Abstract
Aims
In deserts, moss-dominated crusts may play an important role in terrestrial-aquatic and aboveground-belowground connections. Despite its importance, very little is known about moss’s role in biogeochemical cycles and how nutrient pulses (e.g., from N deposition in air pollution) will affect their functional significance as an integrator of nutrient cycling in deserts.
Methods
Moss and soil were sampled from 15 sites in the Sonoran Desert in and around Phoenix, covering the city core subject to N deposition and rural areas to the east and west. Samples were analyzed for C, N, P and micronutrient content to compare moss stoichiometry over a gradient of soil resource availability.
Results
Moss %N and %P were positively correlated with soil N and P. Thus, sites in the city core subject to N deposition tended to have higher soil N and therefore higher moss N than the sites outside the city core. Micronutrient content varied with sampling region but was not related to soil content.
Conclusions
Results suggest that moss can take up excess N,, but overall coverage of moss is lower in the city, limiting its ability to act as a N sink.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Moss-dominated soil crusts are a ubiquitous riparian ground cover in the Sonoran Desert. Mosses are able to withstand the harsh desert environment with a high tolerance for heat stress and lack of water (Proctor et al. 2007; Stark et al. 2009), and following even small precipitation pulses they are quickly activated (Lange 2001; Cable and Huxman 2004). Once metabolically activated, moss can take up nutrients directly from the soil, wet deposition, or dry deposition on its outer surface by passive sorption of mineral ions and metals (Bates 2000). Thus, moss nutrient assimilation and tissue content have been shown to reflect nutrient supply from soil and stream (Solga 2007; Phuyal et al. 2008; Ball and Virginia 2014), though the relationship can be highly variable (Ball and Virginia 2014).
In the urban Sonoran Desert in Phoenix, AZ, the city core receives higher levels of nitrogen (N) deposition than the surrounding rural areas (Lohse et al. 2008; Cook 2014), and commensurate with that, urban soils contain greater levels of soil mineral N than rural soils (Hall et al. 2011). Previous research in this area has shown that excess soil nutrients may be assimilated to a limited extent by vascular plants, via increased growth and modest increases in foliar nutrient content in certain plant species (Hall et al. 2011). However, these responses were largely only observed during periods of prolonged water availability, being limited to the winter rainy season or “wet years” with ample precipitation. Overall vascular uptake of nutrients appears limited, and the ultimate fate of much of the excess N from air pollution is uncertain. It is possible that moss can assimilate some of the excess N, given its ability to become metabolically active shortly after an individual rain event. Because they can take up nutrients from both precipitation and dry deposition, even before nutrient uptake by higher plants, and decompose more slowly than other plants, they may have a very important role in ecosystem nutrient retention (Bates 2000). However, to date, there are no published measurements of moss nutrient content or their potential tolerance of N pollution in this area of the Sonoran Desert.
Desert soil crusts, such as those containing moss, are subject to human disturbance (Belnap and Eldridge 2001). In particular, moss can be sensitive to N deposition in air pollution, and excess N has been shown to decrease moss growth and coverage in some ecosystems (Schröder et al. 2010; Stark et al. 2011; Song et al. 2012). Cities, including Phoenix, are also subject to heavy metal deposition (Zschau et al. 2003), and metals from pollution can become concentrated in moss to negatively affect moss growth (Gerdol et al. 2000; Salemaa et al. 2004). Declines in moss production from human disturbance would limit the capacity of moss to serve as a sink of excess N and trace elements.
Moss has been demonstrated to contribute significantly to aboveground-belowground interactions and stimulate N cycling (Lindo and Gonzalez 2010; Hu et al. 2014), giving moss a potentially significant role in desert biogeochemistry. In fact, such soil crusts play a very important role in global biogeochemistry, accounting for 46 % of biological N fixation and 7 % of terrestrial net primary production (Elbert et al. 2012). Despite its importance, very little is known about moss’s role in biogeochemical cycles, such as the plasticity of their nutrient uptake and stoichiometry, and how urban pollution will affect their functional significance as an integrator of nutrient cycling in deserts. It is important to understand the biogeochemical role of moss to predict the consequences of human disturbance. To begin describing the basic biogeochemical significance of moss in the urbanized Sonoran Desert, we sampled moss from within urban Phoenix, subject to N deposition pollution, and moss from outside the city core. We asked: (1) Does moss macro- and micronutrient content reflect differences in soil resource availability? and (2) Is moss capable of being a sink for excess nutrients deposited from air pollution? We predicted that moss N, phosphorus (P), and micronutrient content would reflect differences in soil content, particularly with higher moss N inside the city core where N deposition increases soil N. We further predict that this uptake will allow moss to act as a sink for excess N from air pollution.
Methods
Study site
Moss and soil were sampled from 15 native desert sites in the Sonoran Desert in and around the city of Phoenix, Arizona (Fig. 1) over 2011–2013. Selected sites were located either within the city core, to the west of the city and east of the city (n = 5 sites per region, with a mix of sites from each region sampled in each year), and all are located within the Central Arizona-Phoenix Long-Term Ecological Research (CAP-LTER) project. These 15 sites have been consistently monitored by the CAP-LTER for N deposition for 15 years. The sites inside the city core receive 5–7 kg N ha−1 year−1 compared to only 4 kg N ha−1 year−1 at the sites east and west of the city (Lohse et al. 2008). These sites also lie along a precipitation gradient, with mean annual precipitation (MAP; averaged over the sampling period of 2011–2013) lowest in western sites (125 ± 9 mm), followed by the city core (144 ± 11 mm), and highest in the eastern sites (197 ± 12 mm; Table 1) (FCDMC 2009). Soils at all sites are Aridisols, with Typic Haplargids occurring across the gradient and additional Typic Camborthids to the west, Typic Paleorthids and Durorthids in the city core, and Typic Calciargids to the east (Table 1).
Map of the 15 sites from which moss and soil samples were collected inside the urban core (circles) of Phoenix, AZ and the surrounding areas west (squares) and east (triangles). See Table 1 for expanded site names
Three discrete moss patches were sampled at each site (n = 3 per site, so n = 15 per region). Moss-dominated crusts tend to be found in discrete patches along the edge of washes, often under the shade of plants (most often creosote (Larrea tridentata) or bursage (Ambrosia spp.), but occasionally palo verdes (Cercidium floridum)), as well as under the shade of rocks. Only a few samples were taken from under legumes such as palo verdes, making it unlikely that leguminous N-fixation would contribute to the average soil N content measured at any given site. While moss is occasionally found away from washes, we focused our sampling there for consistency. Moss frequently co-occurs with cyanobacteria in cryptobiotic crusts, but we intentionally selected moss patches occurring without a visible lichen or cyanobacterial component. Though some cryptic associations were likely present, they were a minor, rather than dominant, component of the crust. Therefore, N-fixation is probably not a significant source of N in these mosses, though it certainly can be in other situations (Gavazov et al. 2010).
Sample collection and processing
Percent cover of moss was measured in the field using the quadrat method (Rosentreter et al. 2001), where ten 25 × 25 cm quadrats were constructed using a wire frame. Fishing line was spaced at 5 cm intervals in order to create a 5 × 5 grid within each wire frame quadrat. Washes were identified as any gullied path that had signs of ephemeral water flow, and a representative selection of both shallow and deeply cut washes were sampled. At each site, ten 10-m transects were placed on the edges of the washes, parallel to the direction of flow, and the ten quadrats were placed at 1 m intervals along each transect. The total percent coverage of each individual quadrat was calculated by summing the coverage in individual sections of the frame. Percent coverage for each site was determined by averaging the 100 frame measurements at each site.
Moss was identified to species using Rosentreter et al. (2007), then analyzed for nutrient content. Several species of moss were sampled ubiquitously across all of the sites (Encalypta vulgaris, Ceratodon purpureus, and Funaria hygrometrica; Table 1). We also sampled two other species of moss: Syntrichia ruralis was only found in the eastern sites, where it was dominant. Bryum caespiticum was less common and only found in the core and western areas. Most moss samples collected were monocultures, but approximately one fifth of the samples were mixtures of two species.
Following Ball and Virginia (2014), when moss patches were located, a moss sample of approx. 3 cm in diameter was collected to a depth that included the entire moss carpet (usually ~1 cm) using a clean plastic spoon and placed in a sterile whirl-pack bag. The soil immediately beneath the moss was collected to approximately 7 cm using a clean plastic scoop and placed in a separate sterile whirl-pack bag. Both moss and soil were transported to the laboratory in a cooler, where they were both stored at 4 °C until processing.
Soil samples were sieved to 2 mm prior to chemical analysis. Gravimetric soil water content (SWC) was estimated by drying 20 g of soil at 105 °C for 24 h. For measurements of extractable phosphate (PO4-P), 10 ± 0.5 g soil were extracted in 50 ml 0.5 M NaHCO3 at pH 8.5. Samples were centrifuged at 17,555 × g for 10 min to remove soil, then 3 ml of 6 N HCl were added to the supernatant. Samples were allowed to degas prior to being frozen until run on a flow injection autoanalyzer (Lachat QC8000, Loveland CO). For extractable inorganic N (NO3 + NO2-N and NH4-N), 20 ± 0.5 g soil was extracted in 50 ml 2 M KCl, centrifuged at 17,555 × g for 10 min, then the supernatant frozen until run on the autoanalyzer. Total and inorganic C and N were measured on soils ground using a sapphire mortar and pestle that were either left unacidified or acidified with HCl respectively. Samples were analyzed on an elemental analyzer (Perkin Elmer PE2400, Wattham MA). Cation micronutrients (K, Ca, Na, Zn, Mg, Mn, and Fe) were measured on 10 ± 0.5 g soil extracted in 50 ml di-H2O. Samples were centrifuged at 17,555 × g for 10 min then filtered to 0.45 μm to remove soil, then acidified to 5 % HNO3 prior to being analyzed using inductively coupled plasma optical emissions spectroscopy (ICP-OES; Thermo iCAP6300, Hudson NH).
Given the amount of sedimentation, moss samples were washed free of as much soil as possible under a dissecting stereomicroscope (Ball and Virginia 2014). Moss samples were then dried at 60 °C before being ground to a fine powder using a mortar and pestle. Total C and N were measured on a subsample of moss on the elemental analyzer. Total P, as well as other cations micronutrients, were measured using a dry ash acid digestion method in which a moss subsample was ashed in a muffle oven that was gradually brought to 475 °C over 1.5 h, held at 475 °C for 4 h, then dropped to 105 °C until digested. Moss ash was then digested in 5 ml of 35 % HNO3. Samples were then centrifuged at 25,250 × g for 10 min, and the supernatant diluted to 5 % HNO3 for measurement on the ICP-OES.
Data analyses
Nutrient content data were analyzed using Analysis of Covariance (ANCOVA) in R 2.7.2 (The R Foundation). A 3-way ANCOVA was used to test for an effect of region (3 levels: core, east, and west), species (6 levels: 5 species and one “unidentified” category), and soil mineral N content on moss %N. Further 3-way ANCOVAs were run for moss %P and all micronutrients, replacing the respective soil nutrient content for mineral N. Moss micronutrient data were sqrt-transformed to meet the assumptions of normality. Given that the 2- and 3-way interactions were not significant, the model was simplified to include only the 3 main effects. If significant, a post-hoc Tukey test determined which regions differed from one another. Similarly, two-way Analysis of Variance (ANOVA) was used to determine whether soil nutrient and micronutrient content differed among the three regions or six moss species, followed by a post-hoc Tukey test if significant. A one-way ANOVA was used to determine whether percent cover (log-transformed to meet the assumptions of normality) differed among regions. A principal components analysis (PCA) was conducted using all moss nutrient data (C, N, P) and all cation micronutrient data from the soil and moss (untransformed) using indirect gradient analysis, focusing scaling on inter-species correlations with species scores divided by standard deviation, and centering by species (Canoco for Windows 4.5). Additionally, to explore the multivariate relationship between nutrient content of moss and soil nutrient resources, a canonical correspondence analysis (CCA) was conducted using all moss nutrient species and all soil and water nutrient environmental parameters (untransformed) using direct gradient analysis, again focusing scaling on inter-species correlations and biplot scaling, with no forward selection or permutation test (Canoco for Windows 4.5). Further, regression analysis was used to compare moss nutrient content with individual environmental nutrient sources from soil (Microsoft Excel 2010).
Results
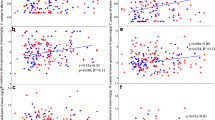
Species identity did not significantly influence moss %N or %P (Table 2; Fig. 2a). As a result, moss data are presented for the entire functional group, rather than by species. There was a significant relationship between soil and moss content for both N and P (Table 2), where moss %N and %P increased as soil content increases (Fig. 3). However, the relationship between soil and moss P was not as strong as it was for N, with a slope only slightly above 0, partially driven by one moss sample high in P (Fig. 3b).
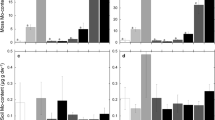
Nitrogen and phosphorus content of moss organized by (a) the different species of moss collected across the sites and (b) in the three regions of sample collection. Also shown is (c) soil nutrient content across the three regions of sample collection. Values are means ± standard error. Moss %N and soil N were significantly influenced by region, and letters above the bars denote differences identified by a post-hoc Tukey HSD test
Sampling region significantly influenced moss and soil N. The core sites had higher concentrations of both soil and moss N than the sites to the east and west (Table 2, Fig. 2b and c), which drove much of the pattern in the regression. However, core sites contained less moss cover (F2,147 = 3.32, P = 0.039, Fig. 4). Sampling region did not influence moss %P or soil P (Table 2; Fig. 2b and c). MAP also varied across the three regions (Table 1), but MAP was not significantly correlated with percent cover or moss N and P content (Appendix 1).
PCA revealed that moss from the different regions also differed in their micronutrient content (Fig. 5a). Specifically, moss from the western sites contained higher percentages of most micronutrients (Ca, Cu, Fe, Mg, Na, and Zn) than the core and eastern sites. The exceptions to this were the moss from the White Tank Mountains (WTM) that were much lower in micronutrient content than the other western moss and grouped with the core and eastern sites, which did not differentiate in their micronutrient content. Unlike with N and P, the variations in moss micronutrient content did not reflect differences in soil content. The PCA of soil micronutrients showed that western sites tended to differ from the other two sites, but western sites were not greater in soil micronutrient content than core and eastern sites (Fig. 5b). In fact, K is the only micronutrient in moss that was significantly related to soil content, and the regression revealed that this is very weak (Table 2; R2 = 0.0084, slope 0.0006, not shown). Soil micronutrients often differed significantly among regions (Table 2), but the patterns varied (Appendix 2).
Discussion
Our prediction that moss nutrient content would reflect differences in soil content was supported for N and P. Moss uptake of both macronutrients appears plastic, in that it takes up more N and P when soil resources are available. Such plasticity has been found in other studies as well (reviewed by Bates 2000; Waite and Sack 2011). Notably, soil P did not vary as much as soil N, so the relationship with moss P identified in the regression is not as strong (i.e., the slope is much lower). It is possible that moss and soil N are correlated as a result of the moss leaching nutrients into the soil, rather than moss uptake of soil nutrients. However, soil nutrient content measured below the moss reflects pattrens measured in soils from the same sites not directly under moss (Hall et al. 2011), suggesting moss leachates are not driving these patterns.
The city core had greater soil N content than outside the city, likely associated with higher levels of N deposition from air pollution (Lohse et al. 2008). Given that moss N is positively correlated with the higher levels of soil N inside the city, it appears to be capable of biologically assimilating N deposited by air pollution. Because moss and other soil crust organisms are quickly activated by precipitation events, and can also take up nutrients from wet and dry deposition, they are more efficient at absorbing nutrients than higher plants (Bates 2000). This may explain why these moss-dominated crusts are more effective at taking up added N than the vascular plants at these sites, which have been shown to be limited in their abilities to take up excess N and P (Hall et al. 2011). However, moss cover along the edges of washes is relatively low, limiting its ability to act as a net sink for that excess N. Particularly, moss is least abundant in the riparian areas of the city core where soil N is higher. This could be due to the fact that moss is sensitive to human disturbance and pollution, including N deposition itself (Song et al. 2012). For example, N deposition can increase tissue N but decrease growth in moss in Europe (Pitcairn et al. 1995; Mitchell et al. 2004). Notably, the washes along which moss-dominated soil crusts grow drain a much larger area, with potentially high levels of N in runoff during precipitation events (Gallo et al. 2013; Hale et al. 2014) that can become concentrated in the tissue of riparian moss-dominated crusts. In this way, moss growing on the terrestrial-aquatic interface may connect soil and stream nutrient cycling, where nutrients in storm runoff are assimilated by riparian moss, which later becomes an organic-matter source for the soil. Such input constitutes a potentially important resource in these carbon-limited soils with limited vascular plant production (Lange 2001). Therefore, their role as an integrator may be larger than their abundance would suggest, but future work would need to estimate their role at this scale.
To understand moss’s overall biogeochemical significance, we also investigated micronutrient biogeochemistry in moss. Interestingly, moss from the western areas was higher in micronutrients than the other areas. The only exception was the western site that is not isolated from the city by mountains (WTM). Notably, micronutrient content in the moss does not reflect that of the soil, as is the case with N and P. While moss from western sites was higher in most micronutrients, soil micronutrient content was often highest in the core or east, suggesting that soil is not the main supplier of micronutrient resources for moss at these sites. Soil is only one potential nutrient source for moss, in addition to wet or dry deposition (Bates 2000), and it is possible that the higher micronutrient content in the moss from west of the city core is the result of elevated amounts in precipitation or dry deposition. However, most of these cations are not an abundant atmospheric component, though they may be present in aeolian dust that lands on soil biological crusts (Reynolds et al. 2006; Beraldi-Campesi et al. 2009). It is possible that the western sites receive a different composition of dust, given their geographic position, to influence moss micronutrient uptake without influencing bulk soil concentrations. Though a western site, WTM is not separated from the core and eastern sites by mountains, which is why it may resemble these sites in cation content rather than the other western sites. Alternatively, rehydration cycles cause moss to leak cations (Brown and Buck 1979; Coxson 1991), and it is possible that moss from the drier western sites have experienced fewer such rehydration events to retain more of these elements. WTM is comparable to the other western sites in MAP over the 3 years of sample collection, but longer-term MAP values suggest WTM receives precipitation amounts comparable to core sites (Hall et al. 2011). Notably, the lack of relationship between soil and moss micronutrient content provides further evidence that moss nutrient leachate is not driving soil nutrient content.
Probably due to the high pH of these soils, soil base cation content (e.g., Ca, K, Mg, Na) is not lower in the core sites where nitrogen deposition is greater, suggesting that base cation depletion is not a consequence of N pollution in this urban area of the Sonoran Desert as it is for other areas subject to N deposition (Matson et al. 2002; Horswill et al. 2008). In fact, core soil is higher in some of these cations. Further, there is no evidence of heavy metal pollution depositing excess amounts of certain micronutrients (Cu, Fe, Mn, Zn) in soils inside the city core compared to outside, as most of these trace elements do not differ across the regions. Therefore, micronutrient loss or addition are not impacted by urban pollution in this region, suggesting that moss-dominated crusts will have the largest role in N dynamics.
The sites we sampled were along a precipitation gradient (increasing from west to east). The western and eastern regions receive ambient levels of N deposition with different levels of precipitation, but there are no significant differences identified for moss or soil N and P between the two sites. The slightly more frequent and larger precipitation events east of the city do not influence moss abundance or its uptake of N or P. This is contrary to many studies that demonstrate that precipitation size and timing influences moss crust biomass and mortality (Coe et al. 2012; Reed et al. 2012; Zelikova et al. 2012), and the associated N dynamics in which they play a role (Reed et al. 2012; Hu et al. 2014). Future climate projections for the southwest are to receive less frequent but more intense precipitation pulses (Seager et al. 2007; Solomon et al. 2007), and the impact of these on moss nutrient dynamics are not predictable from our results.
Overall, moss is able to take up more N where it is more abundant in the urban core soils, but percent cover is lower, which limits its ability to act as a net sink for excess N from air pollution. Given that moss cover in the city core is approximately 50 % lower than the rural areas, the core moss would need to be able to take up enough excess N to compensate for its lower abundance. However, while city core soils contain 2-4× greater soil N than the rural soils, the core moss only contains about 1.2× more N. Therefore, moss have the potential to assimilate nutrient pollution, but the negative impacts on percent cover prevent them from acting as a net sink. Without preservation of moss-dominated soil crusts in urbanized areas to maintain a commensurate biomass, moss will not serve as a significant sink for excess N. Many arguments have been made in favor of preserving fragile soil biocrusts (Belnap and Eldridge 2001; Evans et al. 2001), and we add to that the potential for moss to assimilate and store excess nutrients from pollution.
References
Ball B, Virginia R (2014) The ecological role of moss in a polar desert: implications for aboveground-belowground and terrestrial-aquatic linkages. Polar Biol 37:651–664
Bates JW (2000) Mineral nutrition, substratum ecology, and pollution. In: Shaw AJ, Goffinet B (eds) Bryophyte Biology. Cambridge University Press, New York, pp 225–247
Belnap J, Eldridge D (2001) Disturbance and recovery of biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer Berlin Heidelberg, New York, pp 363–383
Beraldi-Campesi H, Hartnett HE, Anbar A, Gordon GW, Garcia-Pichel F (2009) Effect of biological soil crusts on soil elemental concentrations: implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 7:348–359
Brown DH, Buck GW (1979) Desiccation effects and cation distribution in bryophytes. New Phytol 82:115–125
Cable J, Huxman T (2004) Precipitation pulse size effects on Sonoran Desert soil microbial crusts. Oecologia 141:317–324
Coe KK, Belnap J, Sparks JP (2012) Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology 93:1626–1636
Cook E (2014) Atmospheric nitrogen deposition in a large semi-arid city is lower than expected: Findings from a methods comparison. In: Direct and indirect ecological consequences of human activities in urban and native ecosystems. Dissertation, Arizona State University
Coxson DS (1991) Nutrient release from epiphytic bryophytes in tropical montane rain forest (Guadeloupe). Can J Bot 69:2122–2129
Elbert W, Weber B, Burrows S, Steinkamp J, Budel B, Andreae MO, Poschl U (2012) Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462
Evans RD, Belnap J, Garcia-Pichel F, Phillips SL (2001) Global change and the future of biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer Berlin Heidelberg, New York, pp 417–426
FCDMC (2009) Rainfall information. p. http://www.fcd.maricopa.gov/Rainfall/Raininfo/raininfo.aspx. The Flood Control District of Maricopa County, Phoenix, AZ, USA.
Gallo EL, Brooks PD, Lohse KA, McLain JET (2013) Temporal patterns and controls on runoff magnitude and solution chemistry of urban catchments in the semiarid southwestern United States. Hydrol Process 27:995–1010
Gavazov KS, Soudzilovskaia NA, van Logtestijn RSP, Braster M, Cornelissen JHC (2010) Isotopic analysis of cyanobacterial nitrogen fixation associated with subarctic lichen and bryophyte species. Plant Soil 333:507–517
Gerdol R, Bragazza L, Marchesini R, Alber R, Bonetti L, Lorenzoni G, Achilli M, Buffoni A, De Marco N, Franchi M, Pison S, Giaquinta S, Palmieri F, Spezzano P (2000) Monitoring of heavy metal deposition in Northern Italy by moss analysis. Environ Pollut 108:201–208
Hale RL, Turnbull L, Earl S, Grimm N, Riha K, Michalski G, Lohse KA, Childers D (2014) Sources and transport of nitrogen in arid urban watersheds. Envir Sci Tech 48:6211–6219
Hall SJ, Sponseller RA, Grimm NB, Huber D, Kaye JP, Clark C, Collins SL (2011) Ecosystem response to nutrient enrichment across an urban airshed in the Sonoran Desert. Ecol Appl 21:640–660
Horswill P, O’Sullivan O, Phoenix GK, Lee JA, Leake JR (2008) Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environ Pollut 155:336–349
Hu R, Wang X-p, Pan Y-x, Zhang Y-f, Zhang H (2014) The response mechanisms of soil N mineralization under biological soil crusts to temperature and moisture in temperate desert regions. Eur J Soil Biol 62:66–73
Lange OL (2001) Photosynthesis of soil-crust biota as dependent on environmental factors. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer Berlin Heidelberg, New York, pp 217–240
Lindo Z, Gonzalez A (2010) The bryosphere: an integral and influential component of the earth’s biosphere. Ecosystems 13:612–627
Lohse KA, Hope D, Sponseller R, Allen JO, Grimm NB (2008) Atmospheric deposition of carbon and nutrients across an arid metropolitan area. Sci Total Environ 402:95–105
Matson P, Lohse KA, Hall SJ (2002) The globalization of nitrogen deposition: consequences for terrestrial ecosystems. AMBIO 31:113–119
Mitchell RJ, Sutton MA, Truscott AM, Leith ID, Cape JN, Pitcairn CER, Van Dijk N (2004) Growth and tissue nitrogen of epiphytic atlantic bryophytes: effects of increased and decreased atmospheric N deposition. Funct Ecol 18:322–329
Phuyal M, Artz RRE, Sheppard L, Leith ID, Johnson D (2008) Long-term nitrogen deposition increases phosphorus limitation of bryophytes in an ombrotrophic bog. Plant Ecol 196:111–121
Pitcairn CER, Fowler D, Grace J (1995) Deposition of fixed atmospheric nitrogen and foliar nitrogen content of bryophytes and Calluna vulgaris (L.) Hull. Environ Pollut 88:193–205
Proctor MCF, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD (2007) Desiccation-tolerance in bryophytes: a review. Bryologist 110:595–621
Reed SC, Coe KK, Sparks JP, Housman DC, Zelikova TJ, Belnap J (2012) Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nat Clim Chang 2:752–755
Reynolds RL, Reheis M, Yount J, Lamothe P (2006) Composition of aeolian dust in natural traps on isolated surfaces of the central Mojave Desert-insights to mixing, sources, and nutrient inputs. J Arid Environ 66:42–61
Rosentreter R, Eldridge DJ, Kaltenecker JH (2001) Monitoring and management of biological soil crusts. In: Belnap J, Lange OL (eds) Biological soil crusts: structure, function, and management. Springer Berlin Heidelberg, New York, pp 457–468
Rosentreter R, Bowker M, Belnap J (2007) A Field Guide to Biological Soil Crusts of Westner U.S. Drylands: Common Lichens and Bryophytes. U.S. Government Printing Office, Denver, CO
Salemaa M, Derome J, Helmisaari H-S, Nieminen T, Vanha-Majamaa I (2004) Element accumulation in boreal bryophytes, lichens and vascular plants exposed to heavy metal and sulfur deposition in Finland. Sci Total Environ 324:141–160
Schröder W, Holy M, Pesch R, Harmens H, Fagerli H, Alber R, Coşkun M, De Temmerman L, Frolova M, González-Miqueo L, Jeran Z, Kubin E, Leblond S, Liiv S, Maňkovská B, Piispanen J, Santamaría JM, Simonèiè P, Suchara I, Yurukova L, Thöni L, Zechmeister HG (2010) First Europe-wide correlation analysis identifying factors best explaining the total nitrogen concentration in mosses. Atmos Environ 44:3485–3491
Seager R, Ting M, Held I, Kushnir Y, Lu J, Vecchi G, Huang H-P, Harnik N, Leetmaa A, Lau N-C, Li C, Velez J, Naik N (2007) Model projections of an imminent transition to a more arid climate in southwestern North America. Science 316:1181–1184
Solga A (2007) Seasonal variation in the nitrogen concentration and 15 N natural abundance of a pleurocarpous moss species in dependence on nitrogen deposition dynamics. Cryptogam Bryol 28:93–102
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Regional climate projections. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, 2007 Cambridge University Press, New York, NY,
Song L, Liu W-Y, Ma W-Z, Qi J-H (2012) Response of epiphytic bryophytes to simulated N deposition in a subtropical montane cloud forest in southwestern China. Oecologia 170:847–856
Stark LR, McLetchie DN, Roberts SP (2009) Gender differences and a new adult eukaryotic record for upper thermal tolerance in the desert moss Syntrichia caninervis. J Therm Biol 34:131–137
Stark LR, Brinda JC, McLetchie DN (2011) Effects of increased summer precipitation and N deposition on Mojave Desert populations of the biological crust moss Syntrichia caninervis. J Arid Environ 75:457–463
Waite M, Sack L (2011) Does global stoichiometric theory apply to bryophytes? Tests across an elevation × soil age ecosystem matrix on Mauna Loa, Hawaii. J Ecol 99:122–134
Zelikova T, Housman D, Grote E, Neher D, Belnap J (2012) Warming and increased precipitation frequency on the Colorado Plateau: implications for biological soil crusts and soil processes. Plant Soil 355:265–282
Zschau T, Getty S, Gries C, Ameron Y, Zambrano A, Nash Iii TH (2003) Historical and current atmospheric deposition to the epilithic lichen Xanthoparmelia in Maricopa county, Arizona. Environ Pollut 125:21–30
Acknowledgments
This research was supported by National Science Foundation Division of Environmental Biology grants to the CAP-LTER (CAP3: BCS-1026865), including the CAP-LTER REU program, as well as by the New College of Interdisciplinary Arts & Sciences at Arizona State University. We thank students Jen Bagby, Melanie Kepler, Chelsey Tellez, Jack Ramsey, and Amber Deines for their help in the field and lab. Cathy Kochert, Roy Erickson, and Sara Ryan at the Goldwater Environmental Lab at ASU provided analytical services. Quincy Stewart and Stevan Earl provided logistical support. We thank Sharon Hall for her input on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Zucong Cai.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Ball, B.A., Alvarez Guevara, J. The nutrient plasticity of moss-dominated crust in the urbanized Sonoran Desert. Plant Soil 389, 225–235 (2015). https://doi.org/10.1007/s11104-014-2355-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2355-7