Abstract
Background and aims
Ultramafic soils are characterized by relatively high concentrations of nickel (Ni), chromium (Cr), and cobalt (Co). Globally, some ultramafic outcrops are also rich in copper (Cu) and other metals. The occurrence of Cu-accumulating plants on such soils is a very rare phenomenon so far only described from Sri Lanka. The objective of this study was to evaluate the elemental profiles of plants growing in their natural habitat on polymetallic Cu-rich ultramafic soils, with particular focus on unusual uptake of Cu, and possible co-accumulation of other metals.
Methods
This study focused on Cu-rich ultramafic soils in the Bidu-Bidu Hills (Malaysia) and those in Macedo-Niquelândia and Americano do Brasil (Brazil) where chemical analyses of bedrock, soil and plant leaf samples was undertaken.
Results and conclusions
Although the elemental profile of plants growing on Cu-enriched ultramafic soils reflects that of their environment with elevated concentrations of Co, Cr, Cu and Zn, significant accumulation of these metals is rare. Accumulation of Cu by most plants follows an ‘Excluder’ response, with limited Cu uptake, even by Cu-tolerant plants on soils with high total and extractable Cu concentrations. Some plants show slightly higher uptake than normal, and might act as ‘Indicators’, but true hyperaccumulation of this metal is questionable.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ultramafic outcrops are characterized by their composition of mafic minerals (with high Mg and Fe concentrations), as well as having relatively high concentrations of Ni, Cr and Co (Proctor 2003). The predominant mineral is peridotite, which may be metamorphosed to serpentinite during emplacement and later weathered at the surface to produce red laterite soils (Baillie et al. 2000). The occurrence of Cu minerals in extensive areas of ultramafic (serpentine) rocks is not common, and little attention has been paid to this element in the many studies of ultramafic exposures and their vegetation. However, a number of cases of Cu-ultramafic associations have been documented. For example, Hochstetter in New Zealand in 1860, discussing the geology of Dun Mountain, where he described and named the olivine-based rock type ‘dunite’, noted the presence of native Cu (some pieces weighing nearly 4 kg), red copper oxide and copper pyrites, as ‘nests and bunches’, or lenticular-shaped masses in the ultramafic host rock. This, he said, “is by no means peculiar to the serpentine of New Zealand. In the serpentine district of Cornwall … native copper is found. The Monte Ramazzo, near Genoa, contains copper ores in serpentine; and in North America the same thing occurs (Hochstetter 1860).
In Western Australia the association of Cu with nickeliferous talc serpentinites near Widgiemooltha, south of Kambalda, has been described by Cole (1973; 1991), who also studied the geology and vegetation of areas with Ni-Cu associations in Zimbabwe (Cole 1973; Proctor and Cole 1991). At New Zealand’s North Cape, where there are lateritic soils derived from harzburgite and lherzolite, abnormally high Cu concentrations are found in the ultramafic soils (Shepherd 1983). In Brazil the occurrence of Cu in association with mafic-ultramafic complexes in Goiás State and Bahia State was noted briefly by Trescases et al. (1981), Wernick (1981) and Beurlen and Cassedanne (1981). Analytical data on several ultramafic soils of Goiás State showing elevated Cu concentrations were presented by Brooks et al. (1990; 1992). This is discussed in more detail below.
The typical normal concentrations of Cu in various rock types have been given as 60 μg g−1 (average crustal abundance), 13 μg g−1 (granites), 110 μg g−1 (basalts) and 57 μg g−1 (shales) (Reeves and Brooks 1978). It is therefore not surprising that Cu concentrations in soils of many kinds in many parts of the world lie in a range from about 5 to 60 μg g−1. The majority of ultramafic soils also show Cu concentrations in this range, or only slightly higher, alongside their abnormally high concentrations of Fe, Mg, Ni, Cr and Co. Table 1 summarises the results of analyses of Cu in ultramafic soils worldwide. The table shows that in a wide variety of ultramafic occurrences around the world the Cu concentrations are largely in the normal range that applies to soils of many kinds. Only in occasional samples have concentrations above 100 μg g−1 been observed. The exception from the Table is the lateritic soil of North Cape, NZ, where all soils analysed had at least 760 μg g−1 Cu. It is also noteworthy that, in spite of the recorded occurrences of native Cu and Cu minerals in the ultramafics at Dun Mt., Nelson, NZ, the soils there show only a modest elevation of Cu concentrations above the normal range. In addition to the values in Table 1, we note that (i) Lee et al. (1997) quotes a value of 42 μg g−1 for Cu in the serpentine soil at Red Mountain (NZ), and Lee (1977) in the analysis of a very large number of plants and their corresponding soils from New Caledonia gives soil Cu means for various sites ranging from 56 to 117 μg g−1. Thus the presence of significant amounts of Cu minerals in ultramafic rock assemblages (and of Cu in the derived soils) must be seen as a relatively rare occurrence. Where this does occur, these are often the result of Ophiolite-hosted VHMS (Volcanic-Hosted Massive Sulphide) deposits created by volcanic-associated hydrothermal events in submarine environments. They are predominantly layered accumulations of sulfide minerals that precipitate from hydrothermal fluids on or below the seafloor. Such deposits are known to occur in peridotite-associated ultramafic rock as part of the ophiolite suites in Sabah, Malaysia (Newton-Smith 1967). Abnormally high Cu concentrations (likely of the same geologic origin) have also been observed in Brazil (Brooks et al. 1990, 1992) and Sri Lanka (Rajakaruna and Bohm 2002; Rajakaruna and Baker 2004).
Hyperaccumulators are unusual plants that accumulate trace elements, such as Ni, Mn or Zn, in their shoots (Reeves 2003; Reeves 2006) with different operationally defined threshold values set for each element (Van der Ent et al. 2013). The majority of hyperaccumulators are known for Ni (>1000 μg g−1 foliar Ni) and predominantly found on ultramafic soils (Reeves 2006; Van der Ent et al. 2013). Slightly higher than ‘normal’ foliar accumulation of other elements present in high concentrations in ultramafic soil, such as Co and Cr, are common, but hyperaccumulation of these elements is exceedingly rare. In contrast, hyperaccumulation of Mn, an element often also present in high concentrations in ultramafic soil, has been reported from a number of plants from ultramafic soils in New Caledonia (Jaffré 1977; Brooks et al. 1981).
Hyperaccumulation of Cu was first defined as >1000 μg g−1 foliar Cu (Malaisse et al. 1978), but later revised downwards to >300 μg g−1 foliar Cu (Krämer 2010; Van der Ent et al. 2013) in light of the low concentrations usually encountered in plants. So far, unusual accumulation of Cu from plants growing on ultramafic soils has only been reported from Sri Lanka with 5 plant species, including Geniosporum tenuiflorum (Lamiaceae) with 2299 μg g−1 Cu (Rajakaruna and Baker 2004). Shepherd (1983) recorded Cu data for 308 plant specimens (of 41 species) collected from ultramafic soils in New Zealand containing 760–1430 μg g−1 Cu, as shown in Table 1. In spite of the invariably high Cu in the soil, the uptake of Cu by the plants was limited to the ‘normal’ range: 26% of the 308 specimens had Cu <2 μg g−1, 38 % had 2–4 μg g−1, 19.8% had 4–8 μg g−1, 13.6% had 8–16 μg g−1 and 2.3% had 16–32 μg g−1. A single plant sample was found with 42 μg g−1 Cu.
Although not on ultramafic soils, the phenomenon of Cu hyperaccumulation is best known from the Cu-Co outcrops of the D.R. Congo, which hosts the famous Copper Flora (Duvigneaud 1958; Brooks and Malaisse 1985; Faucon et al. 2010). Extensive research has identified 32 Cu hyperaccumulators from that region (Brooks 1977; Morrison 1980; Brooks et al. 1980, 1982, 1987; Brooks and Malaisse 1985; Reeves 2006). There are also reports of Cu hyperaccumulators from China, with species including Elsholtzia splendens (E. haichowensis) (Lamiaceae) (Jiang et al. 2004), Commelina communis (Wang et al. 2004) and Rumex acetosa (Polygonaceae) (Tang et al. 1999). Brooks et al. (1978) reported a total of 20 species of plants from Salajar Island near Sulawesi (Indonesia) with Cu values exceeding 80 μg g−1 and the highest value was 600 μg g−1 Cu for Laportea ruderalis (Urticaceae). A range of plants with unusual Cu accumulation have also been reported for copper-mineralized areas in Chile, with a number of species with foliar Cu of 100–300 μg g−1 from alpine meadows (Anic et al. 2010) and Oenothera affinis (Onagraceae) reaching 614 μg g−1 (Gonzalez et al. 2008). Compared with hyperaccumulators for Ni (from ultramafic soils) and Zn hyperaccumulators (especially Noccaea caerulescens), Cu hyperaccumulators have been relatively little studied. The famous ‘copper flower’ Haumaniastrum katangense (Lamiaceae) from the DR Congo has, however, been subjected to several experimental studies, which demonstrated that although it is extremely Cu-tolerant it has Excluder-type behavior under controlled conditions (Morrison 1980; Chipeng et al. 2009; Peng et al. 2012) despite Cu hyperaccumulation in field conditions (Paton and Brooks 1996). Similar results were obtained with controlled experiments using Elsholtzia splendens from China, with accumulation of Cu in the roots and low translocation to the shoot, typical for Excluders (Jiang et al. 2004; Weng et al. 2005). These findings re-affirm that Cu hyperaccumulation is difficult to attain when plants are grown in culture (Morrison et al. 1979; Macnair 2003; Faucon et al. 2007; Chipeng et al. 2009; Peng et al. 2012). Nevertheless, the widespread semi-aquatic Crassula helmsii (Crassulaceae) could accumulate more 9000 μg g−1 in its shoot after exposure to 0.6 μg g−1 Cu2+ in nutrient solution (Küpper et al. 2009).
The objective of this study was to evaluate the elemental profiles of plants growing in their natural habitat on polymetallic Cu-enriched ultramafic soils, with particular focus on unusual uptake of Cu, and possible co-accumulation of other transition metals. We hypothesize that even on soils with high Cu availability plant uptake will be strictly controlled and foliar accumulation therefore low. Our paper describes two case studies from tropical ultramafic outcrops in Malaysia and Brazil that have Cu-rich soils and where bedrock, soils and plant leaf samples were collected and analysed.
Materials and methods
Study sites and sample collection
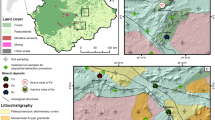
In Malaysia, the only known occurrence of a VHMS deposit is in the Bidu-Bidu Hills and nearby Kuala Kiabau in the Sandakan Division of Sabah. Unique among such occurrences is that this deposit has not been mined, and natural geochemical anomalies are still intact. Cu outcrops in this area are characterized by gossans of secondary minerals (azurite, malachite) formed above disseminated sulfide veins. Two distinct outcrops were studied: (1) iron-rich Cu gossans along the Bangau-Bangau River in the central part of the Bidu-Bidu Hills Forest Reserve (‘Bangau-Bangau’) (2) disseminated Cu sulfide veins outcropping just outside the Forest Reserve near the Labuk River (‘Kuala Kiabau’). The first has intact tall forest vegetation, whereas the second has a disturbed scrub vegetation and open aspect. Fieldwork was carried out in the Bidu-Bidu Hills and Kuala Kiabau in 2012. Figure 1 illustrates the vegetation and bedrock of these natural Cu outcrops in Sabah, Malaysia. Soil samples were collected from 0 to 10 cm depth, packed, brought to the local field station, air-dried at room temperature to constant weight, sieved to <2 mm, shipped to Australia, and gamma irradiated at Steritech Pty. Ltd. in Brisbane following Australian quarantine regulations. Bedrock samples were collected from the bottom of small soil pits.
Natural Cu outcrops in Sabah, Malaysia. Top: Bangau-Bangau River, with vegetation dominated by Machaerina glomerata (Cyperaceae) and Pandanus pectinatus (Pandanaceae) (Myrtaceae), and secondary Cu minerals (malachite) Bottom: Kuala Kiabau with scattered individuals of Neonauclea excelsa (Rubiaceae) and primary Cu minerals in situ (chalcopyrite, bornite)
In Brazil, the main areas visited in the course of field studies in Goiás State in 1988, 1990 and 2005 were: (1) Morro Feio and Crominia-Mairipotaba near Goiânia, where chromite mining has been carried out intermittently; (2) Americano do Brasil, where sulfides of Ni and Cu were mined during the 1970s; (3) Canabrava, the site of extensive chrysotile mining; (4) Macedo, site of major lateritic Ni mining since the late 1970s, and nearby Niquelândia; (5) Barro Alto, where a substantial Ni mining operation has been developed more recently. Several smaller areas were also studied. During the course of the three periods of study more than 800 herbarium-quality plant samples were collected for preservation and analysis (usually in duplicate, or quadruplicate where possible), and more than 120 associated soil samples were also collected for analysis. Soil samples were generally taken from a depth of 0 to 10 cm after removing any loose organic matter on the surface; this can be taken to represent the rooting zone of the herbs and small shrubs. The main purposes of the soil sampling have been (1) to confirm that the plant collections were being made from areas of essentially ultramafic composition, and (2) to show the concentration ranges of the elements that are of most relevance and special interest in the ultramafic soils.
Analysis of rock and soil material
For the Malaysian sites, rock samples (100 mg) were digested with a 5:3:2 mixture of concentrated nitric (70%), hydrochloric (37%) and hydrofluoric (48%) acids in a digestion microwave (Milestone Ethos). Soil samples (300 mg) were digested using freshly prepared ‘reverse’ Aqua Regia (9 mL 70% nitric acid and 3 mL 37% hydrochloric acid per sample) in open vessels in a digestion microwave (Milestone Start), and diluted to 45 mL before analysis. This gave (near) total concentrations of measured elements in the soil samples. Soil pH and electrical conductivity (EC) were obtained in a 1:2.5 soil: water mixture. Potentially plant available concentrations of trace elements (Cr, Co, Cu, Mn, Ni, Zn) were evaluated using 3 types of extraction methods: strontium nitrate (0.1 M) extractable, DTPA-extractable, and organic acid (0.01 M) extractable. The strontium nitrate (Sr(NO3)2) acts as a dilute neutral salt solution and hence provides a measure of the ‘immediately available and exchangeable’ ions in the soil. Diethylenetriamine pentaacetic acid (DTPA) extraction according to Lindsay and Norvell (1978) was undertaken to derive potentially plant-available concentrations of trace element. An ‘organic acid’ extraction solution was prepared by mixing solutions of acetic, citric and malic acids at a final concentration of 10 mM with the molar ratios of the acids 3:2:2 in a soil:solution ratio of 1:5. The samples were then mixed for 16 h. This method is aimed at mimicking plant root exudates and may hence provide a more realistic indication of the amount of trace elements available to plants. Exchangeable cations were extracted with silver-thiorea (Dohrmann 2006) over 16 h. All soil extractions were undertaken in 50 mL PP centrifuge tubes. Soil samples were weighed using a 4 decimal balance and weights recorded. Samples were agitated for method-specific times using an end-over-end shaker at 70 rpm and subsequently centrifuged (10 min at 4000 rpm) and supernatant collected in 10 mL PP tubes. All samples were analyzed with ICP-AES (Varian Vista Pro II) for Ni, Co, Cu, Zn, Mn, Pb, Cd, Fe, Mg, Ca, Na, K, S and P (soils) and Al, As, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, S, Si, Ti, Zn (rocks). Each method run included accredited standards as part of the quality control.
The Brazilian soil samples (n = 122) were air-dried and sieved initially to < 2 mm to remove any stony material. Subsamples of 2–3 g were ground to 70-mesh (<215 μm) and oven-dried at 60 °C. A further subsample of 0.15–0.20 g was weighed to 0.1 mg and transferred to a polypropylene beaker on a water bath at 100 °C for digestion with 10 mL of a 1:1 HF/HNO3 mixture. The use of HF during the digestion of the soil samples from Brazil might result in higher recoveries for some elements, compared to the Aqua Regia digestion method used on the Malaysian soil samples. After the solution had been taken to dryness the residue was dissolved in 10 mL of concentrated HCl, taken to dryness again, and the residue finally dissolved in 10.0 mL of warm 2 M HCl. Further dilutions by a factor of 5–15 were necessary to give solutions with sufficiently low Fe concentration (<1000 mg/L) for multi-element analysis by ICP-AES.
Analysis of plant material
At the Malaysian sites herbarium vouchers were collected from all plants occurring on observable Cu-mineralization in the field (Bangau-Bangau n = 226, and Kuala Kiabau n = 128). The vouchers were brought to the field station, thoroughly washed with demineralized water to remove any dust contamination and then dried at 70 °C for 5 days in a dehydrating oven. Foliar samples for chemical analysis were then collected from the dried herbarium vouchers, packed for transport to Australia and gamma irradiated at Steritech Pty. Ltd. in Brisbane following Australian Quarantine Regulations. Foliar samples were crushed and ground, weighed (300 mg) and digested using 7 mL concentrated nitric acid (70%) and 1 mL hydrogen peroxide (30%) in a microwave oven (Milestone Ethos), and diluted to 30 mL with TDI water before analysis with ICP-AES for Ni, Co, Cu, Zn, Mn, Pb, Cd, Fe, Mg, Ca, Na, K, S and P. This range of elements includes both transition elements that are essential to plants (Cu, Zn, Mn, Fe) and non-essential transition elements (Co, Cr), as well as major plant nutritional and beneficial elements (Ca, K, Mg, Na, S, P).
At the Brazilian sites, samples of leaf material from each number collected were similarly washed for several minutes in deionised water. After drying at 60 °C, 200 mg of leaf/shoot material was set aside in paper packets for analysis; 0.05–0.15 g of the dried plant tissue was then weighed to 0.0001 g and ashed in a muffle furnace over a period of 5 h, with the temperature being raised in stages to 500 °C for the last 2 h. After cooling, the ash was dissolved in 5 mL of warm 2 M HCl for analysis for 14–18 elements by ICP (ARL 34000 and upgraded versions), or in some cases only for Ni and a smaller range of elements by atomic absorption spectroscopy (using a GBC 902 instrument).
Statistical analyses
It was not possible to perform a regression analysis directly between soil Cu and foliar Cu samples because the samples are not paired. Therefore, we regressed site means of soil Cu versus site means of foliar Cu from Brazil and Malaysia (after ln-transformation of the data). All the sites were treated as single entities, except for (a) Americano do Brasil which has a large difference in Cu between the pyroxenite hill and the dunite hill; (b) Macedo which is a group of sites showing a wide range of Cu concentrations, hence we subdivided the sampling areas into low Cu soil (<200 μg g−1), medium Cu (200–1000 μg g−1) and high Cu (>1000 μg g−1). The Ni hyperaccumulator Pfaffia sarcophylla (Amaranthaceae) was omitted because it is the one species that genuinely appears to show very high foliar Cu on high-Cu ultramafic soils, along with Ni hyperaccumulation. A frequency histogram of the Cu concentrations in Brazilian soil samples was produced (after ln-transformation of the data), but this could not be done for the Malaysian data as the number of samples was insufficient. Frequency histograms were also produced for the foliar Cu concentrations in samples from Brazil and Malaysia (after ln-transformation of the data, and normalizing the sample numbers to 100%). We tested whether Ni hyperaccumulator plants accumulated more Cu than non-Ni hyperaccumulator plants with a Student’s t-test with Ni hyperaccumulation delimited at >1000 μg g−1. The analyses were undertaken on 354 foliar samples from the Malaysian sites and 630 foliar samples from the Brazilian sites for the following elements: Al, Ca, Co, Cr, Cu, Fe, K, Mg, Mn, Na, Ni, P and Zn. Specimens that appeared to have retained significant soil contamination, as evidenced by simultaneous high Cr (>60 μg g−1) and Fe (>2500 μg g−1) were omitted. Further, in the Brazilian data 120 samples were analysed by AAS, not with ICP-AES, for a limited range of elements and these samples have also been omitted. For values below the instrumental detection limit, a value half of the detection limit was used. The soil and foliar data was analysed using the software packages STATISTICA Version 9.0 (StatSoft), Excel for Mac version 2011 (Microsoft) and PRIMER Version 6 (PRIMER-E).
Results
Bedrock chemistry
Rock samples collected from the Fe-rich Cu gossans (Bangau-Bangau), and rock samples from the disseminated Cu sulfide veins (Kuala Kiabau) were analysed for their elemental composition. Elemental concentrations of rock samples are given in Table 2. As is characteristic for ultramafic rocks Al (0.1–0.5%), Ca (0.01–0.03%) and Si (0.3–7.9%) were (very) low, whereas Fe (19–51%) and Mg (0.3–8.9%) were extremely high for the bedrock from Bangau-Bangau. The typical Cu-rich gossan from Bangau-Bangau shows malachite veins in iron-crust. Cu concentrations were extremely high, reaching up to 21%, which represents high-grade Cu ore. Relatively high concentrations of Co, Cr and Ni are also typical for ultramafic soils. At Kuala Kiabau the mineralization is not hosted in ultramafic rock, but in associated mafic rocks (dolerite), and Mg is therefore low (0.17%) and Si is high (22%). Cu concentrations are elevated, but not particularly high (0.04%), whereas Ni, Cr, Co are all low.
The ultramafic occurrences of Goiás State are considered to fall into two broad categories: small or medium-sized masses of serpentinised dunite and peridotite, of the so–called alpine type, and large stratiform complexes based on a gabbro-pyroxenite-peridotite association. Areas (1) and (2) (see above) are considered to belong to the first of these categories, while the major layered mafic-ultramafic complexes are those of Barro Alto, Tocantins (Macedo-Niquelândia) and Canabrava (Berbert et al. 1981; Brooks et al. 1990). The site at Americano do Brasil in the São José massif is atypical in providing exposures of Ni and Cu sulfides. The site was studied in detail by Nilson (1981). The main ultramafic rocks here are dunites, peridotites and pyroxenites. Two hills, one of pyroxenite and one of dunite, were visited in the course of the plant work in 1988. Soils from the pyroxenite hill showed lower concentrations of the characteristic ultramafic elements (Fe, Mg, Co, Cr, Ni), but the largest distinction was in the Cu concentrations (mean < 100 μg g−1 over pyroxenite; mean >1000 μg g−1 over dunite) (Brooks et al. 1990). Elemental concentrations of soil samples from Brazil are also given in Table 2.
Soil chemistry
The elemental concentration of soil samples is given in Table 2, and the soils from Bangau-Bangau are characterized by high Mg (mean 2%), high Fe (31%), high Mn (mean 8180 μg g−1), high Ni (mean 6270 μg g−1) and extremely high Cu (range 98–18 600 μg g−1, four samples 98–262 μg g−1and except one exceptionally high sample). The elemental composition of Kuala Kiabau differs from Bangau-Bangau by having relatively high total Ca (mean 0.013% for Kuala Kiabau vs. mean of 0.31% for Bangau-Bangau), low Ni (mean 51 μg g−1), low Co and Cr, lower Fe, but also high Cu (range 247–652 μg/g, mean 423 μg g−1).
Table 3 summarizes the soil chemistry (extractable and exchangeable ions). The pH for the soils from Bangau-Bangau ranged from 5.8 to 6.9 and that for Kuala Kiabau ranged 4.3 to 5.7; hence those from Bangau-Bangau are significantly less acidic. Exchangeable Ca was much higher at Kuala Kiabau, but exchangeable Mg was similar between both localities. Strontium nitrate extractable Co and Cr are very low, whereas extraction of Ni is high in the ultramafic soils from Bangau-Bangau (mean 53 μg g−1), but low in the soils from Kuala Kiabau (mean 2.5 μg g−1), even though the pH is lower in the latter. Extractability of Mn is high in both soils (means 911 and 841 μg g−1 respectively). Although total Cu concentrations are higher at Bangau-Bangau, extractable Cu concentrations are much higher at Kuala Kiabau (mean 28 μg g−1vs. mean 0.1 μg g−1). Such differences are a consequence of the different mineralogies of the two sites. The DTPA-extraction of Co and Ni increases significantly over the strontium nitrate extraction, whereas that of Cr remains very low. Extractability of Zn also increased dramatically for the Kuala Kiabau soils; Cu increased only moderately for the soils from Kuala Kiabau, but one of the soils from Bangau-Bangau (‘gossan 1’) increased to 603 μg g−1. For most elements the organic acid extractions follow a similar pattern to the DTPA extraction, although extractability of Cr increased up to approximately 70-fold.
Most of the 122 soils collected from Goiás State in 1988 and 1990 showed some elevation of Cu concentrations above ‘normal’ levels of 5–60 μg g−1. Table 2 shows the ranges measured at various sites. In several cases soil samples were taken from non-ultramafic sites adjacent to the main ultramafic areas studied. Figure 2 shows that the frequency distribution for the 122 samples is clearly bimodal, with a separation in the region of 200–400 μg g−1. All soil Cu concentrations above 1000 μg g−1 are from the Tocantins complex (Macedo and Niquelândia) and the dunite hill of Americano do Brasil, apart from a single anomalous sample from Morro Feio. The six non-ultramafic samples all showed Cu in the range 19–80 μg g−1.
Plant diversity and vegetation on Cu-enriched ultramafic soils
The Bidu-Bidu Hills is a Class 1 forest reserve (i.e., totally protected) and, like the nearby Meliau Range, a virtual island of intact forest surrounded by palm oil estates. Rare species in the Bidu-Bidu Forest Reserve include Porterandia postarii (Rubiaceae), Paraphalaenopsis labukensis and Paphiopedilum hookerae var. volonteanum (Orchidaceae); this area has 11 endemics for Sabah and 37 endemics for Borneo (J.B. Sugau, pers. comm.). Gymnostoma nobile (Casuarinaceae) and Tristaniopsis grandifolia (Myrtaceae) are particularly common. In waterlogged areas Hopea pentanervia and Shorea venulosa (Dipterocarpaceae) also occur. The vegetation along the Bangau-Bangau River has a typical riparian aspect with Freycinetia robinsonii var. meijeri (Pandanaceae) forming a fringe along the water line. Slightly higher up, the Ni hyperaccumulator Phyllanthus balgooyi is very common, together with Dillenia luzonensis (Dilleniaceae) and Freycinetia robinsonii var. meijeri (Pandanaceae). The Bangau-Bangau River is named after the vernacular name for Borneodendron aenigmaticum (Euphorbiaceae), which is a tree endemic to Sabah and only found on ultramafic soils, and locally common here with Gymnostoma sumatranum and Dipterocarpus lowii. The water of the Bangau-Bangau River has a distinct blue color as a result of finely suspended serpentinite particulates originating from natural landslides above the riverbank. The vegetation of Cu anomalies is tall and extremely species-rich, with 209 species in 71 plant families represented. These diversity figures are remarkable given that 338 specimens were collected from an aggregated total area no larger than 1 ha. No species mono-dominates the Cu-gossans of the Bidu-Bidu Hills, although the sedge Machaerina glomerata (Cyperaceae) typically forms a dense understory, and Pandanus pectinatus (Pandanaceae) is locally common. Neither of these, nor any of the other species recorded from the Cu-gossans is restricted to that habitat.
On the Cu-rich soils of the dunite hill at Americano do Brasil the original vegetation had by 1988 been greatly degraded through mineral exploration and subsequent mining in the late 1960s and the 1970s, when burning exposed the outcrops. Regeneration consisted mainly of tall grasses and a dense shrub cover was developing. The vegetation structure over the ultramafic areas at Macedo and Niquelândia is quite varied. The ultramafic hill just north of Niquelândia town carries an open grassland/shrubland that contrasts strongly with adjacent forested non-ultramafic areas. It is not certain whether this distinction is entirely related to soil chemistry, or whether fire at some stage has contributed to the effect. On the several visits about 100 plant specimens, representing more than 70 different species, mainly of herbs, shrubs and grasses, have been collected from this hill.
At Macedo the vegetation pattern is ever-changing as a result of mining operations, exploration and waste overburden disposal, and occasional fire (of natural or human causes). The structure of the areas of interest available for plant collection falls mainly under the headings of cerrado, campo-cerrado and campo rupestre (herb- and grass-dominated). Apart from the human intervention, variations are related to a complex interplay of soil composition and the nature of the bedrock, altitude and aspect, soil texture and water retention. In some of the valleys and watercourses areas of low forest (mata) can still be found. Outside the areas of mata, shrubs taller than 1–1.5 m are not common. Some of the herbs have well-developed woody rootstocks that enable regrowth after fire. Prominent monocots include species of Paepalanthus (Eriocaulaceae) and Vellozia (Velloziaceae). The flora over that part of the Macedo ultramafic area that has not been destroyed by the mining activity is species-rich. Rare species include the Ni hyperaccumulator Pfaffia sarcophylla (Amaranthaceae), Ophiochloa hydrolithica, Paspalum longiaristatum and P. biaristatum (Poaceae), Microlicia macedoi and Pterolepis sp. nov. (Melastomataceae) and the facultative Ni hyperaccumulator Turnera subnuda (Turneraceae). It has not yet been established if any of these species is particularly associated with the high-Cu areas of the Macedo-Niquelândia ultramafics.
Foliar chemistry
Unusual foliar accumulation of Co, Cr, Cu, Mn and Ni in the Malaysian plants is given in Table 4. At Bangau-Bangau and Kuala Kiabau five species accumulated >75 μg g−1 Co (but the overall mean is 7.7 μg g−1 Co). These five species with unusually high Co concentrations also had high Ni concentrations, and the species with the highest Co accumulation (Aporosa chalarocarpa) is also a Ni hyperaccumulator. This species also accumulated 25 900 μg g−1 Al. For Cr (overall mean is 12 μg g−1 Cr) seven species accumulate >75 μg g−1, and there is no correlation between Cr and Ni accumulation. Four species accumulated >300 μg g−1 Zn (overall mean is 30 μg g−1 Zn), and none of the Zn accumulators has high concentrations of other transition metals. Nickel was accumulated to >500 μg g−1 by eight species (overall mean is 118 μg g−1 Ni) with six species hyperaccumulating the metal (>1000 μg g−1). Aporosa chalarocarpa, Dalbergia beccarii, Glochidion rubrum, G. mindorense and G. cf. lanceisepalum have thus far not been reported as Ni hyperaccumulators. Dalbergia beccarii recorded here with 2620 μg g−1 Ni is interesting, because another species in this genus (D. melanoxylon) together with Combretum hereroense accumulated both high Ni and Cu in Zimbabwe (Cole 1971). In Glochidion there has been one previous report of a species (G. aff. acustylum) with over 6000 μg g−1 Ni on ultramafics of the Soroako-Wasaponda area of Sulawesi (Reeves 2003). Four species in the present study accumulated >5000 μg g−1 Mn, with one species (Urophyllum cf. macrophyllum) reaching hyperaccumulator threshold values (>10 000 μg g−1). Cu in plants ranged from 0.2 to 229 μg g−1 (mean 10.5) μg g−1 with seven species accumulating >75 μg g−1 Cu (Fig. 3 shows boxplots of foliar Cu ranked by families). These low levels of accumulation are remarkable given the extremely high total and extractable concentrations of Cu in the soils. Nevertheless the concentrations >75 μg g−1 Cu are significantly higher than those of most plants, with the small terrestrial herb Argostemma cf. hameliifolium (Rubiaceae) having the highest value at 229 μg g−1, and the large tree Gluta wallichii (Anacardiaceae) the second highest value at 172 μg g−1.
Species with unusual foliar accumulation of Co, Cr, Cu, Mn and Ni in the Brazilian plants are given in Table 4. Cu concentrations in 710 of the 721 specimens analysed fell in the range 1–46 μg g−1, illustrating the remarkable control over Cu uptake exerted by most plants. The values in this range included many specimens taken from soils with elevated Cu concentrations i.e., 400–6400 μg g−1. At Americano do Brasil a species of Cnidoscolus (Euphorbiaceae), was found, following the earlier discovery at Macedo of a species of this genus that accumulated Ni to 100–1020 μg g−1 in its leaves and possessed a latex with >1% Ni. The species at Americano do Brasil has been identified as C. cnicodendron (sometimes regarded as a variety of C. vitifolius.) The specimens from Americano do Brasil did not have a Ni-rich latex, and the leaf Ni concentrations were all in the range < 2–24 μg/g. In spite of the elevated Cu in the soil, the Cu concentrations in leaf samples from 10 plants of this species were never above 8 μg g−1. Cu concentrations in the Ni–accumulator at Macedo, which is still only tentatively identified as C. cf. bahianus were also very low, all being <3 μg/g.
There were notably elevated Cu concentrations (105–298 μg g−1) in four specimens of Pfaffia sarcophylla (Amaranthaceae) from the Macedo area. Five other specimens of this species from Macedo and Niquelândia were in the normal range, with 8–26 μg g−1. This species has already been noted as a Ni hyperaccumulator (Brooks et al. 1992; Reeves et al. 2007); nine specimens analysed to date have shown Ni at 610–8910 μg g−1, with a mean of 3430 μg g−1. Other high Cu levels (in the range 50–106 μg g−1) were found in two specimens of Lippia species (Verbenaceae) from Niquelândia, which also showed Ni hyperaccumulation, in two specimens of Zeyheria species (Bignoniaceae) and in one of a Drosera species (Droseraceae) from Macedo. The single high value reported from Crominia, in Chaptalia integrifolia (Asteraceae), appears dubious: it is believed not to be the result of soil contamination, as the soil Cu concentration is not high, but may be a case of laboratory contamination. Unfortunately, no further specimen is available for analysis at this stage. The same comment may apply to the value of 140 μg g−1 recorded for Dipteryx alata (Fabaceae) at Morro Feio.
A frequency histogram of foliar Cu concentrations (ln-transformed) shows that in both Malaysia and Brazil foliar Cu concentrations are approximately lognormal distributed, and no bimodal pattern, indicative of a distinct physiological response, can be observed (Fig. 4). The Ni hyperaccumulators from Brazil accumulated significantly more Cu than non-hyperaccumulators (mean foliar Cu in Ni hyperaccumulators is 17 μg g−1 and the mean for non-hyperaccumulators is 9 μg g−1, p < 0.01) but this was not the case in Malaysia (mean foliar Cu in Ni hyperaccumulators is 7 μg g−1 and mean for non-hyperaccumulators is 11 μg g−1, p < 0.01). Regression analysis of foliar inter-elemental concentrations revealed no statistically significant correlations (p < 0.01) between foliar Cu and other foliar elements. Site means of Cu soil concentrations versus site means of foliar Cu concentrations from the various sites in Brazil and Malaysia (Table 5 and Fig. 5) are positively correlated (r2 0.83, p = 0.027), if the Americano do Brasil dunite site is excluded. The latter has a very wide range of soil Cu concentrations (782–6360 μg g−1) dominated by a few high values.
Discussion
Cu is essential for normal plant growth, where it is responsible for catalysis of redox reactions, and as such is part of various metalloproteins responsible for electron transport in chloroplasts and mitochondria (Fernandes and Henriques 1991; Yruela 2009). Cu deficiency in plants results in retarded growth and various disease symptoms. Normal Cu concentrations in plants are cited in the literature as 1–5 μg g−1 (Marschner 1995), 3–10 μg g−1 (Clarkson and Hanson 1980), or averaging 10 μg/g (Baker and Senef 1995). Deficiency can occur from plant leaf concentrations below 2–5 μg g−1 (Reuther 1957; Stevenson 1986). Its redox properties, contributing to its essentiality, also cause toxicity at higher concentrations (Kabata-Pendias and Pendias 2001). Toxicity thresholds for plant leaf concentrations can occur above 20 μg g−1 (Stevenson 1986). Yruela (2009) lists the toxic effects as arising from: (a) binding to sulfhydryl groups in proteins, (b) induction of a deficiency of other essential ions; (c) impaired cell transport processes; and (d) oxidative damage. Tolerance mechanisms against Cu toxicity are mainly based on removing Cu from sensitive cellular features. Active and passive mechanisms by which plants can exclude Cu from uptake in the shoot include (a) mycorrhizal association on the roots (b) root exudates resulting in precipitation of Cu ions (c) accumulation of Cu in the roots and restriction of further transport to the shoot via the xylem. Mechanisms inside the cells of the shoot include: (a) storage in the vacuole, (b) enhanced ion pumping of Cu over the plasma membrane (c) chelation by phytochelatins, metallothioneins and organic acids (Hall 2002; Krämer and Clemens 2006; Yruela 2009).
In a recent study by Faucon et al. (2007) supposed Cu and Co hyperaccumulators were collected from the D.R. Congo and the plant leaf material subjected to intensive washing schemes. They concluded that Cu concentrations were much lower than previously reported. Large variations of plant concentrations within a single site, significant linear soil/plant correlation (apparent ‘bioindicator’ behavior that can result from soil contamination) and relatively low concentrations in many specimens are all uncharacteristic for hyperaccumulator behavior. Most of the species from D.R. Congo and China display a high variability of Cu concentrations in leaves. For example, Pandiaka metallorum (Amaranthaceae) from D.R. Congo was found to have Cu ranging from 72 to 6270 μg g−1 in plant leaves (Morrison 1980, Brooks et al. 1987). Such behavior is highly unusual for metal hyperaccumulators, most of which are rather consistent in their accumulation. For instance, the majority of Ni hyperaccumulators appear to be ‘obligate’, meaning they invariably accumulate high Ni concentrations in their shoots, over a considerable range of soil Ni concentrations; so-called ‘facultative’ hyperaccumulators of Ni are less common (Van der Ent et al. 2013; Pollard et al. 2014). Most of the known Cu hyperaccumulators however, appear to be of the facultative type. The erratic Cu accumulation observed could be due to any of several factors: (i) plant accumulation depending on available soil Cu concentrations; (ii) strong influences from other soil properties both physical and chemical, including pH and concentrations of other elements; (iii) contamination of plant leaf samples. The absence of consistently high Cu accumulation in plants growing on soils with a wide variety of Cu concentrations (even when soil Cu is always high) weakens the likelihood of the occurrence of specialized hyperaccumulator behavior for this metal.
In the study of hyperaccumulators from mine and smelter sites, contamination of leaf surfaces always presents a danger to cause spuriously high analytical results, more so for Cu than for Ni. There are several reasons for this. On ultramafic soils, relatively pure Ni secondary minerals are not commonly encountered, so contamination is likely to reflect only the general overall soil composition. Ultramafic soils generally contains Ni at 1000–4000 μg g−1, and the hyperaccumulator plants may contain as much Ni as this, or more, so the risk is only that a contaminated non-accumulator may appear to have elevated Ni. In the case of Cu-rich soils, and around Cu mine sites, very Cu-rich secondary minerals (e.g., malachite, azurite, with >50% Cu) are often observed, and only minute amounts of dusts of these materials can cause apparent Cu levels in ‘normal’ plants to be greatly elevated - even to >1000 μg g−1 (Reeves and Baker 2000). Leaf surfaces vary greatly in the ease with which external contamination can be removed by various commonly used washing regimes. This is supported by experiments by Faucon et al. (2007) and RD Reeves (unpublished data) on Cu hyperaccumulators from the DR Congo, using washing by ultrasonic vibrations on leaves suspended in n-hexane, which found: (i) there were still a few very high values of Cu, although many of the species did not show the levels previously reported, and (ii) analyses of washed and unwashed material showed that there was a general reduction in Cu, often 10–70%, brought about by this (less conventional) washing process.
Conclusion
The overall elemental profile of plants growing on Cu-enriched ultramafic soils reflects that of their environment, with higher than ‘normal’ concentrations of Co, Cr and Cu, but significant accumulation of these metals is rare. The response to Ni is distinct from the other transition metals, with a hyperaccumulation response in some plant species on ultramafic soils. The specificity for Ni accumulation over other metals is remarkable: for example, a specimen of Glochidion rubrum from Malaysia accumulated 5010 μg g−1 Ni, but only 73 μg g−1 Fe, 25 μg g−1 Co, 10 μg/g Cr, 2 μg g−1 Cu, 71 μg g−1 Mn and 27 μg g−1Zn from a soil containing hundreds (Cu, Co, Zn) or thousands (Ni, Mn, Cr) μg g−1 of these metals. The high concentrations of Cu found in some plants reported here (ranging up to 298 μg g−1) are certainly abnormal and exceed widely quoted toxicity thresholds. We hypothesized that even on soils with high Cu availability plant take up will be strictly controlled and foliar accumulation therefore low. We conclude that, in general, the uptake of Cu by most plants seems to follow an ‘Excluder’ response: those species tolerating extremely high (extractable) soil Cu concentrations still manage to maintain their Cu concentrations near the low levels required by normal metabolic processes that require this element. Some species appear to display ‘Indicator’ characteristics, with moderate accumulation of Cu, but this is rare, and still needs thorough checking for the possibility of extraneous contamination.
The occasional occurrence of high soil Cu concentrations in ultramafic regoliths around the world presents an interesting phenomenon that has rarely been studied. Although most plant species growing on such soils limit their uptake of Cu, there appear to be a few exceptions where plants do actually accumulate this metal without showing obvious toxicity effects. Such species are of great scientific interest to better understand Cu metabolism and tolerance mechanisms. However, to avoid contamination problems, future experimental work should use controlled conditions with plants of carefully chosen provenance, natural Cu-rich ultramafic soil, and effective means of preventing soil contamination of the leaves throughout plant growth.
References
Anic V, Hinojosa LF, Díaz-Forester J, Bustamante E, Fuente L, De LM, Casale JF, de la Harpe JP, Montenegro G, Ginocchio R (2010) Influence of soil chemical variables and altitude on the distribution of high-alpine plants: the case of the Andes of central Chile. Arct Antarct Alp Res 42(2):152–163
Baillie I, Evangelista P, Inciong N (2000) Differentiation of upland soils on the Palawan ophiolitic complex, Philippines. Catena 39:283–299
Baker DE, Senef JP (1995) Copper. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, London, pp 179–205
Berbert CO, Svisero DP, Sial AN, Meyer HOA (1981) Upper mantle material in the Brazilian shield. Earth Sci Rev 17:109–133
Beurlen H, Cassedanne JP (1981) The Brazilian mineral resources. Earth Sci Rev 17:177–206
Brooks RR (1977) Copper and cobalt uptake by Haumaniastrum species. Plant Soil 48:541–545
Brooks RR, Malaisse F (1985) The heavy metal-tolerant flora of southcentral africa – a multidisciplinary approach. Balkema, Rotterdam
Brooks RR, Wither ED, Westra LY (1978) Biogeochemical copper anomalies on Salajar Island, Indonesia. J Geochem Explor 10:181–188
Brooks RR, Reeves RD, Morrison RS, Malaisse F (1980) Hyperaccumulation of copper and cobalt: a review. Bull Soc Roy Bot Belg 13:166–172
Brooks RR, Trow JM, Veillon JM, Jaffré T (1981) Studies on manganese-accumulating Alyxia species from New Caledonia. Taxon 30(2):420–423
Brooks RR, Grégoire J, Madi L, Malaisse F (1982) Phytogéochimie de l’anticlinal de Kasonta (Shaba, Zaïre). Geo Eco Trop 6:219–228
Brooks RR, Naidu SD, Malaisse F, Lee J (1987) The elemental content of metallophytes from the copper/cobalt deposits of Central Africa. Bull Soc Roy Bot Belg 119:179–191
Brooks RR, Reeves RD, Baker AJM, Rizzo JA, Diaz Ferreira H (1990) The Brazilian serpentine plant expedition (BRASPEX), 1988. Natl Geogr Res 6:205–219
Brooks RR, Reeves RD, Baker AJM (1992) The serpentine vegetation of Goiás State, Brazil. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation of ultramafic (serpentine) soils, intercept. Andover, UK, pp 67–81
Chipeng FK, Hermans C, Colinet G, Faucon M-P, Ngongo M, Meerts P, Verbruggen N (2009) Copper tolerance in the cuprophyte Haumaniastrum katangense (S. Moore) P.A. Duvign. & Plancke. Plant Soil 328(1–2):235–244
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol Plant Mol Biol 31:239–298
Cole MM (1971) Biogeographical/geobotanical and biogeochemical investigations connected with exploration for nickel–copper ores in the hot wet summer/dry winter savanna woodland environment. J S Afr Inst Mines Metall 71:199–209
Cole MM (1973) Geobotanical and biogeochemical investigations in the sclerophyllous woodland and shrub associations of the Eastern Goldfields area of Western Australia with particular reference to the role of Hybanthus floribundus (Lindl.) F. Muell. as a nickel indicator and accumulator plant. J Appl Ecol 10:269–320
Cole MM (1991) The vegetation of the greenstone belts of Western Australia. In: Roberts BA, Proctor J (eds) The Ecology of Areas with Serpentinized Rocks: A World View. Academic Publishers, Kluwer, pp 343–373
Dohrmann R (2006) Cation exchange capacity methodology II: A modified silver–thiourea method. Appl Clay Sci 34:38–46
Duvigneaud P (1958) La végétation du Katanga et de ses sols métallifères. Bull Soc R Bot Belg 90(2):127–278
Faucon M-P, Shutcha MN, Meerts P (2007) Revisiting copper and cobalt concentrations in supposed hyperaccumulators from SC Africa: influence of washing and metal concentrations in soil. Plant Soil 301:29–36
Faucon M-P, Meersseman A, Shutcha MN, Mahy G, Luhembwe MN, Malaisse F, Meerts P (2010) Copper endemism in the Congolese flora: a database of copper affinity and conservational value of cuprophytes. Plant Ecol Evol 143:5–18
Fernandes JC, Henriques FS (1991) Biochemical, physiological, and structural effects of excess copper in plants. Bot Rev 57:246–27
Gonzalez I, Muena V, Cisternas M, Neaman A (2008) Acumulación de cobre en una comunidad vegetal afectada por contaminación minera en el valle de Puchuncaví. Chile central Rev Chil Hist Nat 81(2):279–291
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53:1–11
Hochstetter F (1860) Dun Mountain Copper Mining Company. Nelson Examiner and New Zealand Chronicle, Volume XIX, Issue 34, 28 April 1860, pp 4
Jaffré T (1977) Accumulation du manganèse par des especes associées aux terrains ultrabasiques de Nouvelle-Calédonie. Comptes Rendus Academie des Science, Paris 284:1573–1575 Série D
Jiang LY, Yang XE, He ZL (2004) Growth response and phytoextraction of copper at different levels in soils by Elsholtzia splendens. Chemosphere 55:1179–1187
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton
Krämer U (2010) Metal hyperaccumulation in plants. Annu Rev Plant Physiol Plant Mol Biol 61:517–534
Krämer U, Clemens S (2006) Functions and homeostasis of zinc, copper, and nickel in plants. In: Tamás M, Martinoia E (eds) Molecular biology of metal homeostasis and detoxification from microbes to man. Springer, Berlin, pp 214–272
Küpper H, Gotz B, Mijovilovich A, Kupper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151(2):702–714
Lee J (1977) Phytochemical and biogeochemical studies on nickel accumulation by some New Caledonian plants. PhD Thesis, Massey University, Palmerston North, NZ
Lee WG, Bannister P, Bastow Wilson J, Mark A (1997) Element uptake in an ultramafic flora, Red Mountain, New Zealand. In: Jaffré, T, Reeves, RD Becquer, T (ed) Écologie des milieu sur roches ultramafiques et sur sols métallifères, Documents Scientifiqueset Techniques No. III/2, ORSTOM, Nouméa, pp 179–186
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Amer J 42:421–428
Macnair M (2003) The hyperaccumulation of metals by plants. Adv Bot Res 40:63–105
Malaisse F, Grégoire J, Brooks RR, Morrison RS, Reeves RD (1978) Aeolanthus biformifolius: a hyperaccumulator of copper from Zaïre. Science 199:887–888
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Morrison RS (1980) Aspects of the accumulation of cobalt, copper and nickel by plants. PhD Thesis, Massey University, NZ
Morrison RS, Brooks RR, Reeves RD, Malaisse F (1979) Copper and cobalt uptake by metallophytes from Zaïre. Plant Soil 53:535–539
Newton-Smith J (1967) Bidu–Bidu Hills area, Sabah: explanation of sheet 5–117–2 and part of 5–117–1: Borneo Reg Malaysia Geol Surv Rept 4
Nilson AA (1981) The nature of the Americano do Brasil mafic–ultramafic complex and associated sulfide mineralization, Goiás, Brazil. PhD Thesis, Department of Geology, University of Western Ontario, London, Ont
Paton A, Brooks RR (1996) A re-evaluation of Haumaniastrum species as geobotanical indicators of copper and cobalt. J Geochem Explor 56(1):37–45
Peng H, Wang-Müller Q, Witt T, Malaisse F, Küpper H (2012) Differences in copper accumulation and copper stress between eight populations of Haumaniastrum katangense. Environ Exp Bot 79:58–65
Pollard AJ, Reeves RD, Baker AJM (2014) Facultative hyperaccumulation of heavy metals and metalloids. Plant Sci 217–218:8–17
Proctor J (2003) Vegetation and soil and plant chemistry on ultramafic rocks in the tropical Far East. Perspect Plant Ecol 6:105–124
Proctor J, Cole MM (1991) The ecology of ultramafic areas in Zimbabwe. In: Roberts BA, Proctor J (ed), The Ecology of Areas with Serpentinized Rocks: A World View, Kluwer Academic Publishers, pp 313–331
Rajakaruna N, Baker AJM (2004) Serpentine: a model habitat for botanical research in Sri Lanka. Cey J Sci (Bio Sci) 32:1–19
Rajakaruna N, Bohm BA (2002) Serpentine and its vegetation: a preliminary study from Sri Lanka. J Appl Botany 76:20–28
Reeves RD (2003) Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 249:57–65
Reeves RD (2006) Hyperaccumulation of trace elements by plants. In: Morel JL, Echevarria G, Goncharova N (eds) Phytoremediation of metal–contaminated soils, Proceedings of the NATO Advanced Study Institute, Třešt’ Castle, Czech Republic, 18–30 August 2002, NATO Science Series: IV: Earth and Environmental Sciences 68. Springer, Berlin, pp 25–52
Reeves RD, Baker AJM (2000) Metal-accumulating plants. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: using plants to clean up the environment. Wiley, New York, pp 193–229
Reeves RD, Brooks RR (1978) Trace element analysis of geological materials. Wiley, New York
Reeves RD, Baker AJM, Becquer T, Echevarria G, Miranda ZJG (2007) The flora and biogeochemistry of the ultramafic soils of Goiás state, Brazil. Plant Soil 293:107–119
Reuther W (1957) Copper and soil fertility. In: Soil, the yearbook of agriculture. U.S. Gov. Printing Office, Washington, D.C. pp 128–135
Shepherd PR (1983) Biogeochemical and geobotanical studies of the ultramafic areas of North Cape. BSc (Hons.) Report, Massey University, Palmerston North, New Zealand
Stevenson FJ (1986) Cycles of soil– carbon, nitrogen, phosphorus, sulfur, micronutrients. Wiley, NewYork
Tang S, Wilke B, Huang C (1999) The uptake of copper by plants dominantly growing on copper mining spoils along the Yangtze River, the People’s Republic of China. Plant Soil 209(2):225–232
Trescases JJ, Melfi AJ, Barros de Oliveira SM (1981) Nickeliferous laterites of Brazil. In: Laterization Processes. Proceedings of the international seminar on laterization processes. Trivandrum, India 1979:170–184
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362(1–2):319–33
Wang H, Shan X-Q, Wen B, Zhang S, Wang ZJ (2004) Responses of antioxidative enzymes to accumulation of copper in a copper hyperaccumulator of Commelina communis. Arch Environ Contam Toxicol 47:1–9
Weng G, Wu L, Wang Z, Luo Y, Christie P (2005) Copper uptake by four Elsholtzia ecotypes supplied with varying levels of copper in solution culture. Environ Int 31(6):880–884
Wernick B (1981) The archaean of Brazil. Earth Sci Rev 17:31–48
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36(5):409–430
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Henk Schat.
Rights and permissions
About this article
Cite this article
van der Ent, A., Reeves, R.D. Foliar metal accumulation in plants from copper-rich ultramafic outcrops: case studies from Malaysia and Brazil. Plant Soil 389, 401–418 (2015). https://doi.org/10.1007/s11104-015-2385-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2385-9