Abstract
Aims
The aim of this study was to determine whether goat grazing in the understory of a pine forest at Doñana Natural Park could accelerate the decomposition of the pine needles accumulated on the soil surface and, if so, through which mechanisms. Specifically, the roles of trampling (mechanical fragmentation) and nutrient enrichment through defecation (fertilization) were evaluated in terms of their effect on pine needle decomposition rates.
Methods
An experiment was conducted featuring the following 4 treatments: 1) intact needles (control), 2) trampled needles, 3) intact needles fertilized with liquid manure, and 4) trampled needles fertilized with liquid manure. Litter decomposition was determined as a function of mass loss over time, using the litter-bag method. Bags were recovered 4, 8, 16, 24 and 36 months after burial in soil, dried and weighed. Needle length, leaf mass per area and C and N concentration were also measured in the buried litter-bags.
Results
Four months after burial, mass loss was greater in the trampled (23–27 %) than non-trampled (14–16 %) treatments. However, from 8 months onwards, decomposition rates in the fertilized treatments were significantly higher than those in the non-fertilized treatments (between 5 % and 15 % less mass loss). Meanwhile, fertilized treatments presented higher N content (2.1 %) than the non-fertilized ones (1.2 %), with a significantly lower C:N ratio also found in the in the fertilized treatment.
Conclusions
Trampling and fertilization during grazing accelerates litter decomposition and thus promotes the incorporation of N into the system. Acceleration of decomposition reduces the accumulation of pine needles on the soil surface, reducing the risk of fire.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effect of livestock grazing on environmental conservation has historically been perceived as detrimental to the environment, due to overgrazing (Mace 1991), desertification (Dregne and Willis 1983), methane emission (Goel et al. 2008) and associated biodiversity loss (Alados et al. 2003). However, grazing is not universally harmful and can actually contribute to the preservation of natural and cultural values and assets. Livestock activity has regained momentum in current forest plans; the incorporation of grazing livestock into fire prevention programs is a feasible and cost-effective method to improve fire prevention strategies (Rigueiro-Rodríguez et al. 2005; Launchbaugh et al. 2008; Ruiz-Mirazo et al. 2011). Additionally, the potential offered by goats in terms of their ability to survive in disadvantaged areas is broadly recognized at national and international level (Devendra and McLeroy 1987; Torrano and Valderrábano 2005; Mosquera-Losada et al. 2006; Celaya et al. 2007; Ruiz-Mirazo et al. 2011; Mancilla-Leytón et al. 2013). Silvopastoral systems incorporating livestock attempt to reconcile the use of natural products and services from the environment with a guarantee of permanence, or attempt to pursue ecological, economic and social stability through an efficient land use and the diversification of structures and products.
During grazing, damage by trampling and nutrient enrichment through faeces and urine deposition can indirectly accelerate litter decomposition. This process is rarely continuous; decomposition alternates between promotion and inhibition stages where chemical, physical and/or biological factors dictate the process (Swift and Anderson 1989). Many studies have evaluated foliar decomposition in relation to different climatic conditions (Silver and Miya 2001) but few have analysed the effect of grazing on foliar decomposition (Olofsson and Oksanen 2002; Garibaldi et al. 2007). Grazing may be of particular relevance in pine forests, where pine needles tend to accumulate abundantly because of their naturally low decomposition rates (Fioretto et al. 1998), and is especially important in nutrient-poor soil ecosystems (e.g. Mediterranean forests and dehesas) where it represents the main source of nutrients for new primary production (Wang and Huang 2008). At the same time, nitrogen (N) availability is widely considered to control the rate of litter decomposition, especially during the early stages in N-poor sites (Liu et al. 2011). A good correlation can often be found between the initial N concentration in litter and the decomposition rate (Dubeux et al. 2006). Furthermore, while detritivores are essential for the initial breakdown of litter (Prescott 1995), trampling during grazing can fragment the litter, thereby facilitating microbial activity and affecting the rate of litter decomposition.
The aim of this study was therefore to determine whether, goat grazing on the understory of a pine forest at Doñana Natural Park could accelerate the decomposition of the pine needles accumulated on the soil surface and to identify the relevant mechanisms. Specifically, the roles of trampling (mechanical fragmentation) and nutrient enrichment through defecation (fertilization) were evaluated in terms of their effect on pine needle decomposition rates.
Materials and methods
Study area
The study was carried out in a reforested pine forest (100 ha) on a private estate located within the Doñana Natural Park, in the southwest of the Iberian Peninsula (37º14′N, 6º20′W). The climate is Mediterranean moderated by the ocean, with wet (80 % of precipitation occurs between October and March) and mild winters (average monthly temperature of 10 °C in December and January) and very hot (average temperature of 25 °C in July and August) and dry (rarely rains in July and August) summers. Average annual rainfall in the study area is 540 mm. The substrate is mainly quartz sand and is extremely nutrient-poor, leading to poorly developed and infertile soils with low water retention capacities.
The vegetation comprises an arboreal stratum of pine trees (Pinus pinea L.) with a mean coverage of 38 %, an average density of 217 trees/ha, and an average tree diameter at breast height (DBH) of 26.92 cm. The understory is dominated by shrubs, with Cistus salvifolius L., Halimium halimifolium (L.) Willk., Halimium calycinum (L.) K., Rosmarinus officinalis L., Pistacia lentiscus L. and Myrtus communis L., the most common species.
The study area is used for timber production, hunting (rabbit, partridge) and grazing. However, wild herbivores (deer) were excluded in 1970 and domestic goats in 2002, with the study area remaining ungrazed for a period of 5 years prior to the reintroduction of goats (2007). During this 5-year period of livestock exclusion, vegetation was unmanaged and consequently grew and expanded rapidly. Afterwards, in spring of 2007, a herd of adult female Payoya goats (average weight of 40–45 kg) was introduced to the area and stocked at 2.7 goats ha−1 year−1 rate (characterized as moderate grazing). This stocking rate may be considered semi-extensive, although in order to exploit the available 100 ha area in a uniform manner, a shepherd actively controlled the herd movement.
Study of litter decomposition using litter-bags
The litter-bag method was used to study the decomposition of the pine needles (Wieder and Lang 1982). In autumn of 2008 (maximum needle fall peak), a total of 45 litter traps were placed in groups of 5 below 9 pine trees (5 traps/tree), and needles were recovered after 1 week. These needles were dried at 80 °C for 48 h, and then mixed together to homogenize the sample before placing the litter into litter-bags. Two grams of the dried needles were placed in plastic mesh bags (pore size of 2 mm2). This mesh size allows a range of detritivorous invertebrates to enter the bag, while retaining most of the fragmented needles. The needles were subject to 4 treatments: 1) intact needles (I), 2) needles trampled by goats (T), 3) intact needles fertilized with liquid manure (IF) and 4) trampled needles fertilized with liquid manure (TF). The mesh bags pertaining to the trampled treatments (T and TF) were placed into hermetically sealed plastic bags and half-buried for 5 days at the entrances and exits of the pen so that the goats could trample them. Afterwards, the bags belonging to treatments TF and IF were submerged for 24 h in a mixture of goat urine and faeces (300 g of faeces/l of urine), while the bags from treatments I and T were submerged in distilled water (also 24 h) in order to obtain a uniform moisture content in all the litter treatments.
A total of 300 bags (75 bags per treatment) were randomly distributed across a flat plot excluded from livestock, buried 3 cm under the soil (December of 2008) and covered with a uniform 2 cm layer of mixed sand and pine needles. Fifteen bags were randomly collected from each treatment after 4, 8, 16, 24 and 36 months and transported intact to the laboratory. Once opened, roots, small invertebrates and soil particles were manually removed from each bag and the needles were dried (80 °C for 48 h) and weighed.
Leaf litter decomposition (k) was determined as a function of mass loss over time (Matus and Rodríguez 1994) and calculated using the equation y = Ao * e-kt, where y is final dry mass, Ao is initial dry mass and t is time of accumulation. From this, litter decomposition rates were calculated as: k = Ln (final mass/initial mass)/t.
Biometric and chemical characteristics of the needles
Needle length and leaf mass per area were measured after decomposition. Leaf mass per area (g/m2) was determined by scanning the surface of the needles with image analysis software (Midebmp) (R. Ordiales, CSIC, Spain, 2000) and recording their weight. Finally, concentrations of carbon (C) and nitrogen (N) were determined from undigested dry samples using an elemental analyser (Leco CHNS-932, Spain).
Leaf litter decomposition in grazed and ungrazed areas
To determine whether the presence of livestock accelerated the decomposition of leaf litter in the pine forest, the decomposition rates of falling residues and of litter accumulated on the soil surface were calculated for both grazed and ungrazed areas. Falling residues were collected in 44 plastic bins (105 cm in height; 2,000 cm2 capture area). These were randomly distributed, with 22 placed in areas excluded from livestock and 22 in grazed areas. The bins were placed below the crowns of the pine trees, fixed to the ground by a metal structure that prevented them from tipping over or being moved by animals. The height and resistance of the bins prevented the herbivores from consuming the tree residues contained within. Residues were collected monthly, transported to the laboratory and dried at 80 °C until reaching constant mass (48 h). Once dried, needles were separated from the other residues. Annual needle fall per unit area of arboreal canopy was estimated by summing the mass of all the needles collected within the same year and expressing this value in g/m2. Average annual needle fall for the pine forest (L) was estimated by multiplying this production value by the canopy area (% pine tree coverage). To quantify the litter accumulated on the soil surface (H), a total of 90 plots of area 0.3 m2 were randomly distributed, 45 across livestock excluded areas and 45 across the grazed areas. Samples (total litter accumulated on each plot) were transferred to the laboratory where they were sieved to remove soil, and dried in a fan oven at 80 °C for 48 h. Once dried, needles were separated from other fractions and the remaining litter was weighed and expressed in g/m2 (H). This procedure was repeated 4 times; once before livestock entered the pine forest, and annually thereafter for 3 years (12, 24 and 36 months). The decomposition rate of the pine forest (k′) was calculated with Olson’s Model, as the ratio of needle fall production (L) to the total amount of litter accumulated on the soil (H) (k′ = L/H). Finally, once the decomposition rate of the pine forest (k′) was estimated, k was calculated through the equation: k = Ln (1-k′) (Olson 1963; Staaf 1987).
Statistical analysis
For each variable (mass remaining (%), needle length, leaf mass per area and C and N content) a repeated measures ANOVA model was generated. The model comprised two factors: treatment (I, T, IF and TF) and time (five sampling dates). The results were adjusted according to the Huynh-Feldt correction. Finally, the linear relationship between remaining mass and leaf mass per area was calculated using a Pearson correlation. All analyses were conducted using the programme SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Leaf litter decomposition
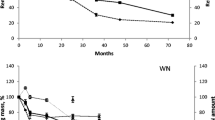
Figure 1 shows the remaining mass (%) of the pine needles throughout the study period. Results show a significant interaction between the different treatments and time (F = 39.43, p ≤ 0.01).
Initially (at 4 months), the treatments with trampled needles (T and TF) registered a greater loss of mass (23–27 %, respectively) than those with intact needles (I and IF, 15–17 %, respectively). From 8 months onwards, the fertilized treatments (IF and TF) lost relatively more mass, with recorded differences between fertilized and non-fertilized treatments of 5–15 %.
Table 1 shows the decomposition rate (k) for each treatment and time period. The highest decomposition rates were recorded after 4 months, with the trampled needle treatments (T and TF) presenting significantly higher rates (0.93 and 0.90 year−1, respectively) than the intact needle treatments (I and IF, 0.64 and 0.70 year−1, respectively). From this time period onwards, however, decomposition rates in the fertilized treatments (TF and IF) were significantly higher than those in the non-fertilized treatments (I and T) (F = 536.46, p ≤ 0.01).
Biometric and chemical characteristics of the pine needles
Throughout the study period, average needle length in the trampled treatments was significantly shorter (T and TF; 8.1 ± 0.3 cm) than those in the non-trampled treatments (I and IF; 9.6 ± 0.2 cm). These differences persisted during the whole study period (F = 29.93, p ≤ 0.01).
In terms of leaf mass per area (LMA), the results showed a significant interaction between the different treatments and time (F = 4.75, p ≤ 0.01) (Figure 2). Initially, both treatments with trampled needles (T and TF) presented significantly lower LMA values (250 and 260 g/m2, respectively) than those with intact needles (I and IF) (300 and 310 g/m2, respectively). From 8 months onwards, the fertilized treatments (IF and TF) presented significantly lower LMA values than the non-fertilized treatments (I and T) (F = 85.23, p ≤ 0.01). These differences (10–20 g/m2) persisted until the end of the study period. The remaining mass of the needles and the LMA correlated positively (r = 0.73, p < 0.01).
Finally, chemical analyses showed similar initial C percentage values (approximately 50 %) in needles across all treatments (Table 2). Over time, the percentage of C decreased across all treatments, but in the fertilized treatments (IF and TF) the loss of C was greater (Table 2). As expected, the initial percentage of N was significantly greater in the treatments soaked with purines (2.1 %) compared to those that were unfertilized (1.2 %) (F = 476.29, p < 0.01). Although all treatments showed an increase in the percentage of N after 8, 16 and 24 months, by the end of the experiment (36 months), the final percentage of N was significantly lower in the treatments that had been soaked with purines (0.7 %) than in those that were unfertilized (0.9 %) (F = 308.01, p < 0.01) (Table 2). Consequently, the C:N ratio was significantly lower in the fertilized treatments (IF and TF) (F = 254.47, p < 0.01) (Table 2).
Decomposition of the pine forest leaf litter
Mean annual litter accumulation (H) in the ungrazed areas was 3 times greater (442.91 g/m2) than in the grazed area (135.83 g/m2). The estimated mean annual needle production of the pine forest (L) was 101.87 g/m2. The mean annual decomposition rate constant (k) of the ungrazed areas (0.26 year−1) did not differ significantly from the control treatment (I) (Table 1). However, the mean annual decomposition rate of the grazed areas (1.40 year−1) was much higher than that of the other treatments (Table 1).
Discussion
Large herbivores mainly affect nutrient cycling through two mechanisms: processing plants into urine and faeces and influencing litter decomposition (Semmartin et al. 2008). Faeces and urine deposition by grazing animals exerts a positive influence on soil nutrient pools and microbial communities (Sankaran and Augustine 2004), thereby endorsing the use of grazers to enhance soil N availability and litter breakdown. Our results show that trampling by goats, which fragments the pine needles, significantly increases their initial decomposition rate, with nutrient enrichment (represented by soaking in purines) becoming more important with time (Table 1).
The decomposition rate of Pinus pinea needles in the control treatment (I) falls within the ranges found in other studies for this genus (Pausas 1997; Moro and Domingo 2000; Li et al. 2007; Harmon et al. 2009). However, the decomposition rates of the trampled and fertilized treatments were much higher. Most studies agree that the physical and chemical characteristics of plant tissues dictate their decomposition (see Prescott 2010). Some studies have shown that the macrofauna plays an important role in decomposition; fragmenters, such as earthworms, millipedes, termites, and isopods, are primarily macrofauna that can affect resource availability by modifying the physical properties of litter (Chapin et al. 2002; Yang et al. 2012). Litter fragmentation has been found to facilitate microbial attack, which in turn may produce increased decomposition rates: by enlarging the surface area available for microbial attack, litter fragmentation by small invertebrates causes a temporary increase in litter decomposition (Gillon and David 2001; Podgaiski and Rodrigues 2010; Yang et al. 2012). This study indicates that needle fragmentation during grazing can significantly increase decomposition rates (Table 1), apparently by enlarging the surface area available for attack by decomposers. However, this effect can be limited to a period of a few months following fragmentation after which rates of C mineralization may fall below the control values.
The chemical characteristics of the litter became more relevant after 4 months of treatment (Table 2). It has been recognized for some time that mineral elements, particularly N, play an important role in controlling decomposition rates in organic material. The magnitude and even the sign of these effects are, however, not universal and the underlying mechanisms are poorly understood (Agren et al. 2001). It is not clear whether the relationship between decomposition and N content is an effect of the N itself, since changing the concentration of N promotes multiple changes in the decomposition process (such as increases in decomposer efficiency, more rapid formation of recalcitrant material and, although less pronounced, decreased decomposer growth rate) (Agren et al. 2001). For instance, Joffre et al. (2001) state that changing the N concentration has little effect on decomposition rates, while Berg and Ekbohm (1991) argue the contrary. Additionally, decomposition rates tend to decline over time with increases in the C:N ratio, the latter being accepted as a general index of quality (Seneviratne 2000). This trend is in accordance with our findings, which show a significant increase in decomposition rates from 8 months onwards in the treatments that were soaked in purines (Table 1). This increase was maintained until the end of the study (36 months).
For a few decades, the consequences of N deposition on soil nutrient renewal rates have often been attributed to microbial activity (Söderström et al. 1983; Arnebrant et al. 1996). The most likely explanation for this increase in decomposer efficiency is that the increased N promotes changes in the decomposer community, especially in N-poor systems such as Doñana Natural Park (Gallardo and Merino 1992; Allison et al. 2007; Treseder 2008; Papanikolaou et al. 2010). This mechanism is supported by numerous studies demonstrating shifts in microbial community composition after N fertilization (see Talbot and Treseder 2012), across nutrient gradients and following the addition of free N from wood ash produced by forest fires (Papanikolaou et al. 2010). We do not fully understand how this relates to the observed changes in decomposition rates, since the community of decomposers has not been analysed; however, this aspect certainly merits further study.
Gallardo and Merino (1992), in a study focusing on the decomposition of Mediterranean shrublands in Doñana, suggested that nitrogen could be imported to the litter by the microbial biomass. This may explain the N increase found as litter decomposition proceeds (Table 2). Similarly, Aber and Melillo (1982) found an inverse-linear relationship between remaining mass and N concentration in litter, this relationship being validated by a large number of litter decomposition studies. Fixation of atmospheric N, contamination by insect frass, through fall/importation from green litter, etc., have all been proposed as possible explanations for this N increase. In this sense, Gallardo and Merino (1992) suggested that tannins could be responsible for N immobilization in leaf litter of Mediterranean shrublands, their effect being relatively more important in nutrient-poor soils with less microbial biomass (Aber and Melillo 1982). During a second step, tannins could precipitate N and immobilize it in the lignin fraction. The maximum amount of immobilized N would be limited by the size of microbial biomass (depending on the site characteristics) and the amount and quality of tannins (as a factor of litter quality) (Gallardo and Merino 1998). Therefore, since the environmental characteristics were shared across all the treatments in our study, the changes found in the N content can be attributed to quality differences in the litter itself (e.g. fertilized vs. unfertilized).
Finally, with respect to decomposition rate in the pine forest (k), the resulting k of the ungrazed areas was similar to that of the control treatment (0.26 year−1), however, the decomposition rate of the grazed areas was far greater than that of the other treatments (IF, T and TF) (Table 1). The differences between these rates are attributed to the constant fertilization and trampling that occurs in the grazed areas throughout the study period (36 months) resulting in a higher decomposition rate, unlike the other treatments that were only fertilized and trampled at the beginning of the experiment. Other factors related to enclosing litter within bags with a small mesh size could have also contributed to the differences found among the different treatments (e.g. the exclusion of macroarthropods from the litter could have slowed down decomposition within the litter-bags). These factors would have been of minor effect, since decomposition in the ungrazed area was similar to that observed in the bags, but they should nevertheless be considered.
In conclusion, contrary to the general assumption that goats only cause environmental degradation, the results of this study indicate an indirect positive effect of goat grazing in the decomposition of leaf litter. Grazing increased the decomposition rate of P. pinea needles, promoting the incorporation of N into the system. The higher decomposition rates recorded in the grazed area could certainly contribute to changes in vegetation composition, creating a positive feedback (plant-soil) in these areas (Bardgett and Wardle 2003). In this sense, grazing could not only benefit vegetation through soil nutrient enrichment, but the acceleration of decomposition could also reduce the accumulation of pine needles on the soil surface, thereby making a valuable contribution to the mitigation of forest fire risk.
References
Aber JD, Melillo JM (1982) Nitrogen immobilization in decaying hardwood litter as afunction of initial nitrogen and lignin content. Canadian J Bot 60:2263–2269
Agren GI, Bosatta E, Magill AH (2001) Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–98
Alados CL, Pueyo Y, Giner ML, Navarro T, Escos J, Barroso F, Cabezudo B, Emlen JM (2003) Quantitative characterization of the regressive ecological succession by fractal analysis of plant spatial patterns. Ecolog Modelling 163:1–17
Allison SD, Hanson CA, Treseder KK (2007) Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biol Biochem 39:1878–1887
Arnebrant K, Bååth E, Söderström B, Nohrstedt HÖ (1996) Soil microbial activity in eleven Swedish coniferous forests in relation to site fertility and nitrogen fertilization. Scand J For Res 11:1–6
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Celaya R, Martínez A, Osoro K (2007) Vegetation dynamics in Cantabrian heathlands associated with improved pasture areas under single or mixed grazing by sheep and goats. Small Rumin Res 72:165–177
Chapin SF, Pamela AM, Harold AM (2002) Principles of terrestrial ecosystem ecology. Springer Press, New York, pp 151–264
Devendra C, McLeroy GB (1987) Goat and sheep production in the tropics. Longman Scientific and Technical Publishers, Singapore
Dregne HE, Willis WO (1983) Dryland agriculture. Agronomy No. 23, American Society of Agronomy, Crop Science Society of America, Soil Science Society of America, Madison
Dubeux JCB, Sollenberger LE, Interrante SM, Vendramini JMB, Stewart RL (2006) Litter decomposition and mineralization in bahiagrass pastures managed at different intensities. Crop Sci 46:1305–1310
Ekbohm BB (1991) Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest. C J Bot 69:1449–1456
Fioretto A, Mussachio A, Andolfi G, Virzo de santo A (1998) Descomposition dynamics of litters of various pine species in a Corsican pine forest. Soil Biol Biochem 30:721–727
Gallardo A, Merino J (1992) Nitrogen immobilization in leaf litter at two Mediterranean ecosystems of SW Spain. Biogeochemistry 15:213–228
Gallardo A, Merino J (1998) Soil nitrogen dynamics in response to carbon increase in a Mediterranean shrubland of SW Spain. Soil Biol Biochem 30:1349–1358
Garibaldi LA, Semmartin M, Chaneton EJ (2007) Grazing induced changes in plant composition affect litter quality and nutrient cycling in flooding Pampa grasslands. Oecologia 151:650–662
Gillon D, David JF (2001) The use of near infrared reflectance spectroscopy to study chemical changes in the leaf litter consumed by saprophagous invertebrates. Soil Biol Biochem 33:2159–2161
Goel G, Makkar HPS, Becker K (2008) Effect of Sesbania sesban and Carduus pycnocephalus and Fenugreek (Trigonella foenum-graecum L.) seeds and their extracts on partitioning of nutrients from roughage-and concentrate-based feeds to methane. Animal Feed Sci Technol 147:72–89
Harmon ME, Silver WL, Fasth B, Chen H, Burke IC, Parton WJ, Hart SC, Currie WS, LIDET (2009) Long-term patterns of mass loss during decomposition of leaf and fine root litter: An intersite comparison. Glob Change Biol 15:1320–1338
Joffre R, Agren GI, Gillon D, Bosatta E (2001) Organic matter quality in ecological studies: Theory meets experiment. Oikos 93:451–458
Launchbaugh K, Brammer B, Brooks ML, Bunting S, Clark P, Davison J, Fleming M, Kay R, Pellant M, Pyke DA, Wylie B (2008) Interactions among livestock grazing, vegetation type, and fire behavior in the Murphy Wildland Fire Complex in Idaho and Nevada, July 2007. United States Geological Survey Technical Report 1214:1–42
Li X, Han S, Zhang Y (2007) Foliar decomposition in a broadleaf-mixed Korean pine (Pinus koraiensis Sieb. Et Zucc) plantation forest: the impact of initial litter quality and the decomposition of three kinds of organic matter fraction on mass loss and nutrient release rates. Plant Soil 295:151–167
Liu K, Sollenberger LE, Silveira ML, Vendramini JMB, Newman YC (2011) Grazing intensity and nitrogen fertilization affect litter responses in ‘Tifton 85’ bermudagrass pastures: II. Decomposition and nitrogen mineralization. Agronomy J 103:163–168
Mace R (1991) Overgrazing overstated. Nature 349:280–281
Mancilla-Leytón JM, Pino Mejías R, Martín Vicente A (2013) Do goats preserve the forest? Evaluating the effects of grazing goats on combustible Mediterranean scrub. App Veget Sci 16:63–73
Matus FJ, Rodríguez J (1994) A simple model for estimating the contribution of nitrogen mineralization to the nitrogen supply of crops from a stabilized pool of soil organic matter and recent organic input. Plant Soil 162:259–271
Moro MJ, Domingo F (2000) Litter decomposition in four woody species in a Mediterranean climate: Weight loss, N and P dynamics. Annals Bot 86:1065–1071
Mosquera-Losada MR, Fernández-Núñez E, Rigueiro-Rodríguez A (2006) Pasture, tree and soil evolution in silvopastoral systems of Atlantic Europe. Forest Ecol Manag 232:135–145
Olofsson J, Oksanen L (2002) Role of litter decomposition for the increased primary production in areas heavily grazed by reindeer: A litter-bag experiment. Oikos 96:507–515
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Papanikolaou N, Britton AJ, Helliwell RC, Johnson D (2010) Nitrogen deposition, vegetation burning and climate warming act independently on microbial community structure and enzyme activity associated with decomposing litter in low-alpine heath. Glob Change Biol 16:3120–3132
Pausas JG (1997) Litter fall and litter decomposition in Pinus sylvestris forests of the eastern Pyrenees. J Veg Sci 8:643–650
Podgaiski LR, Rodrigues GG (2010) Leaf-litter decomposition of pioneer plants and detritivore macrofauna assemblages on coal ash disposals in southern Brazil. European J Soil Biol 46:394–400
Prescott CE (1995) Does nitrogen availability control rates of litter decomposition in forest? Plant Soil 168–169:83–88
Prescott CE (2010) Litter decomposition: What controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Rigueiro-Rodríguez A, Mosquera-Losada MR, Romero-Franco R, González Hernández MP, Villarino-Urtiaga JJ (2005) Silvopastoral systems as a forest fire prevention technique. In: Mosquera-Losada MR, McAdam J, Rigueiro-Rodríguez A (eds) Silvopastoralism and sustainable land management. CABI, Wallingford, pp 380–387
Ruiz-Mirazo J, Robles AB, González-Rebollar JL (2011) Two-year evaluation of fuelbreaks grazed by livestock in the wildfire prevention program in Andalusia (Spain). Agr Ecosyst Environ 141:13–22
Sankaran M, Augustine DJ (2004) Large herbivores suppress decomposer abundance in a semiarid grazing ecosystem. Ecology 85:1052–1061
Semmartin M, Garibaldi LA, Chaneton EJ (2008) Grazing history effects on above-and below-ground litter decomposition and nutrient cycling in two co-occurring grasses. Plant Soil 303:177–189
Seneviratne G (2000) Litter quality and nitrogen release in tropical agriculture: A synthesis. Biol Fertility Soils 31:60–64
Silver W, Miya R (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Söderström B, Baath E, Lundgren B (1983) Canad J Microbiol 29:1500–1506
Staaf H (1987) Foliage litter turnover and earthworm populations in three beech forests of contrasting soil and vegetation types. Oecologia 72:58–64
Swift MJ, Anderson JM (1989) Decomposition. In Lieth H, M.J.A. pp.547–569.
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354
Torrano L, Valderrábano J (2005) Grazing ability of European black pine understory vegetation by goats. Small Rum Res 58:253–263
Treseder KK (2008) Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Wang QS, Huang Y (2008) Comparisons of litter fall, litter decomposition and nutrient return in a monoculture Cunninghamia lanceolata and a mixed stand in southern China. Forest Ecol Manag 255:1210–1218
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642
Yang X, Yang Z, Warren MW, Chen J (2012) Mechanical fragmentation enhances the contribution of Collembola to leaf litter decomposition. European J Soil Biol 53:23–31
Acknowledgments
The authors wish to thank Doñana Natural Park and Dehesa de Gatos S.L. for the logistic support and field facilities. We also thank Dr. R. Fernández-Ales and Dr. J.A. Merino for their useful comments on an earlier version of this manuscript. Dr. R. F. Lo Faso and Keith MacMillan revised the English version of the manuscript. This study was funded by the Consejería de Medio Ambiente (Junta de Andalucía) (OG-052/07). J.M. Mancilla-Leytón gratefully acknowledges a F.P.D.I. grant (Junta de Andalucía).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jeff R. Powell.
Rights and permissions
About this article
Cite this article
Mancilla-Leytón, J.M., Sánchez-Lineros, V. & Martín Vicente, A. Influence of grazing on the decomposition of Pinus pinea L. needles in a silvopastoral system in Doñana, Spain. Plant Soil 373, 173–181 (2013). https://doi.org/10.1007/s11104-013-1788-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1788-8