Abstract
Background and Aims
Amendment of soil by biochar may reduce efficacy of soil-applied herbicides due to sorption.
Methods
Bioassays with Green Foxtail (Setaria viridis) tested the influence of two biochars on phytoavailability of S-metolachlor and sulfentrazone under biochar amendment of 0, 13, 26 and 52 Mg ha-1.
Results
Adsorption of both herbicides was an order of magnitude greater on a high specific surface area (SSA) biochar (EUC-800; SSA 242 m2 g-1) than on a low SSA biochar (BC-1; SSA 3.6 m2 g-1). Herbicide doses near the lowest recommended label rates controlled the weed at 13 and 26 Mg ha-1 of BC-1; sulfentrazone was also effective at 52 Mg BC-1 ha-1. These same herbicide doses controlled weed germination and development only at 13 Mg ha-1 of EUC-800; at herbicide doses near the highest label rates, weed control was also achieved at 26 Mg EUC-800 ha-1, but not at 52 Mg EUC-800 ha-1.
Conclusions
Increased doses of soil-applied herbicides cannot necessarily offset decreases in herbicide phytoavailability in biochar-amended soils, particularly if the biochar has a high SSA. Considering the long half-life of biochar in soil, pest control needs will be best served by low SSA biochars.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Turning biomass wastes into energy while simultaneously addressing CO2-C sequestration in pyrolysis-biochar systems is an intriguing possibility that has attracted much attention of late. Being that the half-life of biochar in soil is estimated to range from 100s, to tens of thousands of years (Zimmerman 2010), soil application of biochar could have long-term C sequestration potential (`Lehmann 2007a, b; Laird 2008; Woolf et al. 2010). Equally intriguing is the mounting evidence that when used along with organic and inorganic fertilizers, different types of biochar can significantly improve soil tilth (Glaser et al. 2002; Chan et al. 2008), crop productivity (Steiner et al. 2008; Graber et al. 2010), nutrient availability to plants (Lehmann et al. 2003; Silber et al. 2010), and protection against plant diseases (Elad et al. 2010; Graber et al. 2010; Kolton et al. 2011). Yet, since many physical/chemical properties of biochars depend on the biomass feedstock and the conditions of pyrolysis, diverse biochars may have dissimilar agronomic effects.
One of the outstanding attributes of many biochars that may contribute to their agronomic performance is their ability to adsorb and retain nutrients. The high adsorption and retention capacity of biochars is not limited to only nutritive elements, but is exhibited also towards many organic compounds, including soil-applied herbicides and insecticides. Qualities of biochar which impact its adsorption ability include the extent of crystallinity of the carbonaceous structure, which grows as pyrolysis temperature increases (Lua et al. 2004). There is also a gradual decrease in -OH and –CH moieties, an increase in C = C moieties, and a transformation of amine-N to pyridine-N as pyrolysis temperature increases (Bagreev et al. 2001; Chan and Xu 2009). Biochar porosity increases significantly with increasing production temperature, leading to increases in specific surface area (SSA), for example, from less than 10 m2 g-1 at production temperatures below 400°C to as much as 400 m2 g-1 at production temperatures of 550–600°C (Brown et al. 2006; Lehmann 2007a). Other adsorption-impacting qualities of biochar that vary as a function of feedstock and pyrolysis conditions include pH, cation exchange capacity (CEC), surface group functionality, and surface heterogeneity (Yang et al. 2004; Gaskin et al. 2008; Amonette and Joseph 2009).
While variations in these characteristics amongst different biochars can substantially influence adsorptive properties, as a general rule, adsorption of organic chemicals to biochars greatly exceeds their sorption to humic substances and soil organic matter (Cornelissen et al. 2004; Sheng et al. 2005; Pignatello et al. 2006; Yu et al. 2006; Zhang et al. 2006; Wu et al. 2007; Yang et al. 2009). Furthermore, desorption kinetics of organic chemicals from biochars is frequently hindered (Braida et al. 2003; Pignatello et al. 2006; Zeng et al. 2006; Sander and Pignatello 2007). A soil amendment with such adsorption characteristics can have either positive or negative impacts on pest management in agricultural soils. On the one hand, enhanced adsorption to the solid phase can reduce leaching of soil-applied herbicides and insecticides (Spokas et al. 2009; Wang et al. 2010; Yu et al. 2010; Zheng et al. 2010; Jones et al. 2011), and protect pesticides from degradation (Cornelissen et al. 2005; Yang et al. 2006; Rhodes et al. 2008; Loganathan et al. 2009; Jones et al. 2011). On the other hand, strong adsorption of pesticides on biochar can result in their inactivation (Toth et al. 1999; Xu et al. 2008; Yu et al. 2009b; Graber et al. 2011), or can potentially increase herbicide injury in rotational crops due to herbicide accumulation in the soil. Kookana (2010) discussed the importance of elucidating these issues to achieve a balance between carbon sequestration and agricultural and environmental stewardship.
Bioassays that specifically address the impact of biochar added to soil on the efficacy of purpose-applied pesticides against their target pests are few. Graber et al. (2011) showed that activity of a soil fumigant (1,3-dichloropropene) against nematodes was not affected by adding 13 Mg ha-1 of a biochar with a low SSA (3 m2 g-1). However, to achieve full pesticidal activity at a biochar amendment level of 26 Mg ha-1, the fumigant dose had to be doubled. It was calculated that the maximum manufacturer’s recommended fumigant dose would not have been effective against the pest had the biochar an adsorption ability greater by half an order of magnitude. This is realistic for a biochars with SSAs of 100 s of m2 g-1 (Bornemann et al. 2007).
The potential for biochar to mitigate crop uptake of insecticides from soils was also tested (Yu et al. 2009a; Yang et al. 2010). Chive and spring onion uptake of chlorpyrifos, fipronil and carbofuran from soils amended with two different biochars at levels up to 1% biochar by weight decreased markedly with increasing biochar content in the soil (Yu et al. 2009a; Yang et al. 2010). The high SSA biochar was particularly effective in reducing phytoavailability of the insecticides, and was suggested to have potential for treating pesticide residues in contaminated soils. Xu et al. (2008) reported that clomazone efficiency against barnyard grass was significantly inhibited in the presence of residues from open burning of rice straw in a field. In common with many biochars, the burned rice straw residue was found to have an herbicide adsorption capacity 3 to 4 orders of magnitude greater than that of the soil (Xu et al. 2008). Despite the obvious importance of soil-applied herbicides in modern intensive agriculture, there have been few reports documenting the extent to which biochar addition may inhibit weed control due to enhanced herbicide adsorption. One recent study has suggested that impact of biochar amendment on herbicide bioavailability will be a function of the herbicide mode of action (Nag et al. 2011).
Considering the very long half-life of biochar in soil, the potential harmful impact of biochar addition on pest control by soil-applied compounds due to adsorption should be well documented before advocating widespread use of biochar in agricultural field soils. For this, it is necessary to determine the qualities of biochar that contribute to pest control failure. Towards these goals, the present study examines the influence of soil amendment with two different biochars on the efficacy of two widely used herbicides, S-metolachlor (henceforth “metolachlor”) and sulfentrazone, against Green Foxtail (Setaria viridis). The biochars were selected for their notably different SSAs, and the herbicides for their appreciably different octanol-water partitioning coefficients (Kow; 2510 and 9.8 for metolachlor and sulfentrazone, respectively; Table 1). Metolachlor [2-chloro-N-2-ethyl-6-methylphenyl)-N-(2-methoxy-1-methylethyl) acetamide] is a broad spectrum chloroacetanilide pre-emergence herbicide used for certain broadleaf weed species as well as many annual grassy weeds (including Setaria spp.) in numerous agricultural food and feed crops, and on lawns and turf, ornamental plants, trees, shrubs and vines, and rights of way (Rivard 2003). Metolachlor is slightly to moderately persistent, ranges from mobile to highly mobile in different soils, and has been detected in groundwater (Rivard 2003). Sulfentrazone [2′,4′-dichloro-5′-(4-difluoromethyl-4,5-dihydro-3-methyl-5-oxo-1H-1,2,4-triazol-1-yl) methanesulfonanilide] is a pre-planting and pre-emergence triazolone herbicide for use in soybeans against a spectrum of broadleaf weeds and annual grass weeds (including Setaria spp.). Sulfentrazone is very mobile and persistent in soil, and has a strong potential to leach into groundwater and move offsite to surface water (USEPA 1997).
Materials and methods
Chemicals
Stock solutions of S-metolachlor (Agan Chemical Manufacturer Ltd., 97.8%, technical grade) and sulfentrazone (Agan Chemical Manufacturer Ltd., 95%, technical grade) were made up in double distilled water (DDW) and diluted to desired concentrations in DDW (range of initial concentrations between 2 to 100 mg/L). Sulfentrazone solutions, being sensitive to photolysis, were made up and kept at 4°C in Al-foil wrapped glassware. Relevant physical and chemical characteristics of the two herbicides are tabulated in Table 1, as is the range of manufacturers’ recommended doses. Dose depends on the herbicide, weed, crop and soil type combination.
Biochar
Two types of biochar were used. BC-1 is a locally-produced wood charcoal made in an earthen pit. Other production details for BC-1 are not available. EUC-800 is a biochar produced from Eucalyptus wood in an in-house pyrolysis reactor operated in indirect retort mode at a highest treatment temperature (HTT) of 800°C. Both biochars were ground into a powder of <0.5 mm particles and stored in a sealed container. pH, electrical conductivity (EC), and dissolved organic carbon (DOC) were determined in a 1:20 w:w biochar:DDW suspension. Ash content of 105°C dry biochar was determined in triplicate by weight loss after heating to 650°C in air for 4 h. Total C, H, N, O, and S were determined in duplicate by element analyzer (Thermo Flash EA-1112 Elemental Analyzer). Biochar SSA was determined by BET-N2 adsorption by the Israel Ceramic and Silicate Institute after degassing at 120°C for 5 h.
Soil
The soil used is a Hamra Red Mediterranean subsoil (Typic Xerochrept) which was air-dried, sieved (1 mm), and stored under ambient laboratory conditions. It consists of 95% sand and 5% clay (determined by hydrometer method), and a soil organic carbon content of 0.26% (determined by the Walkley-Black method).
Seeds
Green Foxtail (Setaria viridis) seeds were stored at 4°C. Before sowing, seeds were soaked for 3 min in a 1% bleach solution to minimize fungal infections, rinsed in DDW very well, and dried in a fume hood.
Bioassays
Soil-biochar mixtures were prepared at levels of 0, 0.5, 1, and 2% biochar by weight, equivalent to 0, 13, 26 and 52 Mg ha-1 biochar respectively, assuming an incorporation depth of 0.2 m and a soil bulk density of 1.3 g cm-3. Each soil and soil-biochar mixture was mixed with either DDW (control) or herbicide solution at a ratio of 1 kg soil to 270 mL solution to give a loose slurry, and allowed to dry in a fume hood for 48 h with mixing and homogenization during and after the drying period. When dried, the prepared soils and soil-biochar mixtures were weighed into pots, a fixed amount (by weight) of seeds was scattered evenly across the surface of the soil, and a further weighed amount of soil was added on top to give a uniform top layer approximately 1 cm thick (weight details below). Pots were arranged randomly on trays, placed in a temperature controlled greenhouse (25 ± 3°C), and watered from below as required during the growing period (14 days). To quantify weed growth, above-ground biomass was harvested, dried at 60°C until weight-loss ceased (about 9 days), and weighed. Results are quantified in terms of aboveground dry biomass from a given herbicide treatment divided by aboveground dry biomass of the equivalent control with the same level of biochar but no herbicide. This is referred to as normalized aboveground biomass.
In bioassays with BC-1 biochar, 200 mL pots were used (bottom soil 150 g, seeds 0.5 g, top soil 40 g). One herbicide dose rate for each herbicide was tested: metolachlor at 325 mL a.i. ha-1, and sulfentrazone at 224 g a.i. ha-1. BC-1 biochar bioassays were duplicated in full sequentially, where each duplicate experiment consisted of 5 replicate pots per treatment. Results of duplicate experiments (total of 10 replicate pots per treatment) were pooled.
To conserve resources for the more extensive EUC-800 biochar bioassay, smaller 100 mL pots were used (bottom soil 65 g, seeds 0.3 g, top soil 18 g). Two herbicide dose rates were evaluated: metolachlor at 325 and 3,290 mL a.i. ha-1, and sulfentrazone at 224 g a.i. ha-1 and 420 g a.i. ha-1. Differences between the duplicated sequential experiments for BC-1 being negligible, the more extensive EUC-800 bioassays were performed one time with 5 replicate pots per treatment. All specified bioassay herbicide doses are nominal concentrations; actual soil concentration was not measured, nor was herbicide degradation over the course of the bioassays investigated.
Sorption experiments
Sorption kinetics (followed over 12 days) and equilibrium sorption experiments on soil and biochar were carried out in glass vials with Teflon-lined silicon septa screw caps. After filling and sealing, the vials were shaken at 25°C ± 1°C in the dark. Each concentration point was tested in three replicate samples and two sorbent-free blanks. For sorption kinetics, a set of samples and blanks was sacrificed at pre-determined time steps, the solid phase allowed to settle, and the supernatant separated from the solid phase by syringe filter (0.45 μm Minisart cellulose acetate filters, Sartorius). No sorption of the compounds to the filters was detected in preliminary evaluations with standard solutions. The filtered supernatants were transferred to 2 mL amber vials equipped with Teflon-lined septa and hole caps. Analysis was by high pressure liquid chromatography (HPLC; Dionex Ultimate 3000 system) and quantification was against external standard curves. For metolachlor, the mobile phase was 90:10 methanol:water, column temperature 30°C, flow rate 0.8 mL min-1, detection at 200 nm. For sulfentrazone, the mobile phase was 50:50 acetonitrile:water at pH 3.15 with acetic acid, column temperature 30°C, flow rate 1.0 mL min-1, detection at 200 nm. Blank losses, compared with freshly made standard solutions, were less than 2% in all cases.
Curve fitting and statistical analysis
Curve fitting and statistical analysis was by OriginPro 7.0 software. Means testing was by one way ANOVA using the Tukey test.
Results
Biochar and soil properties
Biochar physical/chemical characteristics are tabulated in Table 2. BC-1 biochar has a neutral pH (7.4 in a 20:1 water:biochar extract) and an SSA of 3.6 m2 g-1. Biochar pH and SSA increase as pyrolysis HTT increases (Lehmann 2007a). Based on data for pH and SSA as a function of HTT in Lehmann (2007a), and our own unpublished results for biochars prepared from several feedstocks (Eucalyptus wood, olive pomice, greenhouse waste) at different temperatures (350, 450, 600, and 800°C), we estimate that BC-1biochar was produced at an HTT of around 350°C. In contrast, EUC-800, produced at an HTT of 800°C, has a considerably higher SSA (242 m2 g-1) and aqueous suspension pH (11.4), and is more aromatic than BC-1 (H/C ratio of 0.20 versus 0.49 for EUC-800 and BC-1, respectively). EUC-800 ash content (11.6%) is higher than that of BC-1 (3.8%), and accordingly, the EUC-800 aqueous suspension has a higher EC (1.8 vs. 0.12 dS m-1, respectively).
Sorption
BC-1-sulfentrazone suspensions in the sorption experiment had a pH of 7.28, which is 0.72 pH units above the pKa of sulfentrazone (Table 1). Therefore, about 81% of sulfentrazone in solution was in the dissociated form in the sorption experiments. EUC-800-sulfentrazone suspensions had a pH of 9.0, such that virtually 100% of the sulfentrazone was in the dissociated form in those sorption experiments. Metolachlor is not ionizable.
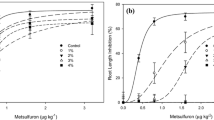
Kinetics of sorption of the herbicides on both biochars was complete within 5 days (out of 12 tested days), and equilibrium sorption isotherms were measured after 7 days. Isotherms are given in Fig. 1 for metolachlor on both biochars and the soil, and for sulfentrazone on the two biochars. The two biochars exhibited strongly non-linear adsorption of the herbicides, with the isotherms being well fit by the Freundlich model (Eq. 1):
where S is adsorbed concentration (mg kg-1), C e equilibrium solution concentration (mg L-1), and K f (mg(1-n) Ln kg-1) and n being fitting parameters. Adsorption data was fit by linear regression to a log-log plot; fitted parameters are given in Table 3.
Over the range of equilibrium solution concentrations, it can be seen from Fig. 1 that adsorption of metolachlor at a given equilibrium concentration was one or more orders of magnitude greater on EUC-800 biochar than on BC-1 biochar. The difference in sulfentrazone adsorption between the two biochars was yet larger (Fig. 1). Adsorption of metolachlor and sulfentrazone on EUC-800 was extremely non-linear, with exponent n values of 0.193 and 0.165, respectively, compared with exponent n values for BC-1 of 0.310 and 0.321, respectively.
Despite the significantly greater Kow of metolachlor than sulfentrazone (2510 vs 9.8, respectively, Table 1), the difference in adsorption between the two is quite small (Fig. 1). The differences between the two herbicides are lower on the high SSA EUC-800 biochar (metolachlor sorption about 1.4 to 1.6 times greater than sulfentrazone sorption) than on the low SSA BC-1 biochar (metolachlor sorption about 2.2–2.4 times greater than sulfentrazone sorption; Fig. 1). Prediction bands at 95% confidence limits (not shown) indicate that all regression lines are distinct.
Sorption of metolachlor on the soil was very low, and best modeled by a single parameter linear isotherm (S = K d C e ; K d being the distribution coefficient), yielding K d = 0.75 ± 0.01 L kg-1 with an R2 = 0.990. The calculated K oc (K d /fraction of organic carbon) is 288 L kg-1, which is comparable to literature values for this herbicide (Table 1). No sorption of sulfentrazone on the soil could be detected.
Bioassays
A photograph of a typical bioassay illustrates these experiments (Fig. 2), and also documents the inhibiting effect of a dose of metolachlor (325 mL a.i. ha-1) on the growth of Setaria viridis at every BC-1 biochar amendment level (Fig. 2b). This dose is near the low end of the recommended use range for metolachlor (Table 1; hereafter referred to as “low dose”). Bioassays were also conducted for metolachlor at 3,290 mL a.i. ha-1, which is near the high end of the recommended use range for metolachlor (Table 1; hereafter referred to as “high dose”). Results of all the metolachlor bioassays (low dose metolachlor in BC-1 amended soil; low dose metolachlor in EUC-800 amended soil; high dose metolachlor in EUC-800 amended soil) are quantified in Fig. 3 (panes A-C) in terms of normalized aboveground biomass.
Normalized weight of above-ground biomass (ordinate) at different biochar amendment rates (abscissa): a pots treated with BC-1 at a low dose of metolachlor (MET; 325 mL a.i. ha-1); b pots treated with EUC-800 at a low dose of MET (325 mL a.i. ha-1); and c pots treated with EUC-800 at a high dose of MET (3,290 mL a.i. ha-1). Columns labeled by different letters indicate significantly different (P < 0.05) results
Metolachlor efficacy at the low dose was not significantly different (P < 0.05) at BC-1 amendment levels of 0, 13, and 26 Mg ha-1 (Fig. 3a). Weed control at a BC-1 amendment level of 52 Mg ha-1 was significantly (P < 0.05) worse than at the other biochar amendment levels (Fig. 3a), but still significantly (P < 0.05) better than the herbicide-free control.
In pots amended with EUC-800 biochar, the low metolachlor dose gave the same level of weed control as the unamended treatment only at the biochar rate of 13 Mg ha-1. Results for the low metolachlor dose were not significantly different (P < 0.05) from that of the herbicide-free treatment at EUC-800 biochar amendment rates of 26 and 52 Mg ha-1 (Fig. 3b). Even at the high dose of metolachlor, which was 10 times greater than the low dose of metolachlor (3290 versus 325 mL a.i. ha-1, respectively) and very near the recommended upper limit for this herbicide (Table 1), weed control in EUC-800 amended soil was significantly (P < 0.05) less good at 52 Mg ha-1 than at the other biochar amendment rates (Fig. 3c). Normalized weed biomass was significantly (P < 0.05) greater at the high metolachlor dose in pots amended by 52 Mg ha-1 of EUC-800 biochar than at the low metolachlor dose in pots amended by 52 Mg ha-1 of BC-1 biochar (Fig. 3c compared with Fig. 3a).
Results of the sulfentrazone bioassays were similar to those of the metolachlor bioassays (Fig. 4a-c). Two sulfentrazone doses, one near the low end of the recommended use range (224 g a.i. ha-1; Table 1), and one at the high end of the recommended use range (420 g a.i. ha-1; Table 1), referred to as “low dose” and “high dose” respectively, were tested. In BC-1 biochar amended soils at the low sulfentrazone dose, no significant (P < 0.05) affect of biochar addition on weed productivity was observed at any biochar loading rate (Fig. 4a). In contrast, in EUC-800 biochar amended pots, the low sulfentrazone dose had the same weed control efficacy as the treatment without biochar (P < 0.05) only at the biochar amendment rate of 13 Mg ha-1 (Fig. 4b). At the high sulfentrazone dose, weed control was similar at 0, 13 and 26 Mg ha-1 EUC-800 biochar levels (P < 0.05), but not significantly different from the herbicide-free treatment at an EUC-800 biochar level of 52 Mg ha-1 (Fig. 4c).
Normalized weight of above-ground biomass (ordinate) at different biochar amendment rates (abscissa): a pots treated with BC-1 at a low dose of sulfentrazone (SFZ; 224 g a.i. ha-1); b pots treated with EUC-800 at a low dose of SFZ (224 g a.i. ha-1); and c pots treated with EUC-800 at a high dose of SFZ (420 g a.i. ha-1). Columns labeled by different letters indicate significantly different (P < 0.05) results
Discussion
There is ample prior evidence in the literature that biochar and biochar-like materials (black carbon) have substantial adsorption ability for many organic compounds (Smernik 2009), pesticides among them (Matsui et al. 2002; Gimeno et al. 2003). Accordingly, both biochars examined in this study exhibit this quality, with the adsorption ability of the high SSA biochar, EUC-800, generally exceeding that of the low SSA biochar, BC-1, by more than an order of magnitude. As a general rule, adsorption ability increases with increasing SSA (Bornemann et al. 2007; Chen and Chen 2009; Wang et al. 2010; Yang et al. 2010). The greater isotherm non-linearity observed on the high temperature, high SSA biochar is also consistent with prior reports in the literature (James et al. 2005).
On both biochars, there was a maximum 2-fold difference in adsorbed concentration of metolachlor as compared with sulfentrazone at a given solution concentration; this difference pales in comparison to the 250-fold difference in KOW between the two herbicides. The similarity in adsorbed concentration is suggestive that the dominant sorption mechanism is pore-filling (Xia and Ball 1998). In pore-filling, the amount of adsorbed metolachlor and sulfentrazone should be nearly the same when identical sorbate volumes are added, assuming that the adsorbate molecules are in the same physical state. The relatively small differences between metolachlor and sulfentrazone can thus be accorded to differences in packing efficiency of the solid sulfentrazone as compared with the liquid metolachlor (Chiou and Manes 1973). Other reasons for the small differences between them can be dissimilarities in specific interactions that the compounds undergo with organic surfaces (Borisover and Graber 2002, 2003), incomplete dissociation of sulfentrazone in BC-1 sorption experiments, differences in molar volume, and differences in aqueous solubility. On balance, the sorption results are consistent with a pore-filling mechanism in both biochars. The importance of pore-filling as the dominant adsorption mechanism in biochar may have particular relevance for eventually predicting the behaviour of other pesticides in biochar-amended soils, but much work remains to be done, particularly as pesticides frequently have multiple modes of interaction with sorbents. Adsorption strength of various chars for non-specifically and specifically-interacting compounds has been found to be a complex function of both SSA and char surface group functionality (Chun et al. 2004; James et al. 2005; Chen et al. 2008).
While strong adsorption of pest control products on a soil additive such as biochar can be desirable in situations where the pesticide is accidently spilled, incorrectly applied, or if residues interfere with seed germination or growth of a sensitive crop, it is not desirable when pesticides are applied for agronomic purposes. As seen in the results presented herein, the adsorption attributes of biochar have a significant impact on the ultimate efficacy of an applied herbicide. Even a substantially increased herbicide dose may be insufficient to offset the loss in herbicide phytoavailability due to adsorption on a strongly adsorbing biochar. This effect is seen for high doses of both metolachlor and sulfentrazone in soil amended with the strongly adsorbing, high SSA biochar added at 52 Mg ha-1 (2 wt%). In that case, herbicide efficacy was hindered even when the herbicides were applied at their maximum or close to maximum recommended use doses. Even at lower amendment rates of high SSA biochar, it was necessary to use much higher herbicide doses to obtain adequate control over the weed. These results are consistent with those of an earlier study examining the effect of biochar on the efficacy of a fumigant (1,3-dichloropropene) against soil nematodes (Graber et al. 2011).
An additional feature of organic compound adsorption on biochar which may adversely affect efficacy of soil-applied pesticides is the oft-reported desorption hysteresis (Braida et al. 2003; Pignatello et al. 2006; Zeng et al. 2006; Sander and Pignatello 2007). If compound desorption is hindered, concentrations in the solution phase (where is it active against the weed or pest) will be even lower than expected under an equilibrium situation, thus reducing compound bio/phytoavailability yet more so. Information on the influence of biochar feedstock and production method, and hence its physical and chemical properties, on the extent of desorption hysteresis is scarce, but suggestive that compounds will exhibit greater desorption hysteresis on higher temperature biochars (Yu et al. 2010; Zhang and He 2010). The extent of hysteresis can be strongly influenced by the nature of the adsorbate and functionality of groups at the adsorbent surfaces. Desorption of aromatic compounds with electron-donating groups was highly hysteretic on activated carbon, while desorption of compounds with electron-attracting groups was reversible (Tamon and Okazaki 1996). Desorption hysteresis featured when the energy difference between the HOMO (highest occupied molecular orbital) of the adsorbate and LUMO (lowest unoccupied molecular orbital) of the adsorbent was small. When carbon surface acidic sites were increased by wet oxidation, desorption hysteresis disappeared, which was attributed to the change of the LUMO energy of the carbon during oxidation (Tamon and Okazaki 1996). This may be very relevant for biochar in soil, as biochar surfaces are known to undergo oxidation over the course of time in the soil environment (Cheng et al. 2006, 2008; Cheng and Lehmann 2009).
Only a few studies have examined how biochar aging under actual field conditions impacts its adsorption properties and potential to interact with herbicides and pesticides. In laboratory investigations using soil-water incubations (either as a suspension or at field capacity), reported reductions in organic compound sorption due to biochar aging are generally relatively small (Yang and Sheng 2003; Kwon and Pignatello 2005; Cheng and Lehmann 2009; Graber et al. 2011). For instance, adsorption of the fumigant 1,3-dichloropropene was unaffected by 10 months incubation at 30°C under field capacity (Graber et al. 2011). Sorption of benzene in a soil-biochar suspension aged 90 days at 45°C was reduced by a factor of 2.7 (Kwon and Pignatello 2005), and diuron adsorption in a soil-biochar suspension aged for 1 year at room temperature decreased by 50–60% (Yang and Sheng 2003). Cheng and Lehmann (2009) reported a decrease in sorption of hydroquinone of about 50% in a 12 month soil-biochar incubation at 30°C, and a more substantial decrease in a system aged at 70°C (Cheng and Lehmann 2009). The higher temperature system, while environmentally not relevant, may represent changes that could be expected under real field conditions over a much longer interval of time. Given the literature results, it seems reasonable to conclude that over a period of a few years, a decrease in pesticide sorption as a result of natural aging of the applied biochar in the soil would not be sufficient to offset the potential deleterious effects of biochar on pest management.
While there is no “standard biochar application rate”, the vast majority of reported field trials with biochar have been conducted at levels of biochar application ranging from 1 Mg ha-1 to 20 Mg ha-1 (Blackwell et al. 2009). The lowest level used in the current study (0.5%, or 13 Mg ha-1) is well within this range, while the highest level (2%, 52 Mg ha-1) is far beyond it. The middle rate, 26 Mg ha-1 (1 wt%) is just about at the upper limit of field biochar application rates, assuming a uniform distribution of biochar in the top 20 cm of the soil and a soil bulk density of 1.3 g cm-1. This study and others (Yu et al. 2009a; Yang et al. 2010; Graber et al. 2011; Nag et al. 2011) have shown that the use of high SSA biochar for agronomic purposes can considerably reduce availability of soil-applied pesticides. In the best case, amendment with a biochar having a high SSA at levels up to 26 Mg ha-1 can greatly increase the pesticide dose required to obtain adequate pest protection. In the worst case, biochar amendment may render soil-applied pest control agents ineffective. In so much as the half-life of biochar in soil is 100s to 1000s of years (Zimmerman 2010), sustainable soil stewardship requires that this effect be taken into account when applying biochar to soils, an essential and non-renewable resource for food production. Until a more comprehensive set of data and predictive models become available, application of the injunction: primum non nocere, first, do no harm, is a precaution worth adopting regarding the use of biochar in field soils. For now, based on the results of this and previous studies, it appears that pest control requirements would be best served by biochars having low SSAs.
References
Amonette JE, Joseph S (2009) Characteristics of biochar: microchemical properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 33–52
Bagreev A, Bandosz TJ, Locke DC (2001) Pore structure and surface chemistry of adsorbents obtained by pyrolysis of sewage-derived fertilizer. Carbon 39:1971–1979
Blackwell P, Riethmuller G, Collins M (2009) Biochar application to soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 207–226
Borisover M, Graber ER (2002) Simplified link solvation model (LSM) for sorption in natural organic matter. Langmuir 18:4775–4782
Borisover M, Graber ER (2003) Classifying NOM—organic sorbate interactions using compound transfer from an inert solvent to the hydrated sorbent. Environ Sci Technol 37:5657–5664
Bornemann LC, Kookana RS, Welp G (2007) Differential sorption behaviour of aromatic hydrocarbons on charcoals prepared at different temperatures from grass and wood. Chemosphere 67:1033–1042
Braida WJ, Pignatello JJ, Lu YF, Ravikovitch PI, Neimark AV, Xing BS (2003) Sorption hysteresis of benzene in charcoal particles. Environ Sci Technol 37:409–417
Brown RA, Kercher AK, Nguyen TH, Nagle DC, Ball WP (2006) Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org Geochem 37:321–333
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Austral J Soil Res 46:437–444
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Chen B, Zhou D, Zhu L (2008) Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ Sci Technol 42:5137–5143
Chen BL, Chen ZM (2009) Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 76:127–133
Cheng CH, Lehmann J (2009) Ageing of black carbon along a temperature gradient. Chemosphere 75:1021–1027
Cheng CH, Lehmann J, Engelhard MH (2008) Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim Cosmochim Acta 72:1598–1610
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488
Chiou CC, Manes M (1973) Application of the Polanyi adsorption potential theory to adsorption from solution on activated carbon. Iv. Steric factors, as illustrated by the adsorption of planar and octahedral metal acetylacetonates. J Phys Chem 77:809–813
Chun Y, Sheng G, Chiou CT, Xing B (2004) Compositions and sorptive properties of crop residue-derived chars. Environ Sci Technol 38:4649–4655
Cornelissen G, Elmquist M, Groth I, Gustafsson O (2004) Effect of sorbate planarity on environmental black carbon sorption. Environ Sci Technol 38:3574–3580
Cornelissen G, Gustafsson O, Bucheli TD, Jonker MTO, Koelmans AA, Van Noort PCM (2005) Extensive sorption of organic compounds to black carbon, coal, and kerogen in sediments and soils: mechanisms and consequences for distribution, bioaccumulation, and biodegradation. Environ Sci Technol 39:6881–6895
Elad Y, Rav David D, Meller Harel Y, Borenshtein M, Ben Kalifa H, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100:913–921
Gaskin JW, Steiner C, Harris K, Das KC, Bibens B (2008) Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 51:2061–2069
Gimeno O, Plucinski P, Kolaczkowski ST, Rivas FJ, Alvarez PM (2003) Removal of the herbicide MCPA by commercial activated carbons: equilibrium, kinetics, and reversibility. Ind Engin Chem Res 42:1076–1086
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fert Soils 35:219–230
Graber ER, Meller-Harel Y, Kolton M, Cytryn E, Silber A, Rav David D, Tsechansky L, Borenshtein M, Elad Y (2010) Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 337:481–496
Graber ER, Tsechansky L, Khanukov J, Oka Y (2011) Sorption, volatilization and efficacy of the fumigant 1,3-dichloropropene in a biochar-amended soil. Soil Sci Soc Am J 75:1365–1373
James G, Sabatini DA, Chiou CT, Rutherford D, Scott AC, Karapanagioti HK (2005) Evaluating phenanthrene sorption on various wood chars. Water Research 39:549–558
Jones DL, Edwards-Jones G, Murphy DV (2011) Biochar mediated alterations in herbicide breakdown and leaching in soil. Soil Biol Biochem 43:804–813
Kolton M, Meller Harel Y, Pasternak Z, Graber ER, Elad Y, Cytryn E (2011) Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl Environ Microbiol 77:4924–4930
Kookana RS (2010) The role of biochar in modifying the environmental fate, bioavailability, and efficacy of pesticides in soils: a review. Austral J Soil Res 48:627–637
Kwon S, Pignatello JJ (2005) Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): pseudo pore blockage by model lipid components and its implications for n-2-probed surface properties of natural sorbents. Environ Sci Technol 39:7932–7939
Laird DA (2008) The charcoal vision: a win-win-win scenario for simultaneously producing bioenergy, permanently sequestering carbon, while improving soil and water quality. Agron J 100:178–181
Lehmann J (2007a) Bio-energy in the black. Front Ecol Environ 5:381–387
Lehmann J (2007b) A handful of carbon. Nature 447:143–144
Lehmann J, da Silva P, Jr J, Steiner C, Nehls T, Zec W, Glaser B (2003) Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central amazon basin: fertilizer, manure and charcoal amendments. Plant Soil 249:343–357
Loganathan VA, Feng YC, Sheng GD, Clement TP (2009) Crop-residue-derived char influences sorption, desorption and bioavailability of atrazine in soils. Soil Sci Soc Am J 73:967–974
Lookchem (2011) Look for Chemicals. www.lookchem.com. last accessed: 12-Jun-2011
Lua AC, Yang T, Guo J (2004) Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J Anal Appl Pyrol 72:279–287
Matsui Y, Knappe DRU, Iwaki K, Ohira H (2002) Pesticide adsorption by granular activated carbon adsorbers. 2. Effects of pesticide and natural organic matter characteristics on pesticide breakthrough curves. Environ Sci Technol 36:3432–3438
Nag SK, Kookana RS, Smith L, Krull E, Macdonald LM, Gill G (2011) Poor efficacy of herbicides in biochar-amended soils as affected by their chemistry and mode of action. Chemosphere 84:1572–1577
PAN Pesticides Database (2010) http://www.pesticideinfo.org/. last accessed: 12-Dec-2010.
Pignatello JJ, Kwon S, Lu YF (2006) Effect of natural organic substances on the surface and adsorptive properties of environmental black carbon (char): Attenuation of surface activity by humic and fulvic acids. Environ Sci Technol 40:7757–7763
PPDB (2011) Pesticide Properties Database. http://sitem.herts.ac.uk/aeru/footprint/en/. last accessed: 3-Jan-2011
Rhodes AH, Carlin A, Semple KT (2008) Impact of black carbon in the extraction and mineralization of phenanthrene in soil. Environ Sci Technol 42:740–745
Rivard L (2003) Environmental fate of metolachlor. Environmental Monitoring Branch. Dept of Pesticide Registration, Sacramento CA, p 14
Sander M, Pignatello JJ (2007) On the reversibility of sorption to black carbon: distinguishing true hysteresis from artificial hysteresis caused by dilution of a competing adsorbate. Environ Sci Technol 41:843–849
Sheng GY, Yang YN, Huang MS, Yang K (2005) Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ Poll 134:457–463
Silber A, Levkovitch I, Graber ER (2010) pH-dependent mineral release and surface properties of cornstraw biochar: agronomic implications. Environ Sci Technol 44:9318–9323
Smernik RJ (2009) Biochar and sorption of organic compounds. In: Lehmann J, Joseph S (eds) Biochar for environmental mangement: science and technology. Earthscan, London, pp 289–300
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a minnesota soil. Chemosphere 77:574–581
Steiner C, Glaser B, Teixeira WG, Lehmann J, Blum WEH, Zech W (2008) Nitrogen retention and plant uptake on a highly weathered central amazonian ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171:893–899
Tamon H, Okazaki M (1996) Desorption characteristics of aromatic compounds in aqueous solution on solid adsorbents. Journal of Colloid and Interface Science 179:181–187
Toth J, Milham PJ, Kaldor CJ (1999) Decreased phytotoxicity of diuron applied over ash of recently burned kangaroo grass (Themeda australis (r.Br.) stapf). Plant Prot Quart 14:151–154
USEPA (1997) Sulfentrazone pesticide fact sheet. Office of Prevention, Pesticides and Toxic Substances (7501C), Washington D.C.
Wang HL, Lin KD, Hou ZN, Richardson B, Gan J (2010) Sorption of the herbicide terbuthylazine in two new zealand forest soils amended with biosolids and biochars. Journal of Soils and Sediments 10:283–289
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:56
Wu C, Zhang XL, Li GB (2007) Effects of humic acid coatings on phenanthrene sorption to black carbon. J Environ Sci—China 19:1189–1192
Xia G, Ball WP (1998) Adsorption-partitioning uptake of nine low-polarity organic chemicals on a natural sorbent. Environ Sci Technol 33:262–269
Xu C, Liu WP, Sheng GD (2008) Burned rice straw reduces the availability of clomazone to bamyardgrass. Sci Total Environ 392:284–289
Yang XB, Ying GG, Peng PA, Wang L, Zhao JL, Zhang LJ, Yuan P, He HP (2010) Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. J Agric Food Chem 58:7915–7921
Yang Y, Hunter W, Tao S, Gan J (2009) Effects of black carbon on pyrethroid availability in sediment. J Agric Food Chem 57:232–238
Yang YN, Chun Y, Sheng GY, Huang MS (2004) pH-dependence of pesticide adsorption by wheat-residue-derived black carbon. Langmuir 20:6736–6741
Yang YN, Sheng GY (2003) Pesticide adsorptivity of aged particulate matter arising from crop residue burns. J Agric Food Chem 51:5047–5051
Yang YN, Sheng GY, Huang MS (2006) Bioavailability of diuron in soil containing wheat-straw-derived char. Sci Total Environ 354:170–178
Yu X-Y, Ying G-G, Kookana RS (2009) Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 76:665–671
Yu XY, Pan LG, Ying GG, Kookana RS (2010) Enhanced and irreversible sorption of pesticide pyrimethanil by soil amended with biochars. J Environ Sci—China 22:615–620
Yu XY, Ying GG, Kookana RS (2006) Sorption and desorption behaviors of diuron in soils amended with charcoal. J Agric Food Chem 54:8545–8550
Zeng GM, Zhang C, Huang GH, Yu J, Wang Q, Li JB, Xi BD, Liu HL (2006) Adsorption behavior of bisphenol a on sediments in xiangjiang river, central-south china. Chemosphere 65:1490–1499
Zhang JH, He MC (2010) Effect of structural variations on sorption and desorption of phenanthrene by sediment organic matter. Journal of Hazardous Materials 184:432–438
Zhang P, Sheng GY, Feng YH, Miller DM (2006) Predominance of char sorption over substrate concentration and soil pH in influencing biodegradation of benzonitrile. Biodegradation 17:1–8
Zheng W, Guo M, Chow T, Bennett DN, Rajagopalan N (2010) Sorption properties of greenwaste biochar for two triazine pesticides. J Haz Mat 181:121–126
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Acknowledgments
Setaria viridis seeds were provided by Prof. Baruch Rubin, and sulfentrazone by Dr. Yael Mishael, both of the Hebrew University of Jerusalem; their generosity is gratefully acknowledged. We greatly appreciate the careful and detailed reviews by the referees. This research was made possible by a grant from the Chief Scientist of the Ministry of Agriculture and Rural Development (project number 301-0693-10). This paper is contribution no. 604/11 of the Agricultural Research Organization, The Volcani Center, Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johannes Lehmann.
Rights and permissions
About this article
Cite this article
Graber, E.R., Tsechansky, L., Gerstl, Z. et al. High surface area biochar negatively impacts herbicide efficacy. Plant Soil 353, 95–106 (2012). https://doi.org/10.1007/s11104-011-1012-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-1012-7