Abstract
Background and aims
Litter decomposition is a critical process in terrestrial ecosystems and, since in natural conditions plant litter occurs in mixtures, understanding the interactive effects of mixed litter is of great ecological relevance. In this context, we test the hypothesis that N transfer between high quality litter to N-poor substrates are at the base of synergistic interactions, positively affecting litter decay rate, temperature sensitivity, and changes of organic C quality.
Methods
We carried out a manipulative experiment using four organic substrates, encompassing a wide range of biochemical quality (Hedera helix and Quercus ilex leaf litter, cellulose strips and woody sticks), each decomposing either separately or in matched pair mixtures for 360 days. Organic substrates were characterized for mass loss, C and N content and by 13C CPMAS NMR to assess biochemical quality changes.
Results
Litter response to mixing was related to the biochemical quality of the components in the mixture: additive when substrates with similarly high (H. helix and Q. ilex) or low (cellulose and wood) N content were paired, but synergistic when substrates with contrasting N content were associated (either of the two leaf litters with either cellulose or wood). Overall, no antagonist effects were observed in this experiment. Interestingly, decomposition of cellulose and wood showed an higher temperature sensitivity, compared to monospecific substrates, when paired with N rich materials. Significant N transfer was found from N rich litter to N poor substrates and 13C CPMAS NMR showed rapid changes of C quality of cellulose and wood sticks only when paired with N rich litter.
Conclusions
Our findings support the hypothesis that mixing litters of different quality, with quality expressed in terms of C/N ratio and N content, increases decomposition rate and temperature sensitivity of the lower quality substrates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litter decomposition is a critical process in terrestrial ecosystems, controlling carbon and nutrient cycling and, as a consequence, primary productivity. Decades of studies confirmed that temperature and water availability are the most important ecological factors affecting decomposition at global and regional levels (Aerts 1997), with biochemical litter quality becoming a relevant factor at a local scale (Bonanomi et al. 2013). Most early decomposition studies used leaf litter from single plant species. However, since in natural conditions plant litter generally occurs in mixtures, in the last decade an increasing research effort was devoted to clarify the decomposition dynamics in litter mixture (Hättenschwiler et al. 2005).
Litter mixtures can decompose as fast as the average of the single components thus showing additive behaviour. In other cases mass loss rate can deviate from the average of its separate components and such non additive effects may produce either synergistic (faster mass loss) or antagonistic (slower mass loss) interactions. Unfortunately, non-additive interactions of decomposing litter mixtures seems to occur idiosyncratically, being rarely predictable from single-species dynamics. Two reviews concerning mixed litter reported that synergistic effects are slightly more common compared to antagonistic and additive ones, both in terrestrial (Gartner and Cardon 2004) and in aquatic ecosystems (Lecerf et al. 2011), but the underlying causal factors are still unclear. Some authors suggest that non-additive interaction is expected to occur more frequently when mixtures include litter with dissimilar biochemical quality (Wardle et al. 1997). In this regards, however, available empirical evidence is contradictory (Hoorens et al. 2003; Wardle et al. 2006; Bonanomi et al. 2010). Interestingly, Chapman and Koch (2007) reported that litter mixtures of functionally similar conifer species decomposed faster than expected from rates of the individual species alone, whereas more functionally diverse mixtures did not show synergistic effects.
Several mechanisms can explain non-additive, synergistic interactions: i. improved microclimatic conditions since litter materials with higher water retention capacity, providing a water buffering effect, may enhance the decay of fast de-hydrating plant residues by favouring moisture persistence in the litter layer (Wardle et al. 2003); ii. creation, in litter mixture, of more structured habitats that may enhance microbial diversity and functionality (Hättenschwiler et al. 2005); iii. a net transfer of nutrients among different litter materials (Schimel and Hättenschwiler 2007). Nitrogen (N) can be transferred passively, through leaching and diffusion, or actively, by fungal mycelia networking (Lummer et al. 2012). In particular, N transfer becomes ecologically relevant during the early phases of litter decomposition, in conditions of C/N ratio of the decomposing substrate above a critical threshold of ~30–35, when microbial N starvation is commonly observed (Taylor et al. 1989). In such conditions, decomposer microbes could get extra N from external sources (e.g. the underlying soil) and transfer it into the N-poor substrate to met their nutritional requirements (Berglund and Ågren 2012). Accordingly, non-additive synergistic interactions are expected to occur when high quality litters (low C/N ratio and high N content) are mixed with N poor substrates, as a consequence of a net N transfer between the two materials. However, this simplistic model does not fully capture the complexity of N related effects on the decomposition process. For instance, a number of studies investigated the effects of N addition on litter decomposition rate, reporting contradictory evidence. In some cases a faster decomposition after N fertilization is observed (e.g. Cornwell et al. 2008), but examples of non significant or even negative responses are common (Berg and McClaugherty 2008). Moreover, a recent meta-analysis (Knorr et al. 2005) reported that, generally, N addition from external sources enhances mass loss of high quality litter, but reduces decay rate of low quality, lignin rich substrates. Such depressive effect has been related to different processes, including the inhibition of oxidative enzymes involved in lignin degradation (Carreiro et al. 2000), the formation of recalcitrant compounds as a result of the interactions between inorganic N and polyphenols (Berg and Matzner 1997), generalised reduced functionality of microbial communities when inorganic N is abundant (Ågren et al. 2001). The complex relationships between litter decomposition dynamics and N availability is furthermore affected by both the type of N source and the biochemical quality of decomposing litter. In a recent study, comparing the effects of substrate N and externally supplied organic and inorganic N on decomposition dynamics, Hobbie et al. (2012) reported contrasting effects. In particular, faster initial decomposition rates were dependent on the level of N availability, but not on its sources and forms. On the other hand, later in decomposition, externally supplied N had a prevalent slowing effect on litter mass loss, contrary to the substrate N.
In the context of litter mixtures decomposition experiments, only few studies have conclusively demonstrated a net N transfer between different starting materials (e.g. Schimel and Hättenschwiler 2007; Lummer et al. 2012). So far, the interactive effects of litter type, in terms of C biochemical quality, and N transfer dynamics have received even less attention. In this work we have carried out a manipulative, laboratory experiment using four organic substrates, encompassing a wide range of biochemical C quality and N content (Hedera helix and Quercus ilex leaf litter, cellulose strips and woody sticks), each decomposing either separately or in matched pair mixtures. We tested the hypothesis that a net N transfer from high quality substrates (leaf litter of H. helix and, to a lesser extent, Q. ilex) to N-poor materials promotes accelerated decomposition rates. A specific hypothesis states that the intensity of synergistic interactions is expected to increase with the difference in N content between the litters in mixture. In particular, a stronger synergistic effect is expected for mixtures containing cellulose strips (N poor and devoid of lignin) compared to woody sticks (N poor, but lignin rich) because, in the latter case, a potential negative interaction between N and lignin may occur (Berg and Matzner 1997; Carreiro et al. 2000; Knorr et al. 2005).
Previous litter mixture studies focused on the effects of mixing on litter mass loss and nutrient dynamics, or on the effects of environmental abiotic and biotic factors on mixture decomposition dynamics (review in Hättenschwiler et al. 2005). So far, no studies investigated if and how monospecific litter and litter mixtures differently affect litter C quality changes during decomposition. In this work, intended as a contribution to fill the gap, undecomposed as well as decomposed materials were characterized by 13C-cross-polarization magic angle spinning (CPMAS) nuclear magnetic resonance (NMR) spectroscopy obtained in solid state. Such method has been applied to characterize organic matter at molecular level and it is useful since it provides a description of the total organic chemical composition of complex matrices, such as plant litter (Kögel-Knabner 2002; Preston et al. 2009). In detail, we tested the idea that mixing N-poor substrates (i.e. cellulose strips and woody sticks) with N-rich litter (i.e. H. helix and Q. ilex leaves) enhances the general functionality of microbial decomposer community by relaxing N starvation. Under such hypothesis, more rapid changes of C quality are expected in cellulose strips and woody sticks when mixed with leaf litter, as compared to unmixed conditions.
Finally, the relationships between litter decomposition and temperature are fairly well established in the literature (e.g. Meentemeyer 1978; Hobbie 1996), with decomposition rates increasing with increased temperatures. However, temperature sensitivity of decomposing litter is a debated issue, with contrasting evidences about the relation between sensitivity and substrate recalcitrance (Hartley and Ineson 2008; Giardina and Ryan 2000). Temperature sensitivity of organic substrates can be reduced in presence of environmental constraints such as water and nutrient shortage, flooding, freezing, as well as organic matter physical and chemical protection (review in Davidson and Janssens 2006). Here, we test the hypothesis that the temperature sensitivity of decomposition of N-poor substrates, when mixed with N-rich litter, increases because the N shortage condition is relaxed. Organic matter decomposition in field conditions mainly depends on organic matter quality, water availability and temperature (Berg and McClaugherty 2008). In this study, to isolate the effects of temperature and litter quality, while minimizing the variability related to water availability, the decomposition experiment was carried out in microcosms under controlled conditions.
Based on the aforementioned considerations, four main hypotheses were tested: (1) in mixtures, N-rich substrates promote mass loss of N-poor materials; (2) a net N transfer occurs from N-rich to N-poor substrates; (3) temperature sensitivity of decomposition is enhanced when N-poor substrates are mixed with N-rich litter; and (4) C biochemical quality of N-poor substrates changes faster when mixed with N-rich litter.
Materials and methods
Plant material collection
Four organic substrate types were selected, showing very different values of C/N ratio, and percent N and lignin content, as follows (values are average ± standard deviations): i) Hedera helix leaf litter, a fast decomposing evergreen vine (N content = 2.02 ± 0.12 %; C/N ratio = 21 ± 2.11; lignin content = 5.7 ± 0.76 %); ii) Quercus ilex leaf litter, a slow decomposing evergreen tree (N content = 1.24 ± 0.21 %; C/N ratio = 31 ± 3.43; lignin content = 18.3 ± 2.91 %) (Bonanomi et al. 2013); iii) woody sticks cut from Q. ilex branch (N content = 0.10 ± 0.02 %; C/N ratio = 440 ± 32.31; lignin content = 34.6 ± 4.54 %); and iv) cellulose strips (N content = 0.09 ± 0.01 %; C/N ratio = 466 ± 21.43; lignin content = 0.00 %). Leaf litter materials were sampled from a natural old-growth holm oak forest located on the southern slope of Mt. Vesuvius (Portici 40° 48’ 43” N – 14° 20’ 49” E, Southern Italy). Freshly abscissed leaves of each species were collected from plants randomly selected at the sampling site (n° plants >20), air dried for 20 days until reaching constant weight and stored afterwards at room temperature.
Decomposition experiment
Microcosms consisted of 300 mL polycarbonate Magenta vessels (Sigma-Aldrich, Co. LLC.) with polypropylene aerated lid. All leaf litter discs, woody sticks and cellulose strips were cut by scissors in order to obtain pieces of the correct mass (100 mg). Every batch was filled with 2 pieces of each material (i.e. experimental units). The organic substrates were introduced into microcosms and twice autoclaved. A microbial inoculum was prepared by mixing 90 g of water with 10 g of soil taken from the top 10 cm layer at the fields where litter was collected. The inoculum was sprayed inside the microcosms in order to improve the start up of the decomposition process. Ten types of experimental units were prepared, each consisting of 2 pieces of either the same organic substrate (4 types) or one of all the possible pairs of different substrates (6 combinations with a loading ratio of 50:50, i.e. a single piece for each materials). In most of previous decomposition experiments mass loss and nutrient dynamics were assessed in lumped mixed substrates, thus obscuring the response of different litter components. Here, instead, we kept separated the mixture components, making possible to assess potential species-specific effects between the different substrate types. The two pieces in each experimental unit were placed at distance of ~10 mm and were never in direct contact. Such spatial arrangement without direct contact between the two pieces, prevented the passive nutrient transfer between different organic substrates (Supplementary Fig. S1). Environmental conditions included not limiting water availability, obtained by watering the experimental units every three days with sterile distilled water until organic substrates were fully soaked, but avoiding connection by moisture between the two pieces in substrate pairs during the experiment, thus preventing nutrient leaching. However, other mechanisms of nutrient transfer between the two substrates in each experimental unit would still be possible, as for example a microbial colonization of the free space in between, creating a bridge between different litter types.
The full experimental design included 10 treatments incubated at two temperatures (12 and 24 °C) and replicated ten times for each of five dates of retrieval after incubation, for a total of 1,000 experimental units. Substrates were retrieved after 10, 30, 90, 180 and 360 days of decomposition. Collected substrates were oven-dried (60 °C until constant weight was reached) and weighted afterwards to the nearest 0.001 g.
Litter chemical analyses
Undecomposed materials were characterized for total C and N content by flash combustion of microsamples (5 mg of litter) in an Elemental Analyser NA 1,500 (Carlo Erba Strumentazione, Milan, Italy). Proximate cellulose and lignin content were quantified as acid hydrolysable fraction and acid unhydrolysable materials, respectively (Gessner 2005). C and N content of materials decomposing in the experimental units were also assessed, separately for each piece of organic substrate, after 90, 180 and 360 days of decomposition. Due to budget limitations, analyses were limited to C and N, omitting other relevant nutrients (e.g. P, K, Ca, etc.).
In order to assess changes of biochemical quality in the organic substrates, undecomposed materials, as well as materials decomposed for 360 days, were characterized by 13C-CPMAS NMR (Kögel-Knabner 2002) obtained in solid state and under the same conditions, thus allowing a comparative analysis of the resulting spectra. The spectrometer used was a Bruker AV-300 equipped with a 4 mm wide-bore MAS probe (for further details see Bonanomi et al. 2013). Spectral regions and corresponding C types have been identified following Bonanomi et al. (2011): 0–45 p.p.m = alkyl C; 46–60 p.p.m. = methoxyl and N-alkyl C; 61–90 p.p.m. = O-alkyl C; 91–110 p.p.m. = di-O-alkyl C; 111–140 p.p.m. = H- and C- substituted aromatic C; 141–160 p.p.m. O-substituted aromatic C (phenolic and O-aryl C); 161–190 p.p.m. carboxyl C. The alkyl C/O-alkyl C ratio (0–45/61–110), considered a robust indicator of the degree of litter decomposition, was calculated following Almendros et al. (2000).
Data analysis
Generalized linear models (GLMs) were used to analyze the results of the decomposition experiments. For each type of organic substrate in the experimental units, main and second-order interactive effects of three experimental factors were tested, considering percent mass remaining, N percent content, and C/N ratio as dependent variables. The type of paired substrate, temperature of incubation, and decomposition time were considered as treatment factors.
Nitrogen transfer was indirectly assessed by quantifying N content in each piece of organic substrate in all the different mixture combinations. In detail, during decomposition, an increase of total N content in a single organic piece, with a corresponding decrease in the piece to which it was paired, was used as evidence of N transfer between the two pieces.
The temperature sensitivity of organic substrates was estimated by calculating Q 10 value (rate of change in the rate of mass loss given by a 10 °C change in temperature). Pair-wise differences at each level of treatment were statistically evaluated by post-hoc Duncan test. Significance was evaluated in all cases at p < 0.05.
Results
Litter mass loss and temperature sensitivity
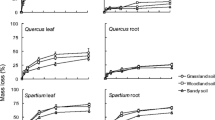
In microcosms with monospecific substrates, decomposition was very rapid for H. helix, intermediate for Q. ilex and very slow for woody sticks and cellulose strips (Fig. 1). Mass loss significantly increased with temperature for all materials (Table 1). Mass loss significantly increased with temperature for all materials, with Q 10 values (average ± standard deviation) of 1.18 ± 0.12, 1.35 ± 0.11, 1.07 ± 0.17, 1.10 ± 0.12 for H. helix, Q. ilex, woody sticks and cellulose strips, respectively.
Decomposition dynamics in experimental microcosms. Variations of mass remaining in cellulose, wood, and leaf litters of Quercus ilex and Hedera helix decomposing for 360 days at two temperatures, paired with four different organic substrates. Data refer to mean ± standard deviation of 10 replicates for each material
Additive mass loss effects were found for both litters of H. helix and Q. ilex, in all mixtures at both incubation temperatures (Fig. 1). In other words, leaf discs decomposed at their own rates, independent of the other substrate in the mixtures (Table 1). Additive effects were also found for mass loss of paired cellulose strips and woody sticks (Fig. 1). In contrast, significantly accelerated decay rates (i.e. synergistic interaction) were observed for both cellulose strips and woody sticks when paired with Q. ilex and H. helix leaf litter (Fig. 1). Overall, no antagonistic interaction was recorded. The magnitude of synergistic interactions was outstanding. In particular, after 360 days of incubation at 24 °C cellulose strips lost 7.1 % of initial mass when unmixed and 6.2 % when paired with woody sticks, but 72 and 65 % when paired with H. helix and Q. ilex, respectively. In the same conditions, woody sticks halved their mass when combined with Q. ilex and H. helix (mass loss equal to 45 and 50 %, respectively), while losing only 14 % when paired with cellulose strips, and 9 % when unmixed. Synergistic interactions occurred at both incubation temperatures but were enhanced at 24 °C. While pairing woody sticks with the two leaf litters did not produce significantly different mass loss dynamics, cellulose strips decomposed faster when combined with H. helix compared to Q. ilex (Fig. 1). Such different patterns were much more evident at 24 °C and over the first half of the decomposition period, and then converging towards similar values of mass remaining. In contrast, at lower temperature mass loss dynamics of cellulose strips paired with H. helix and Q. ilex progressively diverged throughout the observation period, producing significantly different values after 360 days (Fig. 1). Organic substrates showed different temperature sensitivity. Q 10 values of H. helix and Q. ilex leaves were not significantly affected by the paired organic materials (Table 2). In contrast, woody sticks and cellulose strips showed a significantly higher temperature sensitivity when paired with plant leaf litter, compared to other mixtures (Table 2).
N dynamics and transfer between organic substrates
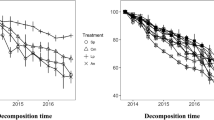
In monospecific microcosms N concentration significantly increased (Fig. 2) and C/N ratio decreased (Fig. 3) during the 360 days of decomposition for all organic substrates.
Nitrogen content variation in litter mixtures. Dynamics of N concentration in cellulose, wood, Quercus ilex and Hedera helix litter decomposing for 360 days at two temperatures, paired with four different organic substrates. Data refer to mean of 10 replicates for each material. Note different scales on Y axis. Standard deviation bars are not reported to improve readability. Descriptive statistics and testing for significant differences are in Supplementary Table S1
C/N ratio dynamics in litter mixtures. Changes of C/N ratio in cellulose, wood, Quercus ilex and Hedera helix leaf litter decomposing for 360 days at two temperatures, paired with four different organic substrates. Data refer to mean of 10 replicates for each material. Note different scales on Y axis. Standard deviation bars are not reported to improve readability. Descriptive statistics and testing for significant differences are in Supplementary Table S2
Concerning N content dynamics in the tested materials, H. helix leaf discs showed additive responses in all mixtures at both incubation temperatures (Fig. 2, Supplementary Table S1). In other words, for H. helix increases of tissue N concentration were independent of the other substrate in the microcosm. A similar pattern of additive interactions was found in the case of Q. ilex leaf discs, but with the important difference of a faster N content increase when paired with H. helix litter, compared to the other materials (Fig. 2, Supplementary Table S1). Cellulose strips showed additive interaction when paired with woody sticks, but remarkable synergistic interactions when combined with both leaf litter types (Fig. 2, Supplementary Table S1). The increase of N concentration in cellulose and wood when combined with N rich litters was evident by the rapid decrease of the C/N ratio during the whole experiment. In the case of cellulose paired with leaf discs, after 360 days of decomposition the C/N ratio decreased to values below or just above 100, while being more than double when the strips were unmixed, or paired with woody sticks (Fig. 3, Supplementary Table S2). A similar pattern was observed in the case of woody sticks, showing a C/N ratio as much as three times lower when paired with leaf discs, compared to sticks unmixed or paired with cellulose strips (Fig. 3, Supplementary Table S1).
Total N content after 360 days of decomposition was lower than the initial value for the two plant litter types, independent of the additional substrate in the mixture, indicating a net N loss (Fig. 4). N release, with values around 50 % of the initial N content, was more prominent for H. helix compared to Q. ilex (Fig. 4). Total N content of cellulose and wood was remarkably different in relation to the additional substrate in the mixture (Fig. 4). In fact, at both incubation temperatures, initial N amount remained almost unchanged when the cellulose and wood materials were unmixed or paired together. In contrast, the final N amount was much higher when such materials were paired with the two leaf litters, indicating a net N immobilization (Fig. 4). Our experiment was specifically planned to control two possible mechanisms of N transfer between different litter types, such as passive diffusion and leaching, but not to precisely assess other possible concurrent mechanisms. In this regard, it is noteworthy that bridges of fungal hyphae rapidly appeared (within 7 days) in the space in between two pieces of material with very different N content, (G.B., personal observation; Supplementary Fig. S2) but not in the pairs of substrates of the same type.
Nitrogen transfer in litter mixtures. Nitrogen content after 360 days of decomposition at 12° and 24 °C, expressed as percentage of initial value (=100 %, indicated by dashed lines) in wood, cellulose, and leaf litters of Hedera helix and Quercus ilex, paired with four organic substrates. Data refer to mean ± standard deviation, different letters indicate statistically significant differences within each organic matter type and incubation temperature (Duncan test; p < 0.05)
Changes of biochemical C quality in organic substrates
The four undecomposed substrates showed remarkably different 13C NMR signals. In detail, cellulose and wood spectra, compared to the two leaf litter types (Bonanomi et al. 2013) showed higher peaks at 61–90 p.p.m., and lower at 0–45 p.p.m., corresponding to O-alkyl C and alkyl C regions, respectively.
After 360 days of decomposition, all the tested materials showed a general trend of decreasing O-alkyl C, mainly associated to sugars and polysaccharides, and increasing alkyl C, related to lipid waxes, cutins, and microbial products as methoxyl and N-alkyl C related to lignin (46–60 p.p.m.). Signals in the, in the di-O-alkyl C (91–110 p.p.m.) and in the carboxyl C (161–190 p.p.m.) regions, as well as those in the two aromatic C regions (111–140 p.p.m. and 141–160 p.p.m.) did not change significantly. However, in the cases of H. helix and Q. ilex litters, such biochemical changes were unaffected by the type of additional substrate present in the mixture (data not shown), whereas for cellulose and wood the observed variations were highly mixture-dependent. In particular, the decrease of O-alkyl-C and the increase of alkyl-C peaks were limited when such materials were paired with N poor substrates, and more rapid in mixtures with N rich leaf litters (Fig. 5).
Variation of C biochemical quality during decomposition. a Relative abundance of seven main classes of organic C assessed by 13C-CPMAS NMR spectroscopy in cellulose strips and woody sticks either undecomposed or after 360 days of decomposition at 24 °C, paired with four different organic substrates. b Spectra of cellulose strips and woody sticks undecomposed or after 360 days of decomposition paired with four different organic substrates
Accordingly, the indicator of stability calculated as the ratio alkyl-C/O-alkyl-C increased for all materials after 360 days of decomposition. Again, in the cases of cellulose and wood, but not for H. helix and Q. ilex, the rate of increase of the indicator was highly affected by the pair composition in the experimental units. In detail, cellulose and wood showed very low initial values of the indicator (0.013 and 0.021, respectively), and a relatively slow increase when the materials were either unmixed (final values of the indicator equal to 0.024 and 0.104) or paired together (0.042 and 0.043). Conversely, the alkyl C/O-alkyl C ratio increased more rapidly in the combinations cellulose-Q. ilex litter (0.057) and cellulose-H. helix litter (0.093), being even faster for wood paired with Q. ilex (0.123) and H. helix (0.216) litters.
Discussion
Our experiment, based on four organic substrates representing a broad range of biochemical quality, demonstrated that the decomposition of mixed residues is affected by additive as well as synergistic, non-additive responses. We showed that mass loss, N dynamics, sensitivity to temperature and changes in C biochemical quality are regulated by the N content of the mixture components. Specifically, additive effects on mass loss and N dynamics were observed when substrates with similarly high (H. helix and Q. ilex) or low (cellulose and wood) N content were paired. In contrast, synergistic interactions were found when substrates with contrasting N content were associated (either of the two leaf litters with either cellulose or wood). Moreover, decomposition of N poor substrates (cellulose and wood) paired with N rich materials showed a higher temperature sensitivity and faster changes of C quality, compared to unpaired substrates, likely as a result of N shortage relaxation. On the other hand, temperature sensitivity and C quality changes of N rich litter (H. helix and Q. ilex) were unaffected by mixing. Overall, no antagonist effects were observed in this experiment. Our findings support the hypothesis that the occurrence in mixtures of high quality litters, with low C/N ratio and high N content, promote mass loss and organic C changes of other, poor quality materials, through N transfer.
Three literature reviews focused on this issue, reporting that litter mixing had mostly non-additive effects on mass loss, with a prevalence of synergistic over antagonistic responses (Gartner and Cardon 2004; Hättenschwiler et al. 2005; Lecerf et al. 2011). In our experiment, non-additive synergistic effects were the rule when mixtures were composed of substrates with contrasting N content. Synergistic interactions among decomposing materials have been often attributed to a net transfer of nutrients from a source litter to a target, usually nutrient-poor substrate (Chapman et al. 1988). Our study, indeed, provides a direct support to such model. In fact, we found an increase in N content in all cases of N-poor materials whenever they were paired with N-rich litters, coupled with a decrease of N content in the latter. This is consistent with a net transfer of N from plant litter to wood and cellulose, which explains the consequent increase of the mass loss rate of the target materials.
Accordingly, the observed pattern of decreasing C/N ratio for all tested materials fits well with the N transfer model. In fact, decaying organic matter releases carbon at a faster rate than N, so that C/N ratio is expected to progressively decrease until a threshold value is attained. Here, C/N ratio of cellulose and wood decreased faster in presence of N-rich litters, likely as a result of N transfer from such sources. N transfer among decomposing litters in mixture has been previously reported (e.g. Schwendener et al. 2005; Schimel and Hättenschwiler 2007). However, the resulting mass loss of mixed litters showed different responses. For instance, Lummer et al. (2012) reported a remarkable transfer of N between litters of the nutrient poor beech (Fagus sylvatica) and the nutrient rich ash (Fraxinus excelsior), but the resulting mass loss was additive. In other cases, mixing N poor with N rich litters resulted in non-additive antagonistic interactions (e.g. Bonanomi et al. 2010; Tan et al. 2013). Nevertheless, it is important to keep in mind that our experimental setup was different from the previous studies, which were carried out with litter-bags placed in direct physical contact with the soil. In this way, alternative sources of N might have been provided, such as N release by soil microbes. Previous results contrast with the outstanding non-additive, synergistic interactions observed in this study at both incubation temperatures. However, such contrasting results are not unexpected, and could be partially attributed to the broader range of N content in the materials used in this experiment, varying from 2.02 % of H. helix to <0.1 % of cellulose and wood, compared to previous studies. Interestingly, we found that the mass loss of the putative N source litters (both H. helix and Q. ilex) remained unaffected by the mixture composition. Such evidence does not support the hypothesis by Berglund and Ågren (2012), suggesting that in two-litter mixtures one litter can decompose faster while the other component can show a slower decomposition compared to unmixed samples. On the other hand, as predicted by the Berglund and Ågren (2012) model, the observed positive effect of N sources was ranked according to their N mineralization rates, being highest for H. helix litter, intermediate for Q. ilex, and null for cellulose and wood that only immobilized N. However, evidence available from the literature is not conclusive. Recently, comparing different pathways of N transfer between two litter species of contrasting N status, Lummer et al. (2012) reported that the transfer was larger for the N poor beech than for the N rich ash, and interpreted such surprising result with the selective development of fungal (more able to transfer N) and bacterial (less able to transfer N) dominated microbial communities over decomposing litter of beech and ash, respectively.
The different responses of litter mass loss recorded in this study can be related also to the C quality of the substrates and, with more detail, to their initial lignin content. External supply of inorganic N, as well as high litter N content, can inhibit mass loss as a result of reduced lignin degradation (Berg and McClaugherty 2008). In fact, it is known that N has a dual, opposite effects on litter decomposition, promoting litter mass loss during the early stages (up to 30–40 % of mass loss) and limiting it thereafter (Berg and Matzner 1997; Bonanomi et al. 2013). In the early decay phases, high N availability may sustain microbial activity to rapidly consume labile C compounds, resulting in a rapid mass loss rate. In contrast, high N concentration in the later stages inhibits mass loss by favoring the formation of recalcitrant chemical complexes with lignin (Hatakka 2001), by inhibiting oxidative enzymes involved in lignin degradation, or reducing the multiple functionality of litter decomposer microbial communities (Hobbie et al. 2012). In our study, N transfer from high quality litter stimulated mass loss of both lignin devoid (cellulose) and lignin rich (wood) substrates throughout the decomposition period (360 days). Such evidence apparently contradicts the previous studies, as well as the findings of a meta-analysis by Knorr et al. (2005) in which, on average, external addition of inorganic N forms reduced the decay rate of low quality, lignin rich substrates. In previous studies it has been showed that the inhibitory effect of N is most prominent at the latest stages of decomposition, usually when it lasts for more than 24 months (Knorr et al. 2005). Here, instead, the very low initial N content of cellulose strips and woody sticks, coupled with the absence of other N sources (i.e. no soil was included in microcosms) likely produced an extreme microbial N starvation, that eventually might have been relaxed once external N was provided. The transfer of N from leaf litter, especially of H. helix, to woody sticks, and the consequent enhancing effect on mass loss rate, bear importance for the decomposition of woody debris in natural woodlands, with potential consequences at ecosystem level. The occurrence of N rich litter (e.g. the vine H. helix which is widespread in many woodland systems) coupled with their rapid N mineralization rate can provide a consistent nutrient flux that may sustain wood decomposition. Previous studies on decomposition of wood debris focused on the role of climatic conditions and substrate quality in terms of wood density, nutrient content and lignin quality (for a review see Weedon et al. 2008). Our results suggest that the variability of N availability at local scale, which is largely related to soil quality and litterfall patterns, might greatly affect wood debris decomposition. Further studies are needed to address the consistency and relevance of N transfer from different litter types to woody debris under field conditions. Our observations of a fungal bridge, connecting spatially separated litter pieces with very different N content, but not in other types of mixtures, suggests that N transfer may be mediated by fungal activity, at least in the tested experimental conditions. However, the occurrence of other mechanisms underlying N transfer, and whether they are active in nature, should be further investigated.
In this study, we observed the strongest synergistic interactions when mixed N-and N-poor substrates were decomposing under warmer conditions. This finding may be related to the fact that microbial, as well as enzymatic activities, are generally faster at higher temperatures, especially under no other ecological limitations. Temperature sensitivity of decomposing litter is a debated issue, with theoretical and empirical evidence suggesting an increasing sensitivity with substrate recalcitrance (Davidson and Janssens 2006; Hartley and Ineson 2008), although opposing evidence are available (e.g. Giardina and Ryan 2000). In our experiment, when decomposed alone, cellulose strips and woody stick showed a limited temperature sensitivity compared with H. helix and Q. ilex leaf litter. This result can likely be explained by the very limited mass loss achieved by cellulose and wood when decomposed alone as a result of the drastic N limitation. In contrast, decomposition rates of both woody sticks and cellulose strips, when mixed with N rich substrates, showed a sharp increase in temperature sensitivity. These results indicate that temperature sensitivity of N poor and/or recalcitrant substrates increases as the environmental conditions affecting litter decomposition (e.g. N availability) improve. Finally, temperature sensitivity of the two leaf litters were unaffected by the type of paired organic materials. In our knowledge, this is the first study that report a significant effect of litter mixture on decomposition temperature sensitivity. This results is consistent with the hypothesis that temperature sensitivity of organic substrate can be reduced in presence of environmental constraints such as water and nutrient shortage, flooding, freezing, as well as organic matter physical and chemical protection (Davidson and Janssens 2006).
Concerning C quality, here, for the first time, we described biochemical changes of C types in different litter mixtures using 13C NMR spectroscopy. The most interesting results are the very small spectral changes showed by both cellulose and wood when decomposed either unmixed or in mixture with other N poor materials, and the increase of such spectral variations when occurring in mixtures with H. helix and Q. ilex. The observed reduction of carbohydrates (O-alkyl C), the increase of alkyl C and the consequent small, but consistent increase of the alkyl C/O-alkyl C ratio indicate a progressive increase of organic matter stability (Almendros et al. 2000). Such rapid changes of C quality of cellulose and wood only in presence of N rich litter could be due to a more active microbial community, able to maximize the consumption of the carbohydrates fraction and allowing, even as a result of microbial by products and spoilage build up, a progressive accumulation of aliphatic compounds (Kögel-Knabner, 2002). Future studies in this direction can unravel if litter mixing has relevant implications for changes of litter C quality and, then, for ecosystem C storage.
Conclusions
Our results provided evidence that litter quality, in particular N content, is the most important factor affecting mass loss and nutrient dynamics in mixture, consistently with previous studies (Wardle et al. 2006; Lecerf et al. 2007). In the context of the debated effects of N on mixed litter decomposition (Hobbie et al. 2012) the finding of synergistic interactions between N poor and N rich substrates, as a result of N transfer and irrespective of the substrate lignin content, provides a new contribution. Future studies can explore the implications of our finding in the context of ecosystem functioning related to plant invasion, since it is well recognized that invasion often increases N pools and accelerates ecosystem N fluxes (Castro-Díez et al. 2014). Previous evidence showed that litter mixing effects are apparently idiosyncratic, both in terms of litter decay rate and nutrient release. The present study indicates that, at least for materials with contrasting N content, additive and non-additive interactions are predictable based on initial N content and C/N ratio. Our study did not identify the mechanisms underlying N transfer, although the formation of fungal bridge connecting N rich to N poor substrates suggests that fungi may play an important role in this process. The variability of N effects on litter mass loss as well as the different temperature sensitivity of litter in mixtures indicate the utility of further studies on this issue, particularly when based on mixture component assessment.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Ågren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98
Almendros G, Dorado J, González-Vila FJ, Blanco MJ, Lankes U (2000) 13C NMR assessment of decomposition patterns during composting of forest shrub biomass. Soil Biol Biochem 32:793–804
Berg B, Matzner E (1997) The effect of N deposition on the mineralization of C from plant litter and humus. Environ Rev 5:1–25
Berg B, McClaugherty C (2008) Plant litter: decomposition, humus formation and carbon sequestration, 2nd edn. Springer, Berlin
Berglund SL, Ågren GI (2012) When will litter mixtures decompose faster or slower than individual litters? A model for two litters. Oikos 121:1112–1120
Bonanomi G, Incerti G, Antignani V, Capodilupo M, Mazzoleni S (2010) Decomposition and nutrient dynamics in mixed litter of mediterranean species. Plant Soil 331:481–496
Bonanomi G, Incerti G, Barile E, Capodilupo M, Antignani V, Mingo A, Lanzotti V, Mazzoleni S (2011) Phytotoxicity, not nitrogen immobilization, explains plant litter inhibitory effects: evidence from solid-state 13C NMR spectroscopy. New Phytol 191:1018–1030
Bonanomi G, Incerti G, Giannino F, Mingo A, Lanzotti V, Mazzoleni S (2013) Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and Lignin/N ratios. Soil Biol Biochem 56:40–48
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Castro-Díez P, Godoy O, Alonso A, Gallardo A, Saldaña A (2014) What explains variation in the impacts of exotic plant invasions on the nitrogen cycle? A meta-analysis. Ecol Lett 17:1–12
Chapman SK, Koch GW (2007) What type of diversity yields synergy during mixed litter decomposition in a natural forest ecosystem? Plant Soil 299:153–162
Chapman K, Whittaker JB, Heal OW (1988) Metabolic and faunal activity in litters of tree mixtures compared with pure stands. Agric Ecosyst Environ 24:33–40
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climatic change. Nature 440:165–173
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gessner MO (2005) Proximate lignin and cellulose. In: Graca MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition. A Practical Guide. Springer Verlag, The Netherlands, pp 115–120
Giardina CP, Ryan M (2000) Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404:858–861
Hartley IP, Ineson P (2008) Substrate quality and the temperature sensitivity of soil organic matter decomposition. Soil Biol Biochem 40:1567–1574
Hatakka A (2001) Biodegradation of lignin. In: Hofman M, Stein A (eds) Biopolymers, vol 1, Lignin, humic substances and coal. Wiley, Weinheim, pp 129–180
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Ann Rev Ecol Syst 36:191–218
Hobbie SE (1996) Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–522
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P (2012) Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137:578–586
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162
Lecerf A, Risnoveanu G, Popescu C, Gessner MO, Chauvet E (2007) Decomposition of diverse litter mixtures in streams. Ecology 88:219–227
Lecerf A, Marie G, Kominoski JS, LeRoy CJ, Bernadet C, Swan CM (2011) Incubation time, functional litter diversity, and habitat characteristics predict litter-mixing effects on decomposition. Ecology 92:160–169
Lummer D, Scheu S, Butenschoen O (2012) Connecting litter quality, microbial community and nitrogen transfer mechanisms in decomposing litter mixtures. Oikos 121:1649–1655
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472
Preston CM, Nault JR, Trofymow JA (2009) Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of “lignin”. Ecosystems 12:1078–1102
Schimel JP, Hättenschwiler S (2007) Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39:1428–1436
Schwendener CM, Lehmann J, de Camargo PB, Luizão RC, Fernandes E (2005) Nitrogen transfer between high-and low-quality leaves on a nutrient-poor Oxisol determined by 15 N enrichment. Soil Biol Biochem 37:787–794
Tan Y, Chen J, Yan L, Huang J, Wang L, Chen S (2013) Mass loss and nutrient dynamics during litter decomposition under three mixing treatments in a typical steppe in inner Mongolia. Plant Soil 366:107–118
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Wardle DA, Nilsson MC, Zackrisson O, Gallet C (2003) Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35:827–835
Wardle DA, Yeates GW, Barker GM, Bonner KI (2006) The influence of plant litter diversity on decomposer abundance and diversity. Soil Biol Biochem 38:1052–1062
Weedon JT, Cornwell WK, Cornelissen JH, Zanne AE, Wirth C, Coomes DA (2008) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12:45–56
Acknowledgments
The 13C-CPMAS NMR measurements were performed at the CERMANU-Interdepartmental Research Centre, University of Napoli Federico II. The work has been supported with grants by MIUR (PRIN 2005–050197 and FISR MESCOSAGR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juha Mikola..
Rights and permissions
About this article
Cite this article
Bonanomi, G., Capodilupo, M., Incerti, G. et al. Nitrogen transfer in litter mixture enhances decomposition rate, temperature sensitivity, and C quality changes. Plant Soil 381, 307–321 (2014). https://doi.org/10.1007/s11104-014-2119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-014-2119-4