached. This critical ψ soil,eff is smaller (more negative) for smaller transpiration rates. For smaller ψ soil,eff , T act /T pot decreases and for a constant K root and ψ leaf , a linear decrease of T act /T pot with a decrease in ψ soil,eff emerges from the hydraulics of the soil-plant system (Couvreur et al. 2012). The plot of T act /T pot versus f dry is a mirror image of the response of T act /T pot to ψ soil,eff .
The agreement for T act /T pot to effective soil water potential and leaf water potential becomes poorer with larger transpiration rates than compared to its relation with f dry . This is probably due to the connection between ψ soil,eff and f dry (Eq. 8), as it does not sufficiently account for the lowering of ψ soil,eff during soil drying. The higher, compensated, water uptake from the irrigated part will lower soil water potentials in the irrigated part.
For the C controlled plant, the direct relations between T act /T pot and ψ leaf are also non-monotonic and for the same T act /T pot more negative ψ leaf are obtained for higher than for lower T pot . Again, the relations between T act /T pot and ψ leaf that were derived from numerical simulations corresponded fairly well with the direct relations. However, the range of T act /T pot that was simulated by the numerical model was not large enough to validate the non-monotonic behaviour. The numerical simulations only indicated that ψ leaf decreased with increasing transpiration reduction or decreasing T act /T pot . The relation between T act /T pot and ψ leaf for the H + C and C controlled plants are qualitatively similar. However, for the C plant, the leaf water potentials were more negative, and varied more with T act /T pot and with T pot which is typical of anisohydric behaviour.
Despite the qualitative similarities in the relations between T act /T pot and ψ leaf for the H + C and C controlled plants, the relations between root zone conditions, ψ soil,eff and f dry , potential transpiration, T pot , and transpiration reduction, T act /T pot were completely different. The C parameterisation leads to a unique relation between T act /T pot and f dry , which is independent of T pot (Fig. 3f) and in which T act /T pot decreases monotonously with increasing f dry (Eq. 10). For the same f dry and the same T act /T pot , the leaf water pressure must be lower for a larger T pot to sustain a larger transpiration flux. This leads to a lower water pressure in the dry root zone and a higher production in signal rate. Since for the C-parameterization both T act (Eq. 5a for ψ leaf < < ψ root,wet ) and the signal production rate (Eq. 1 with ψ lim = 0) are proportional to ψ leaf , which is equal to ψ soil,dry , the higher production rate is offset by a higher dilution so that the chemical concentration and consequently T act /T pot do not depend on T pot . The lower sensitivity of the stomatal closure to chemical concentrations in the C-parameterization led to a small sensitivity of T act /T pot to f dry for f dry smaller than 0.6 (Fig. 3f). The fact that T act /T pot was not dependent on T pot and did not vary a lot with f dry for f dry < 0.6 explains why the leaf water potentials decreased considerably with decreasing T act /T pot before they reached their minimum and were much smaller for larger T pot . The non-monotonic relation between T act /T pot and ψ leaf and the large differences in these relations for different T pot are translated into the relation between T act /T pot and ψ soil,eff . Opposite to the H + C parameterization, the relation between T act /T pot and ψ soil,eff is non-monotonic and the same reduction in T act /T pot occurs for higher T pot at lower ψ soil,eff .
The good comparison between numerical simulation results and direct relationships suggest that the direct relationships can be used to predict plant transpiration as a function of the water status in the root zone for different parameterizations of stomatal regulation by plant chemicals that lead to either isohydric or anisohydric plant behaviour. Isohydric behaviour may be described by Eq. 9 with ψ leaf = ψ lim,H ≈ ψ lim ., whereas anisohydric behaviour, which implies additional chemical signalling, can be explained by Eq. 9 in combination with Eq. 12. However, to derive these relationships several assumptions were made that are discussed in more detail in the following.
Verification of the assumptions
The physiological implications of some model assumptions were evaluated using numerical simulations: Two isolated soil compartments between which no water flow can take place and a steady state condition with a soil compartment that is kept wet were also implemented in the numerical simulations. The distribution of roots and their hydraulic properties were defined so that also assumption (vi) was satisfied in the numerical simulations.
Assumption (i) soil is constituted of two isolated compartments and plant roots do not cross the compartment boundaries
Splitting up the root network in two separated parts was the basis to link the root water potential in the dry soil part to the leaf water potential (assumption iii). This assumption was violated in the horizontal split setups in which the root system crossed the different compartments. However, according to the results shown in Fig. 3, this violation did not affect the agreement between the outcome of the numerical simulations (R-SWMS) and the predictions using the direct relations.
Assumption (ii), ψroot,wet ≈ 0
The root water pressure at the root-soil interface of the irrigated compartment was never lower than -450 hPa (data not shown). Thus assumption (ii) is valid. The water pressure in the xylem, ψ x,wet , however, was always lower than −2000 hPa (Fig. 4), which is due to the radial hydraulic resistance of the root system. While the vertical splits show a marked difference between dry and irrigated xylem water pressure (Fig. 4b, d), the xylem water pressures in the two compartments were very close for the horizontally split domains (Fig. 4a, c). Since there was almost no radial flow in the roots in the dry part, the water pressure in the xylem of the dry part of the root system was nearly equal to the water pressure at the root-soil interface: ψ x,dry ≈ ψ root,dry (data not shown).
Comparison of leaf and root xylem water potential of the dry and irrigated compartment for (a, b) the H + C and (c, d) the C control and Tpot*1 for (a, c) the horizontal splits where the boundary condition is indicated for each fdry in orange for top irrigation and blue for bottom irrigation, respectively; (b, d) depict the vertical splits. It should be noted that the y-axes ranges for (a) and (b) differ from (c) and (d)
Assumption (iii), ψleaf = ψroot,dry
Figure 4 illustrates that this assumption is not fully correct. Although the difference between ψ x,dry ≈ ψ root,dry and ψ leaf is decreasing with larger root fractions in dry soil, a small difference between the dry roots and the leaf water potential remains. For the vertical splits, this difference is due to a small uptake from the dry root zone because equilibrium between the dry root zone and the shoot was not yet reached at the end of the simulations. However, the uptake was smaller than 5 % of the total water uptake (data not shown). Especially for H + C controlled horizontal splits, the assumption is much less well met for small f dry . In contrast to the vertical split scenarios, the shoot segment is not connected in parallel but in series to the dry and wet soil. As a consequence, the xylem root water potentials in the dry and wet compartments are similar for the horizontal splits. When water flows from the wet compartment through root segments in the dry compartment to the shoot (i.e., when irrigation is from the bottom), the water potentials in the dry compartment are closer to the potential in the shoot. However, when the dry compartment is below the wet compartment (irrigation from the top), the water potentials in the shoot are closer to the potentials in the wet root segments, which can especially be observed for higher transpiration rates (Supplementary Material 2).
Assumption (iv), signal production only at the dry side of the root system
For the vertical split, there is a considerable difference between ψ x,dry and ψ x,wet (see Fig. 4b and d). For the H + C case, in which chemical production starts for ψ x < ψ lim , no signal is produced in the wet zone whereas nearly all root segments in the dry zone produce signal (Supplementary Material 3). For the C case, all root segments produce signal but since ψ x,dry < ψ x,wet , most of the signal is produced in the dry zone. For the vertical splits assumption (iv) can be considered valid assuming the wet part is constantly irrigated (Assumption (ii)).
For the horizontal splits, ψ x,dry and ψ x,wet are similar and for irrigation from the top, ψ x,wet is even smaller than ψ x,dry (Fig. 4a and c). Assumption (iv) is therefore clearly violated for the horizontal split experiments. Because the pressure heads are similar in both compartments, it could be assumed that the production of chemical signal takes place in both compartments so that instead of using Eq. 3, the concentration in the leaves could be calculated as:
Deriving direct relations between transpiration reduction and soil and plant parameters using Eq. (15) is straightforward. An evaluation of these functions indicated that the direct relations showed a similar behaviour as shown in Fig. 4 but that an adjustment of the a* parameter might be required. Therefore, we continued to use the equations that were derived based on Eq. 3.
Assumption (v), immediate effect of chemical signal (no transport limitation)
A previous study showed the impact of transport of chemical signalling on transpiration reduction (Huber et al. 2014). Due to transport limitations (when uptake is close to zero) chemicals can be trapped in the roots in dry zones, which can lead to a reduction by an order of magnitude of chemical signal arriving at the leaves as verified experimentally, although not by an order of magnitude (Dodd et al. 2008). Transport limitation can further result in a slight re-opening of stomata during prolonged drying as the trapped signal cannot be effective in stomatal closure as verified experimentally (Stoll et al. 2000). These simulations showed that the effective signal was around 60 % of the total signal produced. This transport related effects are quite minor in comparison to the actual stomatal response and might be accounted for by the adjustment of a*.
Sensitivity analysis
Figure 5 shows the sensitivities of Eqs. 6 and 10 with respect to the parameters K root and s c . Both were varied separately by one order of magnitude. For the H + C parameterisation the slope between relative transpiration and ψ soil,eff is influenced by K root . Further, for a given T act /T pot , the difference between soil and leaf water pressure is decreasing with higher K root . The parameter s c affects slope as well as curvature of the relation between relative transpiration and water potentials. As noted before, for high values of s c this relationship becomes almost linear and has a response comparable to Eq. 9 (isohydric behaviour) whereas for low s c , the variation of ψ leaf with T act /T pot increases and the relation between T act /T pot and ψ soil,eff becomes more nonlinear, and even non monotonous. This resembles more an anisohydric behaviour. The threshold water potential ψ lim determines the convergence point for ψ leaf and ψ soil,eff (Supplementary Material 4).
In the C cases the relation between T act /T pot to ψ soil,eff is independent of K root and non-monotonous. The parameter s c is highly sensitive and responsible for the slope and curvature of T act /T pot (ψ soil,eff ). For lower values of s c the response of stomatal closure to soil drying is almost negligible.
On the meaning of isohydric and anisohydric behaviour
It is important to clarify the definition of isohydric and anisohydric behaviour. There are two different characteristics, which define these behaviours in the literature (Tardieu and Simonneau 1998): The first is whether a plant has the potential to keep the leaf water potential at a certain threshold when soil water potentials and transpiration rates vary. When such a threshold exists, a second relevant characteristic is the level of this threshold. Isohydric behaviour refers to plants that maintain leaf water potentials constant under varying environmental conditions such as soil water potential and transpiration rates. Anisohydric plant behaviour refers to plants in which leaf water potentials vary strongly. The problem with these definitions is that, strictly spoken, they are not discriminative. In other words, it is possible that a plant has both isohydric and anisohydric behaviour. This is the case for a plant that can control leaf water potentials only at a very negative leaf water potential so that large variations of leaf water potential can be observed before this value is reached. Thus, in general isohydric behaviour does not imply an earlier stomatal closure than anisohydric behaviour. C controlled plants with high sc might close their stomata at the same or even higher leaf water potentials then comparably parameterized plants with H + C control. An experimental study by Brodribb et al. (2014) showed for example that H controlled conifer species developed the strategy to keep their stomata open even after prolonged periods of drought, whereas C controlled species showed high levels of ABA and an early onset of stomatal closure.
It is also possible that a plant has neither property. For instance a plant in which stomatal closure is very sensitive to chemical concentrations or in which chemical production in the root zone is strong and very sensitive to root water potentials may keep leaf water potentials at high levels. Such a plant would not have an anisohydric behaviour since leaf water potentials do not vary over large ranges. However, the level of the leaf water potential would not be fixed but depend on the soil water potentials and transpiration rate so that an isohydric behaviour could be assigned neither. In this respect, definitions of anisohydric and isohydric behaviour based on leaf water potential fluctuations are not similar to definitions based on the property of plants to maintain a certain threshold leaf water potential. In this paper, we consider the property of a plant to keep or not to keep a constant leaf water potential under different transpiration demands and soil water potentials to be the difference between isohydric and anisohydric behaviour. A recent study suggests that plant behaviour cannot be strictly classified as either anisohydric or isohydric, but rather in between (Klein 2014), which also emerged from the H + C simulations.
Applicability of these approaches
For H + C parameterisation a reduction in T act occurs for higher ψ soil,eff when T pot is higher and the relation between T act /T pot versus ψ soil,eff may be approximated by a piecewise linear relation. This type of apparent isohydric behaviour is assumed in many soil hydrological models (e.g., Feddes et al. 1978). Couvreur et al. (2014a; 2012) used this approach to describe root water uptake as a function of the hydraulic conductivity of the root system and assumed a fixed leaf water potential. The relations predicted by this approach (Eq. 9 with ψ leaf = ψ lim ) are given by dashed lines in Fig. 6. If stomatal regulation leads to a more or less constant ψ leaf with decreasing T act /T pot , the approach reproduces the course of T act /T pot versus ψ soil,eff relatively well. However, with increasing T pot , and for the considered parameterisation of the stomatal conductance, the stomatal regulation was not ‘able’ to maintain the leaf water potential close to ψ lim but led to considerably smaller ψ leaf values. Consequently, the ratio of T act /T pot was for the same ψ soil,eff considerably larger than predicted assuming that ψ leaf = ψ lim . These results indicate that despite the fact that the transpiration reduction as a function of ψ soil,eff for different T pot shows a behaviour that points at an anisohydric behaviour, the transpiration reduction cannot be predicted well using a model that assumes a perfect isohydric behaviour (Eq. 9 with constant ψ leaf = ψ lim and constant K root ). Further, the sensitivity analysis revealed that with decreasing sensitivities to chemical signalling simulated T act /T pot correspond more to apparent anisohydric behaviour (Fig. 5b). Thus even if the plant is controlled by hydraulic as well as chemical signalling, it does not necessarily have to result in an isohydric behaviour.
C control predicts that the relative transpiration reduction in relation to f dry is independent of T pot . This behaviour was observed by Tardieu and Simonneau (1998) for sunflower, where the relation between stomatal conductance and the concentration of ABA was independent of vapour pressure deficit, which for the conditions in their experiment was linearly related to the potential transpiration. Several species are known to exhibit anisohydric behaviour with different intensities of stomatal closure: sunflower with a low sensitivity (Tardieu and Simonneau 1998), or Eucalyptus gomphocephala (Franks et al. 2007) and an anisohydric cultivar of grapevine (Rogiers et al. 2012) which show pronounced stomatal closure but still high variations in leaf water potential. As f dry can be directly related to the bulk soil water content, model approaches that relate stomatal closure to bulk soil water content like the FAO approach (Allen 1998), might be valid to describe anisohydric plant behaviour.
The behaviour might also change during the lifetime of a plant with changing root architectural conductivity (Sade and Moshelion 2014). The direct relation shows that smaller K root led to a more anisohydric behaviour (see Fig. 5a). It will be of interest how these relationships change dynamically, as K root depends on root hydraulic conductivities, which change with age but also with environmental conditions in time-scales as fast as hours (Chaumont and Tyerman 2014), and on root architecture.
Conclusion
Two simple equations (Eqs. 6 and 10) were derived to describe transpiration of plants of which stomata are controlled by hydraulic and/or chemical signalling. Both are in good agreement with relations derived from simulations with a numerical mechanistic model, which solved the plant hydraulics as well as chemical signalling and transport explicitly.
The results indicate that a completely different relation between the reduction of plant transpiration, soil water status and potential transpiration rate is obtained depending on how the production of chemical signals in the root zone and the sensitivity of stomatal closure to chemical concentrations are parameterized.
The commonly used piece-wise linear relationship between the ratio of the actual to the potential transpiration and the effective water potential at the soil root interface is based on the assumption of a constant leaf water potential when transpiration is reduced due to low soil water availability. H + C controlled plants can keep leaf water potential constant when T pot is not too large and the conductivity of the root system (K root ) and the sensitivity to chemical concentrations (s c ) are sufficiently large. Otherwise, leaf water potential depends strongly on the effective soil water potential at the soil root interface and the potential transpiration (Eq. 6).
A simplified equation (Eq. 10) relating actual and potential transpiration for C controlled plants was derived by omitting the water pressure threshold for signal production and stomatal regulation. Contrasting to H + C controlled plants, for C-controlled plants the ratio of the actual to the potential transpiration rate is a unique function of the fraction of roots in dry soil. This emerging behaviour is to some extent analogous to eco-hydrological models that describe root water uptake only as function of the lumped soil water content in the root zone (Rodriguez-Iturbe and Porporato 2005). It implies that for the same soil water availability or the same amount of roots in dry soil, the actual transpiration is larger with a lower leaf water potential for a high than for a low potential transpiration rate.
Based on these direct relationships, it could be concluded that datasets in which the transpiration rates and leaf water potentials are measured for the same soil water availability but with different potential transpiration offer great potential to discriminate between contrasting plant behaviour and parameterize their control mechanisms. A next step would be to investigate the impact of different control mechanisms on total water uptake, plant growth, and field water balances over an entire growing season. This would require simulations that consider more realistic spatial and temporal distributions of the soil water content.
References
Allen RG (1998) In: Allen RG (ed) Crop evapotranspiration : guidelines for computing crop water requirements. Food and Agriculture Organization of the United States, Rome
Ball JT, Woodrow I, Berry J (1987) A model predicting stomatal conductance and its contribution to the control of photosynthesis under different environmental conditions. In: J Biggins (ed) Progress in photosynthesis research. Springer Netherlands
Brodribb TJ, McAdam SAM, Jordan GJ, Martins SCV (2014) Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc Natl Acad Sci U S A 111:14489–14493. doi:10.1073/pnas.1407930111
Caldeira CF, Jeanguenin L, Chaumont F, Tardieu F (2014) Circadian rhythms of hydraulic conductance and growth are enhanced by drought and improve plant performance. Nat Commun 5. doi: 10.1038/ncomms6365
Carsel RF, Parrish RS (1988) Developing joint probability-distributions of soil-water retention characteristics. Water Resour Res 24:755–769. doi:10.1029/WR024i005p00755
Chaumont F, Tyerman SD (2014) Aquaporins: highly regulated channels controlling plant water relations. Plant Physiol 164:1600–1618. doi:10.1104/pp. 113.233791
Choat B, Jansen S, Brodribb TJ, Cochard H, Delzon S, Bhaskar R, Bucci SJ, Feild TS, Gleason SM, Hacke UG, Jacobsen AL, Lens F, Maherali H, Martinez-Vilalta J, Mayr S, Mencuccini M, Mitchell PJ, Nardini A, Pittermann J, Pratt RB, Sperry JS, Westoby M, Wright IJ, Zanne AE (2012) Global convergence in the vulnerability of forests to drought. Nature 491:752–755, http://www.nature.com/nature/journal/v491/n7426/abs/nature11688.html#supplementary-information
Clausnitzer V, Hopmans JW (1994) Simultaneous modeling of transient three-dimensional root growth and soil water flow. Plant Soil 164:299–314
Couvreur V, Vanderborght J, Beff L, Javaux M (2014a) Horizontal soil water potential heterogeneity: simplifying approaches for crop water dynamics models. Hydrol Earth Syst Sci 18:1723–1743. doi:10.5194/hess-18-1723-2014
Couvreur V, Vanderborght J, Draye X, Javaux M (2014b) Dynamic aspects of soil water availability for isohydric plants: focus on root hydraulic resistances. Water Resour Res. doi:10.1002/2014WR015608
Couvreur V, Vanderborght J, Javaux M (2012) A simple three-dimensional macroscopic root water uptake model based on the hydraulic architecture approach. Hydrol Earth Syst Sci 16:2957–2971. doi:10.5194/hess-16-2957-2012
Damour G, Simonneau T, Cochard H, Urban L (2010) An overview of models of stomatal conductance at the leaf level. Plant Cell Environ 33:1419–1438. doi:10.1111/j.1365-3040.2010.02181.x
Dodd IC, Egea G, Davies WJ (2008) Abscisic acid signalling when soil moisture is heterogeneous: decreased photoperiod sap flow from drying roots limits abscisic acid export to the shoots. Plant Cell Environ 31:1263–1274. doi:10.1111/j.1365-3040.2008.01831.x
Dodd IC, Theobald JC, Richer SK, Davies WJ (2009) Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate. J Exp Bot 60:4029–4039. doi:10.1093/jxb/erp236
Doussan C, Pierret A, Garrigues E, Pages L (2006) Water uptake by plant roots: II - Modelling of water transfer in the soil root-system with explicit account of flow within the root system - Comparison with experiments. Plant Soil 283:99–117. doi:10.1007/s11104-004-7904-z
Doussan C, Vercambre G, Pagès L (1998) Modelling of the hydraulic architecture of root systems: an integrated approach to water absorption—distribution of axial and radial conductances in maize. Ann Bot 81:225–232. doi:10.1006/anbo.1997.0541
Feddes RA, Kowalik PJ, Zaradny H (1978) Simulation of field water use and crop yield. Pudoc, Wageningen
Franks PJ, Drake PL, Froend RH (2007) Anisohydric but isohydrodynamic: seasonally constant plant water potential gradient explained by a stomatal control mechanism incorporating variable plant hydraulic conductance. Plant Cell Environ 30:19–30. doi:10.1111/j.1365-3040.01600.x
Gardner WR, Ehlig CF (1963) Influence of soil water on transpiration by plants. J Geophys Res 68:5719
Gowing DJG, Davies WJ, Jones HG (1990) A positive root-sourced signal as an indicator of soil drying in apple, malus x domestica-borkh. J Exp Bot 41:1535–1540. doi:10.1093/jxb/41.12.1535
Herkelrath WN, Miller EE, Gardner WR (1977) Water-uptake by plants.1. divided root experiments. Soil Sci Soc Am J 41:1033–1038
Huber K, Vanderborght J, Javaux M, Schröder N, Dodd I, Vereecken H (2014) Modelling the impact of heterogeneous rootzone water distribution on the regulation of transpiration by hormone transport and/or hydraulic pressures. Plant Soil: 1–20. doi: 10.1007/s11104-014-2188-4
Jarvis AJ, Mansfield TA, Davies WJ (1999) Stomatal behaviour, photosynthesis and transpiration under rising CO2. Plant Cell Environ 22:639–648. doi:10.1046/j.1365-3040.1999.00407.x
Javaux M, Couvreur V, Vanderborght J, Vereecken H (2013) Root water uptake: from three-dimensional biophysical processes to macroscopic modeling approaches. Vadose Zone J 12. doi: 10.2136/vzj2013.02.0042
Javaux M, Schröder T, Vanderborght J, Vereecken H (2008) Use of a three-dimensional detailed modeling approach for predicting root water uptake. Vadose Zone J 7:1079–1088
Klein T (2014) The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct Ecol. doi:10.1111/1365-2435.12289
Martin-Vertedor AI, Dodd IC (2011) Root-to-shoot signalling when soil moisture is heterogeneous: increasing the proportion of root biomass in drying soil inhibits leaf growth and increases leaf abscisic acid concentration. Plant Cell Environ 34:1164–1175. doi:10.1111/j.1365-3040.2011.02315.x
Puértolas J, Alcobendas R, Alarcón JJ, Dodd IC (2013) Long-distance abscisic acid signalling under different vertical soil moisture gradients depends on bulk root water potential and average soil water content in the root zone. Plant Cell Environ 36:1465–1475. doi:10.1111/pce.12076
Richards LA (1931) Capillary conduction of liquids through porous mediums. Phys J Gen Appl Phys 1:318–333. doi:10.1063/1.1745010
Rodriguez-Iturbe I, Porporato A (2005) Ecohydrology of water-controlled ecosystems: soil and moisture and plant dynamics. Cambridge Univ. Press, Cambridge
Rodriguez-Iturbe I, Porporato A, Laio F, Ridolfi L (2001) Plants in water-controlled ecosystems: active role in hydrologic processes and response to water stress: I. Scope and general outline. Adv Water Resour 24(7):695–705
Rogiers SY, Greer DH, Hatfield JM, Hutton RJ, Clarke SJ, Hutchinson PA, Somers A (2012) Stomatal response of an anisohydric grapevine cultivar to evaporative demand, available soil moisture and abscisic acid. Tree Physiol 32:249–261. doi:10.1093/treephys/tpr131
Sade N, Moshelion M (2014) The dynamic isohydric–anisohydric behavior of plants upon fruit development: taking a risk for the next generation. Tree Physiol. doi:10.1093/treephys/tpu070
Schröder N, Lazarovitch N, Vanderborght J, Vereecken H, Javaux M (2013) Linking transpiration reduction to rhizosphere salinity using a 3D coupled soil-plant model. Plant Soil 1–17. doi: 10.1007/s11104-013-1990-8
Simonneau T, Barrieu P, Tardieu F (1998) Accumulation rate of ABA in detached maize roots correlates with root water potential regardless of age and branching order. Plant Cell Environ 21:1113–1122. doi:10.1046/j.1365-3040.1998.00344.x
Stoll M, Loveys B, Dry P (2000) Hormonal changes induced by partial rootzone drying of irrigated grapevine. J Exp Bot 51:1627–1634. doi:10.1093/jexbot/51.350.1627
Tardieu F, Davies WJ (1993) Integration of hydraulic and chemical signaling in the control of stomatal conductance and water status of droughted plants. Plant Cell Environ 16:341–349. doi:10.1111/j.1365-3040.1993.tb00880.x
Tardieu F, Simonneau T (1998) Variability among species of stomatal control under fluctuating soil water status and evaporative demand: modelling isohydric and anisohydric behaviours. J Exp Bot 49:419–432. doi:10.1093/jexbot/49.suppl_1.419
Tardieu F, Zhang J, Gowing DJG (1993) stomatal control by both aba in the xylem sap and leaf water status - a test of a model for droughted or aba-fed field-grown maize. Plant Cell Environ 16:413–420. doi:10.1111/j.1365-3040.1993.tb00887.x
Van Genuchten MT (1980) A closed form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci Soc Am J 44:892–898
van Lier QJ, Metselaar K, van Dam JC (2006) Root water extraction and limiting soil hydraulic conditions estimated by numerical simulation. Vadose Zone J 5:1264–1277
Acknowledgments
This work is a contribution of the Transregio Collaborative Research Center 32, Patterns in Soil-Vegetation-Atmosphere Systems: Monitoring, Modelling and Data Assimilation, which is funded by the German research association, DFG.
Helpful comments on a previous version of this manuscript were provided by two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Susan Schwinning.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2734 kb)
Rights and permissions
About this article
Cite this article
Huber, K., Vanderborght, J., Javaux, M. et al. Simulating transpiration and leaf water relations in response to heterogeneous soil moisture and different stomatal control mechanisms. Plant Soil 394, 109–126 (2015). https://doi.org/10.1007/s11104-015-2502-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2502-9