Abstract
Background and aims
Mixing effects during litter decomposition could occur between two or more different litter species because of the potential nutrient transfer. However, evidence of mixing effects is variable and the underlying mechanisms remain unclear. Using a three-year decomposition experiment, we aim to examine for the effects of litter mixing and position on decomposition rates and nitrogen (N) and phosphorus (P) dynamics.
Methods
We studied litter decomposition of Stipa krylovii (Sk) and Astragalus galactites (Ag), two dominant species with contrasting litter quality, in a typical steppe of northern China in both single decomposition and three mixing treatments. The three mixing treatments included thorough mixing (Sk-Ag), Ag over Sk (Ag/Sk), and Sk over Ag (Sk/Ag).
Results
Both the Sk-Ag and the Sk/Ag mixture had negative mixing effects on the mass loss of the litter mixture, while the Ag/Sk mixture had a neutral mixing effect. The percent mass loss was higher when the litter species was placed at the top (25.0 and 51.9 % of mass remaining for Ag and Sk, respectively) than at the bottom (38.3 and 61.8 % of mass remaining for Ag and Sk, respectively). The Sk/Ag mixture had negative effects on the release of N while all three mixing treatments had positive effects on the release of P.
Conclusions
Our results indicate that: (1) mixing treatments can induce different mixing effects; (2) environmental factors likely play an important role in controlling the mixing effect; and (3) litter-mixtures have different non-additive effects on N and P, which may further increase the heterogeneity of N and P availability as the two litter species may fall differentially in terms of space and time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In most terrestrial ecosystems, litter decomposition is a fundamental ecological process that provides soil nutrients for plant biological activity (Swift et al. 1979). Due to its importance in nutrient cycling, the dynamics of plant litter decomposition and the controlling factors have been the focus of a considerable number of studies. Previous studies have revealed that litter decomposition could be affected by a series of abiotic factors, such as precipitation (Austin and Vitousek 2000), UV-radiation (Austin and Vivanco 2006), water table depth (Moore et al. 2007), and soil nutrient availability (Dent et al. 2006; Hobbie and Vitousek 2000). In addition, biotic factors, such as litter quality (Berg and Ekbohm 1991), soil animals (Bradford et al. 2002), and soil microbes (Daniel and Anderson 1992), could have great impact on litter decomposition. In semiarid environments, litter decomposition is more complex and additional factors such as vegetation structure and soil moisture could also play important roles (e.g., Throop and Archer 2009; Wang et al. 2009). Among these factors, it is often believed that litter quality, i.e., nitrogen (N) content, C/N ratio, and lignin/N ratio, is the most important factor in determining the rate of litter decomposition especially in a terrestrial ecosystem (Aerts 1997; Vivanco and Austin 2006).
Most of the previous studies, however, have only focused on the decomposition of single litter species, while litter layer in natural ecosystems is in fact a mixture of different plant species. Decomposition of one litter species is inevitably influenced by the presence of adjacent litter species, resulting in so-called mixing effects which may either facilitate or inhibit the rate of overall litter decomposition (Jonsson and Wardle 2008; McTiernan et al. 1997; Wardle et al. 1997). Such mixing effects can also be regulated by environmental factors (Gartner and Cardon 2006; Madritch and Cardinale 2007) and litter quality of the component species, though the results are inconsistent among different studies (Hättenschwiler and Vitousek 2000; Hector et al. 2000; Hobbie et al. 1999; Hoorens et al. 2003). Most studies compared the observed with the expected mass loss rate based on the decomposition rates of single component litter; few of these studies explored the underlying mechanisms inducing the mixing effects (Hättenschwiler et al. 2005; Wardle et al. 1997). Even fewer studies attempted to separate the component litter species from the litter mixture to investigate how the identity of the neighboring litter species affected decomposition interactively (Barantal et al. 2011; Hoorens et al. 2010; Wardle et al. 2003).
In this study, we selected two dominant species, Stipa krylovii (Sk) and Astragalus galactites (Ag), with contrasting litter quality in terms of N content, from a semiarid typical steppe ecosystem in Inner Mongolia, China, to examine whether there were significant mixing effects on the decomposition of litter mixtures. In order to understand which factors determined the observed litter mixing effects, the two litter species in the litter mixture were mixed in three different treatments (thorough mixing, Sk on top of Ag, and Ag on top of Sk). We hypothesized that (1) the three mixing treatments would have different mixing effects as position could play an important role; (2) the high quality Ag litter would release nutrients while the low quality Sk litter would immobilize nutrients; and (3) the presence of Ag litter would facilitate the nutrient release whereas the Sk litter induce immobilization in the mixture.
Materials and methods
Study site
The experimental site was located at a temperate steppe in Duolun County, Inner Mongolia (42°02′N, 116°17′E, 1,324 m above sea level), China. This area is in a typical temperate zone characterized by a semiarid continental monsoon climate. Long-term mean annual precipitation is 385 mm, and mean annual temperature is 2.1 °C with monthly mean temperature ranging from −17.5 °C in January to 18.9 °C in July. Annual rainfall is 198.5 mm in 2007, 314.4 mm in 2008, and 172.6 mm in 2009, and small daily rainfall events (0–2 mm) account for 67, 50, and 68 % of total rainfall from 2007 to 2009, respectively (Fig. 1). The sandy soil of the study site is classified as chestnut according to the Chinese classification, or Haplic Calcisols according to the Food and Agriculture Organization (FAO) classification. The surface 10 cm soil layer is slightly alkaline (pH = 7.2) and contains 20.4 g kg−1 total C, 1.63 g kg−1 total N with 10.35 mg kg−1 total available N, and 0.31 g kg−1 total phosphorus (P) (Liu et al. 2010). Mean soil bulk density is 1.31 g cm−3.
Experimental design
The selected species in this study, Stipa krylovii (Sk) and Astragalus galactites (Ag), are two dominant species in this typical steppe ecosystem in Inner Mongolia grassland. S. krylovii is a perennial bunchgrass, and A. galactites is a perennial forb and an important legume species in the studied grassland system (Yang et al. 2011). The two litter species have quite different chemical and physical properties. The initial N concentration of the litter of Ag is about four times that of Sk, and the initial P concentration of Ag is also significantly higher than that of Sk (Table 1).
In late September 2006, the senescent leaf litters from the current year of the two species were collected, air-dried, and placed in polyethylene litterbags (15 × 20 cm, 1 mm mesh). Each bag was filled with 15 g litter either from a single litter species (Ag or Sk), or a 1:1 mixture of the two litter species. Two litter species were either mixed thoroughly (Sk-Ag) or separated by a polyethylene sheet (0.20 mm mesh size) in the litterbags. For the two mixing treatments with a divider, either Sk or Ag was placed on top of the other (Sk/Ag and Ag/Sk). In total, there were five treatments: two single litter treatments (Ag and Sk) and three litter mixed treatments (Sk-Ag, Sk/Ag, and Ag/Sk). The initial litter weight (15 g air-dried litter) in each bag was chosen based on the following considerations: (1) experimental duration (∼3 years), (2) potential decomposition rate in this area, and (3) sufficient remaining mass for chemical analysis after decomposition. The choice of the litterbag size was constrained by the limited space among plant clumps in the studied community, i.e., it would be difficult to use litterbags larger than 15 × 20 cm.
On 27 October 2006, 200 litterbags were deployed in five replicate plots, with 40 in each plot. Litterbags were retrieved after 162, 252, 341, 620, 706, 911, 991, and 1,072 days, during the following 3 years. At each collection date, one set of litterbags, i.e., one litterbag of each single litter species and of each of the three mixing treatments from each replicate plot (25 total each time), was randomly collected, and then transported back to the laboratory.
In the laboratory, five sub-samples of each type of the original litter were oven-dried at 70 °C for 48 h before initial deployment to determine the ratio of air-dried versus oven-dried mass. This ratio was used to calculate the initial oven-dried mass of each litterbag from the air-dried mass. After the litterbags were collected, extraneous matter such as in-growth plant materials and small animals were removed from the decomposing litter. At the same time, the mixture of Sk/Ag and Ag/Sk were separated by species. The soil mixed with the litter sample was rinsed in cold water, and the litter was then oven-dried at 70 °C for 48 h to determine remaining dry mass.
Chemical analyses
To determine the initial litter chemistry, total C, N, and P of the five original non-decomposed sub-samples were measured. Only N and P concentrations were examined for litter samples harvested during decomposition. All the litter samples were ground using a ball mill (Retsch MM 400; Retsch, Haan, Germany), and passed through a No. 4 sieve. Total C content was determined using H2SO4–K2Cr2O7 oxidation method (Nelson and Sommers 1996), and total N concentrations with an Alpkem autoanalyzer (Kjektec System 1026 Distilling Unit, Sweden). After sub-samples had been digested in the mixture of H2SO4 and H2O2, total P was measured using the molybdenum blue colorimetric method at 880 nm.
Statistical analyses
The expected percent mass remaining of each litter mixture (Sk-Ag, Sk/Ag, and Ag/Sk) were calculated as:
where R 1 and R 2 are the percent remaining mass (%) of the singly decomposed litter species 1 and 2, respectively, and M 1 and M 2 are the initial dry mass of each litter species in the mixture (Hoorens et al. 2003).
The expected percent N and P mass remaining in the litter mixtures were determined, similarly based on N and P concentrations of each single litter species at each collection date. Any significant deviation from expected values indicates an interactive effect, either positive or negative, between the mixing litter species.
Repeated measures ANOVA was used to test the significance of overall differences between the observed and the expected percent mass remaining values and between different litter mixing treatments (i.e., Sk-Ag, Sk/Ag, and Ag/Sk) across the whole decomposing period, where time was treated as within-subject factor. Repeated measures ANOVA was also used to test the differences of the mass loss and nutrient concentration of the same litter species placed at different positions (top or bottom) during the whole decomposing period. One-way ANOVA was used to test the nutrient concentration differences between the two positions in the litterbag at each collection date. The regressions between time and the ratios of the percent remaining mass of the top and bottom treatments of the same species were also conducted. The significant level of α = 0.05 was used. Data analyses were performed using SPSS (v.16.0).
Results
Mass loss of single-component litter and the mixtures
After 1,072 days, an average of 74.3 % of the initial mass of the singly decomposed Ag litter was lost, compared with a 48.7 % loss of the single Sk litter (Fig. 2a).
Among the three types of litter mixing treatments, Sk-Ag had a significant negative mixing effect on mass loss (P = 0.031). The amount of the observed percent mass remaining of the Sk-Ag treatment was 42.8 % compared with the expected value of 41.0 % at the end of this study. Sk/Ag also had a significant negative mixing effect on the mass loss (P < 0.001), and the observed percent mass remaining was 45.1 %, higher than the expected value of 41.0 %. In contrast, Ag/Sk did not show significant mixing effect on the mass loss (P > 0.05) (Fig. 2b; Table 2).
Litter placed on the top, either Sk or Ag, decomposed much faster than that at the bottom. There were significant differences in the percent mass remaining between the top and the bottom positions for both Sk and Ag at all collection dates except the one at day 162 (Fig. 3a, b). The ratios between top and bottom percent remaining mass showed a significant exponential decrease with time for both Sk and Ag, and tended to level off at the end of this study (r 2Sk = 0.84, r 2Ag = 0.69; Fig. 3a, b).
Percent mass remaining of the component species in the two mixing treatments. a Sk from Sk/Ag (means Sk on the top) and Ag/Sk (means Ag on the top), respectively; b Ag from Ag/Sk and Sk/Ag, respectively. Insets indicate the regressions between time and the ratios of the percent mass remaining of the top and bottom treatments. All the data are mean ± 1SE, n = 5. Sk Stipa krylovii; Ag Astragalus galactites
Nutrient concentrations of the component litter species and their nutrient release patterns
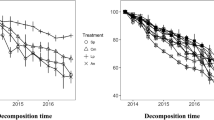
N concentration of the litter species placed on the top was much higher than that of the same species placed at the bottom for both Sk and Ag (P = 0.011 for Sk; P < 0.001 for Ag; Table 3; Fig. 4a, b) during decomposition. However, results of one-way ANOVA showed that significant difference in N concentrations of the two positions for the Sk litter was found only at the last collection date (Fig. 4a), while significant difference was found for the Ag litter retrieved on days 252, 341, 706, and 991 (Fig. 4b). N concentrations of Sk in Sk/Ag and Ag/Sk showed a small decrease at the beginning, increased continuously afterwards, and decreased again when it approached the end of the observation period (Fig. 4a). N concentrations of Ag in Ag/Sk and Sk/Ag showed remarkably large fluctuations during the decomposition period, although there was an overall increasing trend after a dip at the beginning (Fig. 4b). Because the two component species could not be separated from each other in the thorough mixture Sk-Ag, we were unable to separately investigate the change of N and P concentrations of each decomposing litter species in the mixture.
The N and P concentration of the component species Sk and Ag in the litter mixture across the decomposition period (mg.g−1), a N concentration of Sk; b N concentration of Ag; c P concentration of Sk; d P concentration of Ag. Data are means ± 1SE, n = 5. *P < 0.05; **P < 0.01; ***P < 0.001. Sk Stipa krylovii; Ag Astragalus galactites
Repeated measures ANOVA showed that there was no overall difference in P concentration between the top and the bottom positions for both species (P = 0.535 for Sk; P = 0.163 for Ag; Table 3; Fig. 4c, d). In addition, there was no significant difference in P concentrations of the two positions for the Sk litter at any collection date (Fig. 4c, d). However, one-way ANOVA results showed that the P concentration of the Ag litter on the top was significantly higher than that of the Ag litter at the bottom collected on days 620, 706, and 911 (Fig. 4d).
For both single decomposition and mixing treatments, there was a releasing phase for both N and P of the Ag litter. A leaching phase of N and P was found followed by immobilization and then a release phase for the Sk litter (Fig. 5a–d). When Sk decomposed with Ag, a stronger immobilization of N was found at both top and bottom positions (Fig. 5a; P = 0.006). There was no significant difference in P release between the Sk litter decomposing singly and at the bottom position of the mixture, but the percent P remaining of the Sk litter at the upper position was significantly lower than the above two positions (Fig. 5d; P = 0.002). For the Ag litter, the release of N was the fastest when Ag was placed above Sk. It became the slowest when Ag was placed below Sk (Fig. 5b; P < 0.001). The release of P during the Ag litter decomposition showed a similar pattern to that of N (Fig. 5e; P < 0.001).
The nutrient dynamics (% of initial values) in the Sk and the Ag single decomposition and the three mixing treatments. a, d Percent N and P remaining of the Sk litter decomposing in single decomposition and in mixtures with Ag; b, e percent N and P remaining of the Ag litter decomposing in single decomposition and mixtures with Sk. c, f percent N and P remaining of the observed and the expected values of the mixing litter. Values are mean ± 1SE, n = 5. Sk Stipa krylovii; Ag Astragalus galactites
Neither Sk-Ag nor Ag/Sk showed a significant mixing effect on N release (Table 2; P > 0.05). However, there was a negative effect of the Sk/Ag mixture on N release (Table 2; P < 0.001), which was indicated by the higher observed percent N remaining value compared to the expected value over the whole decomposing period. Overall, N of the litter in the three mixtures was continuously released throughout the whole study period (Fig. 5c). All three mixtures had an overall significantly positive mixing effect on P release (Table 2). In addition, P was released throughout the study period with exception of a large increase in the percent remaining after the first year’s decomposition and a small increase for the last ∼200 days of decomposition (Fig. 5f).
Discussion
Mass loss in the mixtures
In natural terrestrial ecosystems, litter species in the litter layer usually decompose in mixtures rather than singly, and the decomposition of litter mixture may be either enhanced or retarded by the component litter species. Previous studies have indicated that there were mixing effects on the decomposition of different litter species in fen ecosystems (Hoorens et al. 2003), boreal forests (Nilsson et al. 1999), grasslands, and agricultural systems (Wardle et al. 1997). In this study, consistent with our first hypothesis, there were different mixing effects for different mixing treatments. We found significant negative mixing effects on the mass loss of the Sk/Ag mixture and significant but weaker negative mixing effects on the mass loss of the Sk-Ag mixture, while no significant mixing effect on the mass loss of the Ag/Sk mixture (Table 2). We also found that the percent mass remaining was significantly different between the two mixing positions for both Sk and Ag after 162 days (Fig. 3). The ratios of percent mass remaining between the top and the bottom litter species, for both Sk and Ag, decreased exponentially with time but tended to level off later (Fig. 3 inset). The average difference of the eight collection dates showed that Sk in the Ag/Sk treatment was 5.4 % higher in percent mass remaining than that of the single Sk, while Ag in the Ag/Sk treatment was 5.0 % lower in percent mass remaining than that of the single Ag. This difference between the two component litter in the Ag/Sk treatment counterbalanced each other. However, Sk in the Sk/Ag treatment was 3.5 % lower in percent mass remaining than the single Sk, while Ag in this mixture was 12.1 % higher than the single Ag, resulting in a lower overall mass loss in the Sk/Ag mixture. Therefore, the difference in mixing effects on mass loss in different litter mixtures might be a result of the change in mixing positions.
Studies have shown that litter quality and the environmental factors regulated mixing effects. Litter chemistry can influence the decomposition rates of the component litter species through the transfer of nutrients and secondary chemicals among different litter species. This movement of materials can simply be caused by leaching (Fyles and Fyles 1993; McArthur et al. 1994), and may also be mediated by the growth of fungi (McTiernan et al. 1997). Rainfall and soil moisture also play an important role in dryland decomposition (e.g., Wang et al. 2009; Yahdjian et al. 2006). The frequency of small rainfall events (less than 2 mm) accounted for more than 50 % of the total rainfall in this semi-arid steppe area over the 3 years of observation, and may induce little effect on N leaching from the top litter to the bottom litter. Under this situation, the rain water might rarely reach the bottom litter species, and might hardly produce sufficient moisture conditions for the decomposition of the bottom litter species. Wardle et al. (2003) found that a positive mixing effect was largely caused by low quality component litter, due to its contribution to enhancing the moisture status in the litter layer. Their study results, supported by others, also suggested that the changes in environmental conditions affected the progress of decomposition and litter-mixing effects (Gartner and Cardon 2006; Jonsson and Wardle 2008; Madritch and Cardinale 2007).
The mixing effect in this study could be affected by our specific litterbag design, as the top and bottom layers of the two species were separated by a polyethylene barrier with 0.2-mm mesh size. This separation could lead to difference in moisture content of the top and the bottom litter as the upper litter might receive more water than the bottom litter when the amount of rainfall was small. At the same time, this was a full factorial experimental design, i.e., Ag/Sk treatment, Sk/Ag treatment, and thorough mixing (Sk-Ag). The same negative mixing effect was detected in both Sk/Ag and Sk-Ag treatments. Therefore, the effect of the experimental design on the mixing effects may not be significant.
Our results showed that the litter placed on the top, which received more rainfall and solar radiation, decomposed much more quickly at the early period than the same litter species placed at the bottom. Berg and Laskowski (2006) also showed that early stage mass loss could be stimulated by climatic factors and the availability of nutrients such as N and P. Since there was no difference in chemical concentration between the two positions at the beginning of the decomposition, we concluded that the environmental factors might induce significant differences in mass loss between the two positions since the very beginning of the decomposition. In addition, the overall higher decomposition rate of the component species on the top could be mainly due to the faster mass loss at the early stage, as the effect of position seemed to level off at the later stage (Fig. 3 inset).
Nutrient dynamics during decomposition
Studies have shown a typical triphasic pattern for nutrient release during litter decomposition. However, not all the three phases will occur during decomposition. For example, only phase III will occur in the decomposition of litter species with high nutrient concentration (Berg and Laskowski 2006; Prescott 2005). Sometimes, only phases II and III occur during the decomposition of nutrient-poor litter species.
The increase in N concentrations of the remaining litter during decomposition was common in most previous studies, and this has generally been interpreted as microbial immobilization. In this study, we also found that there was a significant increase in N concentrations of both the Sk and the Ag litter (Fig. 4a, b). Fewer studies have shown that litter P concentrations also increase during litter decomposition, as most studies only focused on N. Results from this study indicated that P concentrations of the Sk litter also increased throughout the decomposition (Fig. 4c). However, there was not much net immobilization as the remaining P did not exceed the initial litter P content (Fig. 5d). According to some of the previous studies, the increase in P concentrations and percent remaining have also been interpreted as microbial immobilization, especially when the availability of P is high due to fertilization (Liu et al. 2006; McGroddy et al. 2004) or high P content in the co-existing litter (e.g., Ag in this study).
Our results showed that when Sk and Ag decomposed separately, the Ag litter generally released N and P over the 3-year decomposition. The Sk litter, however, released N and P at the early stage of decomposition, followed by an immobilization period, and then released nutrients again (Fig. 5a, b, d, e). This generally agreed with our second hypothesis. Both litter species showed similar N and P release patterns regardless of their positions in the mixtures. When they were mixed, N was continuously released throughout the whole decomposition period regardless of the mixing treatments, which was similar to the pattern of the individual Ag litter (Fig. 5b, c). The release pattern of P in the mixtures, on the other hand, was more similar to the pattern of individual Sk litter with short-term immobilization in the middle stage of decomposition (Fig. 5d, f). Therefore, the release of N from the mixtures during decomposition was primarily determined by the fast-decomposing Ag litter while the release of P was largely determined by the slow-decomposing Sk litter. In particular, when Ag litter was placed at the bottom of the litterbags, the decomposition of the Sk/Ag mixture was slowed down significantly, resulting in significant negative mixing effects on the N release. This also suggests that, when the litter layer of a natural community has large amounts of Sk over Ag litter, high N retention will likely occur.
Although P was immobilized in the individual Sk litter (Fig. 5d) and in the mixtures (Fig. 5f) at the middle stage of decomposition, the immobilization in the mixture was not as strong as in the Sk litter of the single decomposition treatment (Fig. 5d). This indicates that the presence of the Ag litter during the Sk litter decomposition alleviated P limitation for microbial needs. Sk-Ag, Ag/Sk, and Sk/Ag mixtures all had significant positive effects on P release (Table 2), although the mixing effects on mass loss were only found significantly negative for the Sk-Ag and the Sk/Ag mixtures, suggesting that the presence of Ag might stimulate P release of the Sk litter. This also partially confirmed our third hypothesis. Liu et al. (2006) found that the addition of P significantly accelerated the decomposition of Sk, indicating limitations of P in this area during litter decomposition. Therefore, mixing of different litter types may have different effects on the release of N and P. Litter mixing had different non-additive effects on N and P dynamics during litter decomposition, which was also found by Ball et al. (2009). This was possibly due to nutrient limitations or different capacities of N and P uptake by microbial communities (Hobbie and Vitousek 2000).
In this semiarid typical steppe ecosystem, litter fall is a gradual process at the end of the growing season. Different litter species may be layered randomly either on the top or at the bottom position to form different types of litter mixtures, and this was generally neglected in most studies on the decomposition of litter mixtures. Gartner and Cardon (2004) proposed that the potential for interaction among different litter species may be determined by how leaves are mixed in litterbags (i.e., whether the leaves are layered in the order of the leaf fall or are thoroughly mixed). The results from this study clearly showed that there were different mixing effects with different mixing treatments.
Conclusions
Based on a 3-year decomposition experiment, this study demonstrated that different mixing treatments of litter species had different mixing effects on the mass loss and the release of N. All three mixing treatments had positive effects on the release of P. We found that the release of N from the mixtures was primarily determined by the fast-decomposing Ag litter while the release of P was largely determined by the slow-decomposing Sk litter. The fast decomposition at the early stage more or less determines the overall rate of the mass loss. Based on these results, we think it is necessary to consider the litter placement in the litter layer when evaluating the decomposition of the mixtures and the cycling of nutrients. Our results also suggest that different mixing positions of litter species will result in different non-additive effects on the release of nutrients such as N and P, which may further increase the heterogeneity of the nutrient distribution pattern in the studied steppe ecosystem.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Austin AT, Vitousek PM (2000) Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J Ecol 88:129–138
Austin AT, Vivanco L (2006) Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–558
Ball BA, Bradford MA, Hunter MD (2009) Nitrogen and phosphorus release from mixed litter layers is lower than predicted from single species decay. Ecosystems 12:87–100
Barantal S, Roy J, Fromin N, Schimann H, Hättenschwiler S (2011) Long-term presence of tree species but not chemical diversity affect litter mixture effects on decomposition in a neotropical rainforest. Oecologia 167:241–252
Berg B, Ekbohm G (1991) Litter mass-loss rates and decomposition patterns in some needle and leaf litter types - long-term decomposition in a Scots pine forest. VII. Can J Bot 69:1449–1456
Berg B, Laskowski R (2006) Litter decomposition: A guide to carbon and nutrient turnover. Elsevier, San Diego, pp 102–183
Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE (2002) Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos 99:317–323
Daniel O, Anderson JM (1992) Microbial biomass and activity in contrasting soil materials after passage through the gut of the earthworm Lumbricus rubellus hoffmeister. Soil Biol Biochem 24:465–470
Dent DH, Bagchi R, Robinson D, Majalap-Lee N, Burslem DFRP (2006) Nutrient fluxes via litterfall and leaf litter decomposition vary across a gradient of soil nutrient supply in a lowland tropical rain forest. Plant Soil 288:197–215
Fyles JW, Fyles IH (1993) Interaction of Douglas-fir with red alder and salal foliage litter during decomposition. Can J For Res 23:358–361
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246
Gartner TB, Cardon ZG (2006) Site of leaf origin affects how mixed litter decomposes. Soil Biol Biochem 38:2307–2317
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol Evol 15:238–243
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition interrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH (2000) Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos 90:357–371
Hobbie SE, Vitousek PM (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Hobbie SE, Shevtsova A, Chapin FS (1999) Plant responses to species removal and experimental warming in Alaskan tussock tundra. Oikos 84:417–434
Hoorens B, Aerts R, Stroetenga M (2003) Does initial litter chemistry explain litter mixture effects on decomposition? Oecologia 137:578–586
Hoorens B, Coomes D, Aerts R (2010) Neighbour identity hardly affects litter-mixture effects on decomposition rates of New Zealand forest species. Oecologia 162:479–489
Jonsson M, Wardle DA (2008) Context dependency of litter-mixing effects on decomposition and nutrient release across a long-term chronosequence. Oikos 117:1674–1682
Liu P, Huang J, Han X, Sun OJ, Zhou Z (2006) Differential responses of litter decomposition to increased soil nutrients and water between two contrasting grassland plant species of Inner Mongolia, China. Appl Soil Ecol 34:266–275
Liu P, Huang J, Sun OJ, Han X (2010) Litter decomposition and nutrient release as affected by soil nitrogen availability and litter quality in a semiarid grassland ecosystem. Oecologia 162:771–780
Madritch MD, Cardinale BJ (2007) Impacts of tree species diversity on litter decomposition in northern temperate forests of Wisconsin, USA: a multi-site experiment along a latitudinal gradient. Plant Soil 292:147–159
McArthur JV, Aho JM, Rader RB, Mills GL (1994) Interspecific leaf interactions during decomposition in aquatic and floodplain ecosystems. J North Am Bentholl Soc 13:57–67
McGroddy ME, Silver WL, de Oliveira RC Jr (2004) The effect of phosphorus availability on decomposition dynamics in a seasonal lowland Amazonian forest. Ecosystems 7:172–179
McTiernan KB, Ineson P, Coward PA (1997) Respiration and nutrient release from tree leaf litter mixtures. Oikos 78:527–538
Moore TR, Bubier JL, Bledzki L (2007) Litter decomposition in temperate peatland ecosystems: the effect of substrate and site. Ecosystems 10:949–963
Nelson D, Sommers L (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour MA, Tabatabai MA, Johnston CT, Sumner ME (eds) Methods of soil analysis. Part 3, Chemical methods. Soil Science Society of America, American Society of Agronomy, Madison, pp 961–1010
Nilsson MC, Wardle DA, Dahlberg A (1999) Effects of plant litter species composition and diversity on the boreal forest plant-soil system. Oikos 86:16–26
Prescott C (2005) Decomposition and mineralization of nutrients from litter and humus. In: BassiriRad H (ed) Nutrient acquisition by plants. Springer, Berlin, pp 15–41
Swift M, Heal O, Anderson J (1979) Decomposition in terrestrial ecosystems. University of California Press, Los Angeles
Throop HL, Archer SR (2009) Resolving the dryland decomposition conundrum: Some new perspectives on potential drivers. In: Lüttge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany. Springer, Berlin, pp 171–194
Vivanco L, Austin AT (2006) Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia 150:97–107
Wang L, D’Odorico P, Manzoni S, Porporato A, Macko S (2009) Soil carbon and nitrogen dynamics in southern African savannas: the effect of vegetation-induced patch-scale heterogeneities and large scale rainfall gradients. Clim Change 94:63–76
Wardle DA, Bonner KI, Nicholson KS (1997) Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos 79:247–258
Wardle DA, Nilsson MC, Zackrisson O, Gallet C (2003) Determinants of litter mixing effects in a Swedish boreal forest. Soil Biol Biochem 35:827–835
Yahdjian L, Sala O, Austin A (2006) Differential controls of water input on litter decomposition and nitrogen dynamics in the Patagonian Steppe. Ecosystems 9:128–141
Yang H, Wu M, Liu W, Zhang Z, Zhang N, Wan S (2011) Community structure and composition in response to climate change in a temperate steppe. Glob Change Biol 17:452–465
Acknowledgements
The authors would like to thank Ang Li and Jianyang Xia for their constructive comments on an earlier version of this manuscript. We are also extremely grateful for two anonymous reviewers and the section editor Tim Moore for their constructive comments. This study was financially supported by the National Basic Research Program of China (2009CB421102) and the National Natural Science Foundation of China (41073056). We also thank the Duolun Restoration Ecology Research Station for permission to access the study site and for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim Moore.
Rights and permissions
About this article
Cite this article
Tan, Y., Chen, J., Yan, L. et al. Mass loss and nutrient dynamics during litter decomposition under three mixing treatments in a typical steppe in Inner Mongolia. Plant Soil 366, 107–118 (2013). https://doi.org/10.1007/s11104-012-1401-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1401-6