Abstract
Aims
To survey the occurrence of nodulated legumes in the arid and semi-arid areas of Western Rajasthan and to characterize their associated symbiotic bacteria.
Methods
Herbaceous annual species were excavated whole, while tree species were studied as seedlings in the field or as trap plants in pot experiments. Nodules were examined by microscopy to confirm their effectiveness and to determine their internal structure. Bacteria isolated from the nodules were authenticated on their original hosts and were identified on the basis of 16S rRNA sequencing. Phylogenetic trees were inferred using the neighbour-joining method.
Results
We studied 35 of more than 50 species of native legume reported from these areas. Legumes are drought escaping (annual species), drought tolerant perennials or trees possessing deep root systems and other adaptations to arid conditions. Nodulation was recorded in all members of the Papilionoideae and Mimosoideae, but only one species of Caesalpinioideae. Internal structure of nodules varied within these groups, especially with respect to the presence or absence of uninfected cells in the infected region. Full 16S rRNA gene sequencing revealed that the nodules harboured a range of nodulating bacteria belonging to the genera Sinorhizobium, Rhizobium and Bradyrhizobium, within which they formed separate sub clades.
Conclusions
This study extends the range of legumes known to grow and nodulate in semi-arid regions, and provides information about their endosymbionts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drought and salinity are generally considered by agronomists to be a problem for nodulated legume crops, and yet there are many wild legumes that grow in deserts, some of which are also saline and/or of high pH. With the current levels of desertification and climate change, such areas are extending rapidly, and are now estimated to occupy up to 45% of the earth’s land surface (Schimel 2010). Studies on nodulated legumes have been conducted in a number of arid and semi-arid areas of Africa, including Egypt, Ethiopia, Kenya, Senegal, Sudan and Tunisia (Zahran 2001; Zakhia et al. 2004; Wolde-meskel et al. 2004; Odee et al. 1997; Nick et al. 1999). Very drought and saline tolerant species of Prosopis have been studied in the Americas (Sprent 2009), as have Mimosa spp. in the semi-arid Caatinga biome of Brazil (dos Reis Junior et al. 2010). The plants studied in Africa were mainly either pasture species, or trees, especially African acacias. Some shrubby legumes such as Caragana spp have been studied in high altitude deserts in China (Hou et al. 2009). However, there are many other potentially nodulated legumes native to arid and semi-arid areas. Some of these have been cited in a review of nine major arid and semi-arid areas around the world (Sprent and Gehlot 2010). Diverse rhizobial genera have been isolated from 19 wild legume species growing in the Tunisian infra-arid zone in which rainfall does not exceed 180 mm (Zakhia et al. 2004). Native (endemic) legumes are widely distributed throughout India (Sanjappa 1992), including the arid and semi-arid regions of the Indian Thar Desert (Bhandari 1990). This desert, the 18th largest in the world, is 200,000 km2 in area and extends from India into Pakistan. It contains a number of legume genera not previously studied for their in situ nodulating ability or the identity of the bacteria nodulating them. The present study aims firstly to survey nodulation in these legumes, secondly to characterise the nodules formed, and thirdly to identify the rhizobia that nodulate them.
Material and methods
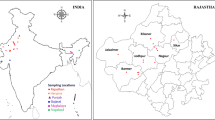
Studies were carried out in several districts of the state of Rajasthan. Figure 1 gives a general map of the area, together with a more detailed map of the Jodhpur district and surroundings. The current work was undertaken at the marked sites (Table 1 gives details). Overall the area is alkaline, being based on limestone. The soil in arid zone regions of Western Rajasthan has been classified as ‘desert soil’ (Ray Choudhury et al. 1963); this comes under aridisol in the USDA soil classification system (Dhir 1977). Compared with the more arid and sometimes acid desert areas in the Southern hemisphere, the soils are relatively rich in plant nutrients, including potassium and phosphorus, although much of the latter is not in a plant available form. Minimum monthly average temperatures vary from 10 to 29°C and maxima from 26 to 43°C. Rainfall varies greatly from year to year, in dry years being less than 300 mm and in wet years over 400 mm. Most of it falls in the monsoon season, between July and August. In the dry season, relative humidity is very low (around 40%), but rises to over 80% in the monsoon period. Light winds (5–10 km h−1) occur in the summer.
Determination of soil properties
Soil properties were estimated by standard methods as follows: pH (aqueous extract) and cation exchange capacity (CEC) according to Jackson (1973); electrical conductivity (EC) according to Richards (1954), organic carbon by wet digestion (Walkley and Black 1934), and available phosphorus and potassium by the methods of Olsen et al. (1954) and Pratt (1982), respectively.
Sampling methods
Field studies were carried out during the monsoon and post monsoon season (July–August) in years 2007, 2008 and 2009. Native legumes were identified using Flora of the Indian Desert (Bhandari 1990). The general classification of legumes follows Lewis et al. (2005) and the taxonomy of the Indian species is from Bhandari (1990) and Kumar and Sane (2003). Whole plants of the smaller species were excavated. Nodulated roots of 10–20 plants of each species were kept moist and brought to the laboratory for counting of nodules, making photographic records, preparation for microscopy and isolation of rhizobia. For mature tree species it was not possible to see nodules in the field because of their deep root systems. Nodulation for these was studied in seedlings growing in the field as well as in trap seedlings grown in pots with soil taken from sites where adult plants were growing. Seeds of all species were grown in pots in order to record stages of nodule development. After thorough washing, nodules were photographed under low magnification. A selection of nodules was halved and examined for pink internal colouration, indicative of leghaemoglobin and presumed nitrogenase activity (dos Reis Junior et al. 2010). Healthy nodules were halved and prepared for microscopy according to James et al. (2001). Herbarium specimens of all sampled plants were lodged in the Department of Botany, J.N. Vyas University, Jodhpur, and samples of seeds were deposited at the regional station of the National Bureau of Plant Genetic Resources, India at the Central Arid Zone Research Institute, Jodhpur. Nodulation records were checked against the database http://www.ars-grin.gov/~sbmljw/cgi-bin/taxnodul.pl and against our own records, updated from Sprent (2009).
Isolation of bacteria and cultural conditions
Bacterial strains were isolated from root nodules after surface sterilization by immersion in 0.1% (w/v) sodium hypochlorite for 5–7 min followed by immersion in 70% ethanol for one minute. After washing six times in sterile water the nodules were then crushed in one drop of sterile water and the suspension was streaked on yeast extract mannitol agar (YEMA). Bacterial colonies were grown on YEMA and on tryptone yeast (TY) agar at 28°C for 3–8 days according to the methods of Vincent (1970) and Somasegaran and Hoben (1994). They were routinely checked for their purity by repetitive streaking and Gram staining.
Authentication on original host
Isolates were tested for nodulation on their original hosts in glasshouse pot experiments as described by Yates et al. (2004). After six weeks the host plants were harvested to record nodulation. Rhizobia were reisolated from nodules and confirmed to be the inoculant strain by PCR fingerprinting using RPO1 primers (Richardson et al. 1995).
Molecular fingerprinting
Bacterial cells were used as a source of DNA for templates in all PCR reactions. Cells from a freshly grown culture in 1/2 Lupin Agar (or LA, which is described as Medium 1 in Howieson et al. 1988 and Yates et al. 2007) were concentrated to an OD600 of 6.0 in 0.89% (w/v) saline for molecular fingerprinting. PCR reaction volumes of 20 μL contained 1 μL of concentrated cells and 2.5 Units of TAQ-1 DNA polymerase (Biotech International, Perth, Australia), 1 μM of RPO1 primer, 1.5 mM MgCl2, 5X PCR Polymerisation buffer (PB-1, Biotech International, Perth, Australia).
All the primers used in this study are listed in Table 2. The reaction mixture for amplification with the RPO1 primer (Richardson et al. 1995) was held at 94°C for 5 min followed by 5 cycles at 94°C for 30 s, 50°C for 20 s, 72°C for 90 s and then 30 cycles at 94°C for 30 s, 55°C for 20 s and 72°C for 90 s and a final extension at 72°C for 5 min. A Bio-Rad Sub-Cell GT Agarose gel electrophoresis system was used to analyse the band pattern of the amplified products. Electrophoresis using a 1.5% (w/v) agarose gel prepared in TAE buffer (40 mM Tris acetate, 2 mM EDTA, pH 8.0) was carried out at 80 V for 3 h. The molecular weight marker used was 100 bp DNA Ladder (Cat. No. G5711, Promega). Gels were pre-stained by incorporating Sybr Safe (Invitrogen) (8 μL/100 ml) in molten agarose. The gels were visualised using a UV transilluminator and a digital image of the gel was captured using GEL-DOC 2000 (BioRad, USA).
16S rRNA gene sequencing and bacterial identification
Cells from a freshly grown culture in ½LA were concentrated to an OD600 of 2.0 in 0.89% (w/v) saline solutions. The intragenic fragments of 1475 bp from the 16S rRNA gene were amplified using the primers 18 F and 1492R. The PCR reaction mixture was held at 94°C for 5 min followed by 35 cycles of 94°C for 30 s, 53°C for 30 s, 72°C for 60 s and a final extension at 72°C for 5 min. The amplified gene products were purified using the QIAquick™ PCR purification kit (QIAGEN, Melbourne, Australia). The primers 18F, 800F, 820R and 1492R were used for sequencing of the amplified DNA. 4 μL of purified amplified template was added to 4 μL of Big-Dye (version 3.1) with the relevant primer. All DNA sequencing was carried out as described by the manufacturer (Applied Biosystems, Melbourne, Australia) by using a dye terminator cycle sequencing ready reaction kit and automated sequencer (ABI Model 377A).
Sequence contigs were assembled using the Geneious software (www.geneious.com). Databases at the National Centre for Biotechnology Information (NCBI) were searched using BLASTN (Altschul et al. 1990) for similar DNA sequences. All primers were synthesized by GeneWorks (Adelaide, Australia).
atpD gene amplification and sequencing
The housekeeping atpD gene which codes for ATP synthase subunit β of size 500 bp was amplified using the primers atpD-294 F and atpD-771 described by Gaunt et al. (2001). 5μL of lysed cell suspension was added to a 50 μL PCR mixture (1.5 mM MgCl2, 10 pmol each primer, 1 U Taq DNA polymerase and 5X PCR Polymerization buffer) and incubated at 94°C for 5 min followed by 30 rounds of thermal cycling (94°C for 45 s, 55°C for 60 s and 72°C for 90 s) and final extension at 72°C for 5 min. The amplified product was confirmed by electrophoresis using 4 μL products on a 1% agarose gel at 80 V for 2 h. 100 bp DNA Ladder (Promega) was used to estimate the length of the amplification products. The purified DNA was sequenced as described above in for the 16S rRNA gene.
Phylogenetical analysis
Sequences of type strains and/or NCBI reference sequences (NR) were included in the analyses. The GenBank accession numbers are listed in parentheses for the 16S rRNA and atpD genes. To perform molecular phylogenetic analyses, reference sequences required for comparison were downloaded from the NCBI . All the sequences of 16S rRNA and atpD were aligned using the multiple sequence alignment program CLUSTAL W (Thompson et al. 1997) and were saved as molecular evolutionary genetics analysis (MEGA) format in software MEGA v5.1 (Tamura et al. 2011). The pair wise evolutionary distances were computed using the Kimura 2-parameter model (Kimura 1980). Phylogenetic trees were inferred using the neighbour-joining method (Saitou and Nei 1987). To obtain the confidence values, the original data set was resampled 1000 times using the bootstrap analysis method. The MEGA 5.1.0 package (Tamura et al. 2011) was used for construction of phylogenetic trees, inferring distances and percent similarity.
Results
Nodulation
Nodulation of 35 native species recorded at the different sites is given in Table 3. Of the four caesalpinioid legumes found, nodules were only seen on Chamaecrista pumila. Nodules were absent in all investigated species of Cassia or Senna. All mimosoid species found are from genera known to nodulate and all were nodulated in our study sites. Nodulation of Acacia jacquemontii is a new report. Similarly, all papilionoid species found are from known nodulating genera and all were found to nodulate in the present work. Two species of Rhynchosia, were found, one of which, R. aurea, is a new report of nodulation. Seven species of the large pan-tropical genus Indigofera were recorded, as well as the related Cyamopsis tetragonoloba, a very important food legume also known as clusterbean. Tephrosia is another large pan-tropical genus well represented here, and T. falciformis is a new report of nodulation.
The type genus of tribe Crotalarieae contains a large number of species, many of which have xerophytic characters, as in the two species found here. The woodiest papilionoid species studied is the valuable timber tree Dalbergia sissoo. This species is not particularly well adapted to dry conditions and may be at the limit of its habitat range, although it is often listed as drought tolerant. Two species of tribe Trifolieae, Melilotus indicus and Trigonella corniculata (synonym T. balansae Boiss. & Reut.), are also on the borderlines of their normal habitat. Taverniera is a small genus in tribe Hedysareae that is largely restricted to drier areas and deserts. Table 4 lists the major morphological features associated with drought tolerance found in the present study and Fig. 2 illustrates some of these.
Plants from the Thar desert, illustrating xerophytic characters; (a) Acacia jacquemontii, a small drought-tolerant tree which has large spines and sheds its leaves in the dry season (b) Alysicarpus vaginalis, a drought-escaping annual which has a prostrate habit and a short life cycle (2–3 months); (c) Crotalaria burhia, a drought-tolerant shrub which has thick multibranched photosynthetic stems, and small leaves (arrows) that become thick and scaly under dry conditions; (d) Indigofera sessiliflora, a drought-escaping annual with hairy leaves (arrows) and a short life cycle (2–3 months); (e) Tephrosia villosa, a drought-tolerant shrub with hairy subcoriaceous leaves, and profusely hairy pods (arrow). See Table 3 for further details about these species and other nodulated legumes native to the Thar Desert
The number of nodules per plant varied in the field depending on growing conditions, the sample site and type of plant species (herbaceous annual, perennial or trees). However, during authentication experiments in pots, host plants inoculated with their respective isolated microsymbionts had between 20 and 35 nodules per plant, depending on host species and inoculant strains (data not shown).
Nodule morphology and anatomy
Nodule morphology is shown in Fig. 3. Nodules with determinate growth and surface lenticels were found in Alysicarpus (not shown) and Rhynchosia (Fig. 3a). Except for Dalbergia sissoo, which has aeschynomenoid nodules (not shown, but see Sprent 2009), a type with a form of determinate growth that is associated with emergence of lateral roots, all other species had nodules that showed indeterminate growth (Fig. 3b–f), although they varied greatly in the degree of branching. Nodules on species of Acacia are usually branched (Sprent 2001), unlike the example shown here. Indigofera nodules are particularly interesting as they appeared classically determinate at early stages and then became elongated, but retained the lenticels typical of other determinate nodules (Fig. 3f). This is the first report illustrating this type of nodule, although it has also been found in South African species of Indigofera (I. Law, pers. comm.; Sprent, unpublished data). The surfaces of all nodules were dark in colour, due to drying soil. This has been shown in other nodules from dry areas to arise by production of bark from a cortical phellogen (Fig. 3a & d), which also produces aerating tissue in the wet season (see for example Fig. 3.5 in Sprent 2001).
Morphology of root nodules of (a) Rhynchosia aurea; (b) Chamaecrista pumila; (c) Crotalaria burhia; (d) Tephrosia leptostachya; (e) Acacia jacquemontii; (e); (f) Indigofera linifolia. All the nodules are indeterminate, except for those on R. aurea (a). Note the bark (*) on (a) and (d) that has arisen from a cortical phellogen, and the pronounced lenticels (arrow) on (f). Bars = 5 mm
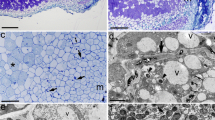
All nodules collected were red in internal colour and therefore assumed to be active in fixing nitrogen. This was confirmed by immuno-labelling of NifH protein in bacteroids (data not shown here, but similar to that shown in other work from our laboratory e.g. dos Reis Junior et al. 2010). The general structure of nodules for species of Chamaecrista, Acacia, Rhynchosia and Mimosa was similar to previous descriptions given by Naisbitt et al. (1992), Räsänen et al. (2001), Garau et al. (2009) and dos Reis Junior et al. (2010), respectively. Here we illustrate the nodule structure of three genera, in particular the species Crotalaria burhia, Indigofera linnaei and Tephrosia wallichii (Fig. 4). All have a distinct apical meristem, an inner cortex containing vascular bundles and an outer cortex with some indications of phellogen activity (Fig. 4b, d, f). Where they differ is in the region behind the apex, where cells become infected with rhizobia and in the arrangement of the cells in the active nitrogen fixing tissues. In C. burhia (Fig. 4a) no uninfected (interstitial) cells are present in the infected tissue, whereas in I. linnaei (Fig. 4c) and T. wallichii (Fig. 4e) the latter consists of a mixture of infected and uninfected cells. The absence of interstitial cells appears to be a characteristic of nodules from tribe Crotalarieae (Sprent 2007). Thus, the nodules of C. burhia and Ch. pumila that may appear similar morphologically (Fig. 3a, b) are internally quite different, as the Chamaecrista nodules have interstitial cells (not shown, but see Naisbitt et al. 1992).
Light microscopy of longitudinal sections of nodules of Crotalaria burhia (a, b); Indigofera linnaei (c, d) and Tephrosia wallichii (e, f); (a, c, e) illustrate for each species details of the nodule meristem (m) and the nitrogen-fixing infected zone (iz), whereas (b, d, e) are higher magnification views of the cortex, including vascular bundles (arrows). Asterisks mark uninfected cells in the infected zone in (c – f). Bars = 100 μm (a, c, e) and 30 μm (b, d, f)
Bacteria isolated from nodules and their identification on the basis of 16S rRNA sequences
A collection of 70 rhizobial isolates was obtained from the five genera and 8 nodulated species of legumes. Of these we focussed on isolates from Mimosa hamata, as the genus Mimosa is known to have the betaproteobacterial genus Burkholderia as its major symbiont in Brazil (dos Reis Junior et al. 2010), on Prosopis cineraria, because of the world wide occurrence of this genus in arid areas (Sprent 2009), on species of Tephrosia, as they are the first to resume growth after monsoonal rains, and on Rhynchosia aurea and Acacia jacquemontii, as both are new reports of nodulation. In addition, R. aurea is of interest, as it is from a genus that is also known to nodulate with Burkholderia (Garau et al. 2009). Selected isolates from the aforementioned plants were sequenced for their 16S rRNA genes based on groups obtained by RPO1 fingerprints (RAPDs patterns). The selected isolates were initially identified through BLASTN searches using their partial (>500 bp) 16S rRNA gene sequences, and then a nearly full length fragment (>1300 bp) of the 16S rRNA gene was amplified and sequenced for sixteen selected isolates (Table 5). All nodulating isolates were from the Alphaproteobacteria, and they included species of Sinorhizobium, Bradyrhizobium and Rhizobium, with Sinorhizobium spp. being particularly common (Table 5). In the case of strains identified as Bradyrhizobium on the basis of their 16S rRNA sequences, another housekeeping gene, atpD, was also sequenced in order to more precisely determine their phylogenetic relationships (Table S1). All rhizobial isolates were authenticated on their original hosts.
Sequence analysis and phylogeny
The sequences of the 16 selected strains were aligned and compared with 16S rRNA and atpD gene sequences of rhizobial type strains available in the GenBank database. Dendrograms showing the phylogenetic relationships of the unclassified Thar desert rhizobia with those of previously designated species of rhizobia are shown in Figs. 5a, b and S1. On the basis of 16S rRNA gene sequences our results indicate that strains from Mimosa hamata (JNVU MH3a & JNVU MH8) and Acacia jacquemontii (JNVU AJ10) were 99.8% similar to Sinorhizobium sp. ORS 1085 (AJ295078) from nodules of Acacia tortilis ssp. raddiana growing in Sahara, Africa (Ba et al. 2002). These three strains showed a similarity to the type strain of S. saheli LMG 7837 (NR_026096) between 99.7 to 99.8%, but formed a separate group from the latter. Strains from species of Tephrosia, such as T. wallichii (JNVU TW8 & JNVU TW10), T. falciformis (JNVU TF7) and T. villosa (JNVU TV3), as well as those from Prosopis cineraria (JNVU PC2) and Rhynchosia aurea (JNVU RA9) were very close (with 99.7% similarity) to Sinorhizobium sp. ORS 3178 (AY875975), which was isolated from Acacia nilotica in Senegal. Although these strains had 99.2 to 99.6% similarity with sequences of the type strains of S. terangae LMG 7834 (NR_044842) and S. saheli LMG 7837 (NR_026096), respectively, they formed two lineages in a subclade together with S. terangae strain LMG 7834 and S. saheli strain LMG 7837 (Fig. 5a). On the other hand, strains from T. purpurea (JNVU TP13 & JNVU TP18) formed separate lineages from the other Tephrosia sinorhizobia, and had 99.7% similarity with S. terangae PHABA-2 (GU784793) isolated from Phaseolus vulgaris in Cuba, and 99.3 and 99.6% similarity with the S. terangae (NR_044842) and S. saheli (NR_026096) type strains, respectively. In addition, isolate JNVU TV1 from T. villosa showed 99.7% similarity with Sinorhizobium sp. ORS 3180 (AY875976) isolated from Acacia nilotica in Senegal and with the type strain of S. terangae (NR_044842), forming yet another separate lineage in the same (S. terangae) clade. In summary, nine of the 12 Sinorhizobium strains isolated and described during this study formed four lineages in a subclade together with the S. terangae type strain, whereas the remaining three strains (all from M. hamata and A. jacquemontii) formed another lineage in a subclade of S. saheli (Fig. 5a).
Neighbour-joining phylogenies for 16S rRNA gene sequences of rhizobia isolated from legumes in the Thar Desert together with those of reference strains. The trees were generated using MEGA version 5.0 with default parameters, K2P distance model and the Neighbour-Joining algorithm. Bootstrap values calculated for 1,000 replications are indicated. The scale bars indicate 1% substitutions per site. Accession numbers from GenBank are in parenthesis. B., Bradyrhizobium; R., Rhizobium; S., Sinorhizobium; E., Ensifer; M., Mesorhizobium; T, type strain and (NR), NCBI Reference sequence. (a) Sinorhizobium strains; (b) Rhizobium and Bradyrhizobium strains. The tree is rooted using the sequence from Rhodobacter capsulatus
Of the four non-sinorhizobial symbionts isolated during this study (Table 5), isolate JNVU TP3 from T. purpurea was identified as a Rhizobium strain with 99.2% similarity to Rhizobium sp. ORS 3441 (EU584258) isolated from Acacia senegal in Senegal (Fall et al. 2008), and 99.1% similarity with the type strain R. mesosinicum CCBAU 25010 (NR_043548) forming a separate subclade (Fig. 5b). The remaining three strains, all from Tephrosia spp., were identified as bradyrhizobia. Strains JNVU TF17 from T. falciformis and JNVU TV4 from T. villosa showed 99.7 and 99.2% similarity respectively with the B. yuanmingense type strain B071 (NR_028768). Strains JNVU TF17 and JNVU TV4 showed 99.9 and 99.5% similarity respectively with B. yuanmingense strain M11 (AB601666) isolated from Vigna radiata in Nepal, but had separate lineages within this clade (Fig. 5b). Isolate JNVU TV11 was also identified as a species of Bradyrhizobium with 99.5% similarity to Bradyrhizobium sp. strain M16 (AB601671), which was isolated from V. radiata in Nepal, and 99.2% similarity to the B. iriomotense type strain EK05 (AB300992), although it formed a separate subclade within B. iriomotense (Fig. 5b). Two of the bradyrhizobial isolates from Tephrosia (JNVU TV4 & JNVU TV11) were selected on the basis of their locations within separate 16S rRNA groups (Fig. 5b) for further sequencing of their atpD housekeeping gene in order to more clearly define their phylogenetic positions within the genus Bradyrhizobium (Table S1). Phylogenetic analysis of the atpD gene showed that isolate JNVU TV4 had 99.3% similarity with the type strain of B. yuanmingense LMG 21827 (FM253140) isolated from Lespedeza cuneata in China, whereas the other isolate, JNVU TV11, had 95.2% similarity to B. yuanmingense CCBAU 33112 (FJ418711) isolated from V. angularis in China, and 95.6% similarity to the type strain of B. yuanmingense (LMG 21827) (Fig. S1).
Discussion
Nodulation of native legumes of the Thar desert
Plants have a number of ways in which they adapt to dry conditions, basically by tolerating or avoiding drought. Legumes are no different in this respect (Fig. 2, Table 4). Comparison of our data with the known occurrence of potentially nodulating genera in other alkaline desert soils shows that the same genera are often found. For example in dry areas of Madagascar (Du Puy et al. 2002) and Soqotra, an island off the Yemeni archipelago (Miller and Morris 2004), the genera Acacia (s.l.), Crotalaria, Indigofera and Tephrosia are all well represented. They are also common in arid areas of Southern Africa, in soils with a range of pH values (Jürgens et al. 2010). It is now thought that legumes evolved in seasonally dry areas bounding the Tethys Sea about 55 Mya (Schrire et al. 2005). From here they extended into other biomes, including arid ones, and evidence suggests that these processes are continuing (Crisp et al. 2004). Thus it is not surprising that legumes are found in deserts today. What is surprising is that their nodulation characters (rather than their taxonomic ones) have frequently been ignored. We found all known types of nodule morphology, plus an additional intermediate determinate/indeterminate form (in Indigofera). Nodule structure also showed the known range of variation, and new observations on Tephrosia, Crotalaria and Indigofera confirm the proposed taxonomic and evolutionary significance of these characters (Sprent 2007). There appears to be no particular advantage of a specific type of nodule in desert environments, even though indeterminate nodules have the capacity to re-grow after adverse conditions, whereas determinate nodules normally have to be replaced (Sprent 2001).
On the other hand, the nodules in our study did show some morphological and structural adaptations to their semi-arid environment, such as a covering of bark on the determinate nodules of R. aurea and the indeterminate nodules of T. leptostachya; this arises from a corky phellogen. Indeed, in their study of endemic Mimosa spp. in the seasonally dry Cerrado and Caatinga regions of Brazil, dos Reis Junior et al. (2010) also observed a thickened cortex in the nodules on woody perennial species that were subjected to prolonged periods of drought, and hypothesised that such a “corky” layer would help the nodules to retain moisture. Interestingly, some of the nodules, such as those on Indigofera linifolia also displayed adaptations to inundation, such as lenticels (James et al. 2001). This highlights the highly seasonal nature of precipitation in such environments, which necessitates adaptations to both drought and flooding.
Thar desert legumes are predominantly nodulated by Sinorhizobium (Ensifer) strains
As with other recent studies from natural arid and semi-arid environments (Nick et al. 1999; Odee et al. 1997; Wolde-meskel et al. 2004; Zahran 2001; Zakhia et al. 2004, 2006; Romdhane et al. 2006), we found a wide range of bacteria to be present inside nodules, but with a particularly high incidence of Sinorhizobium (Ensifer) strains. Interestingly, the type of rhizobia associated with some of the native legumes of the Indian Thar desert are similar to those found in leguminous trees in seasonally dry regions of Sudan and Kenya (Nick et al. 1999). On the basis of 16S rRNA gene sequencing, Nick et al. (1999) reported that 25 Sudanese and 5 Kenyan strains isolated from root nodules of Acacia senegal and Prosopis chilensis showed similarity with Sinorhizobium saheli and Sinorhizobium terangae, but were sufficiently different from these established species to be included in two new species, Sinorhizobium arboris and S. kostiense. Similarly, Benata et al. (2008) isolated a diverse range of bacteria from root nodules of Prosopis juliflora growing in two arid soils in eastern Morocco that again were dominated by sinorhizobia, including strains of S. kummerowiae and S. fredii (although strains of R. multihospitium, R. tropici and R. giardinii were also isolated). Furthermore, Deng et al. (2011) have reported diverse rhizobia belonging to eight putative species in the genera Mesorhizobium, Rhizobium and Sinorhizobium to be nodulating a single legume species, Sphaerophysa salsula, from two regions of the semi-arid Loess Plateau of China.
On the other hand, the aforementioned studies, as well as the present one, contrast with an extensive study of legumes from dry areas of Senegal by Doignon-Bourcier et al. (2000) which found that species in many of the genera that we have studied in India, such as Chamaecrista, Crotalaria, Indigofera, Rhynchosia and Tephrosia, were exclusively nodulated by strains of Bradyrhizobium. In addition although the Rhynchosia spp. in our study were nodulated by Sinorhizobium spp., a recent study on a South African species of Rhynchosia has shown that it is nodulated by Burkholderia (Garau et al. 2009). This difference might be explained by the fact that unlike those in our work, the species (R. ferulifolia) examined by Garau et al. (2009) was native to acid soils which could favour the growth of nodulating Burkholderia (dos Reis Junior et al. 2010). This is also likely be the case with the native Indian Mimosa species, M. hamata, which is nodulated by Sinorhizobium rather than by Burkholderia strains. Mimosas in their native environments are commonly nodulated by Burkholderia, as was recently shown in a study of 70 Mimosa species growing in acidic soils in central Brazil (dos Reis Junior et al. 2010). Thus it is likely that local soil factors play an important role in defining the bacteria nodulating indigenous legumes.
Concluding remarks
From the results of the current investigation it appears that the native rhizobia of arid regions of the Indian Thar desert belong to both fast- and slow-growing rhizobial species, but also that the legumes may have a particular preference for Sinorhizobium. Indeed, the fast growing sinorhizobia characterized in this study are phylogenetically close to those reported from semi-arid regions of African countries (Sudan, Kenya, Tunisia, Morroco, Senegal). Interestingly, as with the African sinorhizobia from semi-arid regions, the Thar desert sinorhizobia reported here appear to have evolved to be promiscuous symbionts, as they can nodulate several native legumes (6 species) as well as crop legumes, such as Vigna radiata, V. aconitifolia and V. unguiculata (data not presented here). Our data further suggest that our strains may constitute two to three new species of Sinorhizobium, and possibly also a new species each of Rhizobium and Bradyrhizobium. However a polyphasic approach, including DNA-DNA hybridization and GC% analyses, is needed to confirm this.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Ba S, Willems A, de Lajudie P, Roche P, Jeder H, Quatrini P, Neyra M, Ferro M, Promé JC, Gillis M, Boivin-Masson C, Lorquin J (2002) Symbiotic and taxonomic diversity of rhizobia isolated from Acacia tortilis ssp. raddiana in Africa. Syst Appl Microbiol 25:130–145
Benata H, Mohammed O, Noureddine B, Abdelbasset B, Abdelmoumen H, Muresu R, Squartini A, El Idrissi MM (2008) Diversity of bacteria that nodulate Prosopis juliflora in the eastern area of Morocco. Syst Appl Microbiol 31:378–386
Bhandari MM (1990) Flora of the Indian desert. MPS Repros, Jodhpur
Crisp M, Cook L, Steane D (2004) Radiation of the Australian flora: what can comparisons of molecular phylogenies across multiple taxa tell us about the evolution of diversity across multiple plant lineages. Phil Trans Roy Soc London B 359:1551–1171
Deng ZS, Zhao LF, Kong ZY, Yang WQ, Lindstrom K, Wang ET, Wei GH (2011) Diversity of endophytic bacteria within nodules of the Sphaerophysa salsula in different regions of Loess Plateau in China. FEMS Microbiol Ecol 76:463–475
Dhir RP (1977) Western Rajasthan soils, their characteristics and properties. In desertification and its control, ICAR 102–115
Doignon-Bourcier F, Williams A, Coopman R, Laguerre G, Gillis M, de Lajudie P (2000) Genotypic characterization of Bradyrhizobium strains nodulating small Senegalese legumes by 16S-23S rRNA intergenic gene spacers and amplified fragment length polymorphism fingerprinting analyses. Appl Environ Microbiol 66:3987–3997
dos Reis FB Jr, Simon MF, Gross E, Boddey RM, Elliott GN, Neto NE, Loureiro Mde F, de Queiroz LP, Scotti MR, Chen W-M, Noren A, Rubio MC, de Faria SM, Bontemps C, Goi SR, Young JPW, Sprent JI, James EK (2010) Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytol 186:934–946
Du Puy DJ, Labat VN, Rabevohitia R, Villiers J-F, Bosser V, Moat J (2002) The Leguminosae of Madagascar. Royal Botanic Gardens, Kew
Fall D, Diouf D, Ourarhi M, Faye A, Abdelmounen H, Neyra M, Sylla SN, El Idrissi MM (2008) Phenotypic and genotypic characteristics of Acacia senegal (L.) Willd. root-nodulating bacteria isolated from soils in the dryland part of Senegal. Lett Appl Microbiol 47:85–97
Garau G, Yates RJ, Deiana P, Howieson JG (2009) Novel strains of nodulating Burkholderia have a role in nitrogen fixation with herbaceous legumes adapted to acid, infertile soils. Soil Biol Biochem 41:125–135
Gaunt MW, Turner SL, Rigottier-Gois L, Lloyd-Macgilp SA, Young JPW (2001) Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int J Syst Evol Microbiol 51:2037–2048
Hou BH, Wang ET, Li Y, Jia RZ, Chen WF, Man CX, Sui XH, Chen WX (2009) Rhizobial resource associated with epidemic legumes in Tibet. Microb Ecol 57:69–81
Howieson J, Ewing M, D’Antuono M (1988) Selection for acid tolerance in Rhizobium meliloti. Plant Soil 105:179–188
Jackson ML (1973) Soil chemical analysis. Prentice Hall of India Pvt Ltd, New Delhi
James EK, Loureiro M de F, Pott A, Pott VJ, Martins M, Franco AA, Sprent JI (2001) Flooding tolerant legume symbioses from the Brazilian Pantanal. New Phytol 150:733–738
Jürgens N, Schmiedel U, Hoffman MT (2010) Biodiveristy in Southern Africa, vol 1. Klaus Hess, Hamburg
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kumar S, Sane PV (2003) Legumes of South Asia: a check list. Royal Botanic Gardens, Kew
Lewis G, Schrire B, Mackinder B, Lock M (2005) Legumes of the world. Royal Botanic Gardens, Kew
Miller AG, Morris M (2004) Ethnoflora of the Soqotra archipelago. Royal Botanic Gardens, Edinburgh
Naisbitt T, James EK, Sprent JI (1992) The evolutionary significance of the genus Chamaecrista as determined by nodule structure. New Phytol 122:187–192
Nick G, de Lajudie P, Eardly BD, Soumalainen S, Paulin L, Zhang X, Gillis M, Lindström K (1999) Sinorhizobium arboris sp. nov. and Sinorhizobium kostiense sp. nov., isolated from leguminous trees in Sudan and Kenya. Int J Syst Bacteriol 49:1359–1368
Odee DW, Sutherland JM, Makatiani ET, McInroy SG, Sprent JI (1997) Phenotypic characterization and composition of rhizobia associated with woody legumes growing in diverse Kenyan conditions. Plant Soil 188:65–75
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. US department of Agriculture, Circular, pp 939
Pratt PF (1982) Potassium. In: Page AL, Miller RH, Keeny DR (eds) Methods of Soil Analysis Part II chemical and microbiological properties. Madison, Wisconsin, USA, pp 225–246
Räsänen IA, Sprent JI, Lindström K (2001) Symbiotic properties of sinorhizobia from Acacia and Prosopis nodules in Sudan and Senegal. Plant Soil 235:193–210
Ray Choudhury SP, Agarwal RK, Gupta NK, Thomas PK (1963) Soils of India. ICAR, New Delhi, p 495
Richards LA (1954) Diagnosis and improvements of saline and alkali soils. Agricultural Handbook No. 60, USDA, Washington, D. C.
Richardson AE, Viccars LA, Watson JM, Gibson AH (1995) Differentiation of Rhizobium strains using the polymerase chain reaction with random and directed primers. Soil Biol Biochem 27:515–524
Romdhane SB, Nasr H, Samba-Mbaye R, Neyra M, Ghorbal MH, De Lajudie P (2006) Genetic diversity of Acacia tortilis ssp. raddiana rhizobia in Tunisia assessed by 16S and 16S-23S rDNA genes analysis. J Appl Microbiol 100:436–445
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sanjappa M (1992) Legumes of India. Bisan Singh Mahendra Pal Singh, Dehra Dun
Schimel DS (2010) Dry lands in the earth system. Science 327:418–419
Schrire BD, Lewis GP, Lavin M (2005) Biogeography of the Leguminosae. In: Lewis G, Schrire B, Mackinder B, Lock M (eds) Legumes of the world. Royal Botanic Gardens, Kew, pp 21–54
Somasegaran PS, Hoben HJ (1994) Handbook for Rhizobia. Springer, New York
Sprent JI (2001) Nodulation in legumes. Royal Botanic Gardens, Kew
Sprent JI (2007) Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol 175:11–25
Sprent JI (2009) Legume nodulation: a global perspective. Wiley-Blackwell, Oxford
Sprent JI, Gehlot HS (2010) Nodulated legumes in arid and semi-arid environments: are they important? Plant Ecol Divers 3:211–219
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Vincent JM (1970) A manual for the practical study of root nodule bacteria. Blackwell Scientific, Oxford
Walkley A, Black CA (1934) An examination of Degtijareff methods for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Wolde-meskel E, Berg T, Peters NK, Frostegård A (2004) Nodulation status of native woody legumes and phenotypic characteristics of associated rhizobia in soils of southern Ethiopia. Biol Fertil Soils 40:55–66
Yanagi M, Yamasato K (1993) Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett 107:115–120
Yates RJ, Howieson JG, Nandasena KG, O’Hara GW (2004) Root-nodule bacteria from indigenous legumes in the north-west of Western Australia and their interaction with exotic legumes. Soil Biol Biochem 36:1319–1329
Yates RJ, Howieson JG, Reeve WG, Nandasena KG, Law IJ, Brau L, Ardley JK, Nistelberger HM, Real D, O’Hara GW (2007) Lotononis angolensis forms nitrogen fixing, lupinoid nodules with phylogenetically unique, fast growing, pink-pigmented bacteria, which do not nodulate L. bainesii or L. listii. Soil Biol Biochem 39:1680–1688
Zahran HH (2001) Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J Biotechnol 91:43–53
Zakhia F, Jederz H, Domerguei O, Willems A, Cleyet-Mareh J-C, Gillis M, Dreyfus B, de Lajudie P (2004) Characterisation of wild legume nodulating bacteria (LNB) in the infra-arid zone of Tunisia. Syst Appl Microbiol 27:380–395
Zakhia F, Jederz H, Domerguei O, Willems A, Cleyet-Mareh J-C, Gillis M, Dreyfus B, de Lajudie P (2006) Diverse bacteria associated with root nodules of spontaneous legumes in Tunisia and first report for nifH-like gene within the genera Microbacterium and Starkeya. Microb Ecol 51:375–393
Acknowledgments
Dheeren Panwar, Indu S. Sankhla, Neetu Poonar and Nisha Tak would like to thank the University Grants Commission (UGC) and Council of Scientific and Industrial Research (CSIR) for financial assistance in the form of a junior research fellowship, while Alkesh Tak acknowledges CSIR for financial support in the form of a senior research fellowship. We also acknowledge the Department of Biotechnology, Govt. of India for providing infrastructure, facilities and grants to the Department of Botany (BT/PR11461/AGR/21/270/2008). The Department of Meteorological and Soil Science Central Arid Zone Research Institute, Jodhpur are acknowledged for their help in providing data. Mahendra S. Rathore is thanked for valuable suggestions, assistance with field work, production of figures and computing expertise.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Neighbour-joining phylogenetic tree based on atpD gene sequences. Bootstrap values, expressed as a percentage of 1000 replicates, are given at branching points. The scale bar indicates 2% nucleotide substitutions per site. GenBank accession numbers are shown in parenthesis. (B., Bradyrhizobium and T, type strain) (JPEG 47 kb)
Table S1
BLASTN results of atpD housekeeping gene of Bradyrhizobium species (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Gehlot, H.S., Panwar, D., Tak, N. et al. Nodulation of legumes from the Thar desert of India and molecular characterization of their rhizobia. Plant Soil 357, 227–243 (2012). https://doi.org/10.1007/s11104-012-1143-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1143-5