Abstract
The application of pyrogenic carbon, biochar, to agricultural soils is currently discussed as a win-win strategy to sequester carbon in soil, thus improving soil fertility and mitigate global warming. Our aim was to investigate if biochar may improve plant eco-physiological responses under sufficient water supply as well as moderate drought stress. A fully randomized greenhouse study was conducted with the pseudo-cereal Chenopodium quinoa Willd, using three levels of biochar addition (0, 100 and 200 t ha−1) to a sandy soil and two water treatments (60% and 20% of the water holding capacity of the control), investigating growth, water use efficiency, eco-physiological parameters and greenhouse gas (GHG) fluxes. Biochar application increased growth, drought tolerance and leaf-N- and water-use efficiency of quinoa despite larger plant–leaf areas. The plants growing in biochar-amended soil accumulated exactly the same amount of nitrogen in their larger leaf biomass than the control plants, causing significantly decreased leaf N-, proline- and chlorophyll-concentrations. In this regard, plant responses to biochar closely resembled those to elevated CO2. However, neither soil- nor plant–soil-respiration was higher in the larger plants, indicating less respiratory C losses per unit of biomass produced. Soil-N2O emissions were significantly reduced with biochar. The large application rate of 200 t ha−1 biochar did not improve plant growth compared to 100 t ha−1; hence an upper beneficial level exists. For quinoa grown in a sandy soil, biochar application might hence provide a win-win strategy for increased crop production, GHG emission mitigation and soil C sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Atmospheric CO2 concentrations have already increased from 275 ppm in preindustrial times to 387 ppm today which is higher than at any time during the past 20 million years, resulting in global warming (IPCC 2007b). At the same time, arable land areas worldwide decline by soil erosion, drought, salinization, loss of soil organic carbon (SOC) contents (Lal 2004; IPCC 2007a; Kimetu et al. 2009) or other forms of degradation. Global warming and a fast-growing world population intensify the need to develop solutions for our future food and energy needs (Mathews 2008; Hansen et al. 2008; Lal 2009).
In recent years, application of black, charred carbon (in the following termed ‘biochar’) has been increasingly discussed as a mitigation strategy for sequestering recalcitrant carbon into agricultural soils, which can, at the same time, improve soil fertility (Glaser et al. 2002; Marris 2006; Lehmann 2006, 2007a, b). The idea originates in Amazonian dark earth or Terra preta research (Glaser et al. 2001; Marris 2006). Terra preta (TP) soils enable several harvests per year without extra fertilization, or the need to move and cut new forest after a few years (Glaser 2007; Steiner et al. 2008). TP soils have distinct bacterial communities with a significantly greater species richness (Kim et al. 2007), exhibit significantly larger cation exchange capacities (Glaser et al. 2001; Steiner et al. 2008), contain significantly higher phosphorus amounts, and have larger stocks of soil organic matter besides the black carbon than near-by Ferralsols (Glaser et al. 2001), suggesting that additional C sequestration in soil organic matter has occurred.
Modern pyrolysis techniques, which are currently undergoing a rapid technical development (Laird et al. 2009) allow energy production from syngas (mainly CO, H2 and CH4 and other hydrocarbons) and/or liquid–fuel production while simultaneously generating different types of biochar (abbreviated BC in the following; Gaunt and Lehmann 2008; McHenry 2009). The resulting biochars can greatly differ in their material properties (CHO-concentrations, aromaticity, cation exchange capacity, pH, nutrient contents, porosity, energy density etc.), depending on feedstock and pyrolysis conditions (Amonette and Joseph 2009, Downie et al. 2009). Experimental evidence so far shows that (a) BC is quite stable and hence principally suitable for C sequestration (Cheng et al. 2008; Kuzyakov et al. 2009; Major et al. 2010), (b) BC addition often promotes plant growth, in particular combined with N-fertilizer addition in poor soils (Blackwell et al. 2009; Major et al. 2010), (c) it reduces nutrient leaching (Chan et al. 2007, 2008; Laird et al. 2009; Steiner et al. 2008). Additionally it could be shown (d) that the cation exchange capacity (CEC) of soils increases with BC addition (Liang et al. 2006), in particular over time as the functional groups are oxidized (Cheng et al. 2006).
Although using BC seems to be promising, and despite the fact that several international projects have been initiated, there is still a considerable lack of knowledge on its effects and their causes (Blackwell et al. 2009). In particular, the plant physiological response (other than crop yield) to BC in soils remains poorly understood and has, to our knowledge, seldom been investigated (Elad et al. 2010; Graber et al. 2010).
Before BC can be applied large scale in agricultural practice, possible counterproductive effects must be investigated. Negative effects could theoretically lead to increased greenhouse gas (GHG) fluxes of CO2, CH4 or N2O, (Wardle et al. 2008; Clough et al. 2010) or reduced plant stress resistances, e.g. drought tolerance with improved water supply. However, first lines of evidence suggest that N2O emissions may decline rather than increase with BC addition (Lehmann 2007a; Spokas et al. 2009; van Zwieten et al. 2010). Increased soil CO2 effluxes may result from soil organic carbon (SOC) decomposition via "priming" of old soil carbon (Kolb et al. 2009; Wardle et al. 2008). In addition, suboptimal large application rates of BC may lead to other negative effects, or less positive responses (compare Rondon et al. 2007), e.g. by nutrient immobilization at BC surfaces or pH changes (Chan and Xu 2009; Laird et al. 2009).
Beside the investigation of possible productive BC effects on arable land, in particular BC effects on less productive soils must be investigated because there is also need to extend arable land into less suitable areas, or to avoid further desertification. Drought is a worldwide problem, seriously constraining global crop production and quality. It is well known that soil characteristics influence plant communities through water relations (Sperry and Hacke 2002). The addition of BC to sandy soil changes soil characteristics such as its texture and porosity. Hypothetically, finer textured soils (after BC addition) in arid climates should be associated with more negative plant and soil water potentials during drought, inducing a greater resistance of xylem to cavitation, and shallower root systems than coarse soils.
Therefore, the aim of this study was the investigation of BC application effects on plant responses such as water relations, C-, N-content or gas exchange and plant–soil interactions including greenhouse gas (GHG) fluxes of CO2 and N2O under high and low water availability.
In recent years there has been a growing interest in introducing alternative crops in Europe able to resist conditions of nutrient-poor soil, drought, salinization or other forms of degradation. One such crop is quinoa (Chenopodium quinoa Willd) originating from the South American highlands and therefore considered as a hardy plant with good drought tolerance (Galwey 1989; Jacobsen and Stølen 1993; Jensen et al. 2000). It was chosen as a suitable candidate for this study because it exhibits the attributes of drought tolerance, sustainability in the context of global changes and a high economic potential. Until now, few investigations have been performed to study the drought tolerance of quinoa under controlled conditions.
We studied the validity of the following hypotheses: (1) BC addition will promote comparatively more plant growth under limited water supply than under good water supply. (2) BC amendment will not alter basic plant parameters such as the leaf N or C content, the relative chlorophyll content, transpiration or respiration. (3) Soil-derived CO2 effluxes and N2O emissions (based on denitrification) may be stimulated at first, due to priming (Wardle et al. 2008) or to initial oxidation of labile C fractions on the BC surfaces (Cheng et al. 2006). Later (4), N2O emissions might be reduced, but CO2 effluxes of the plant–soil systems might continue to increase, when BC-grown plants (with an unchanged respiration per leaf area) become larger than controls. (5) A very large BC application dose will have negative effects, e.g. via N immobilization (Chan and Xu 2009).
Material and methods
Experimental setup and growth conditions
The greenhouse study was designed as a completely randomized experiment with n = 4 replicates per treatment, with sufficient or reduced water supply and 0 (control), 100 or 200 t ha−1 BC application rates (n = 24 pots in total). The unusually high rate of 200 t ha−1 (compare Chan et al. 2007, 2008) was chosen to investigate if there is an upper limit of BC addition, which has negative effects on plant growth. Temperature, relative humidity (RH) and light regimes were set to 22 ± 2°C, 60 ± 5% RH and >10.000 lux at a 16 and 8 h day-night cycle, respectively.
To obtain a poor sandy soil medium, 1/4 (v/v) of a sandy loam brown earth (obtained from Kieswerk Gießen, Germany) was mixed with 3/4 of pure washed sand (<1.4 mm particle size). Each of the 24 pots (inner diameter 10.2 cm, height 20 cm) was filled with 2 kg of air-dried sandy soil (=1.872 ± 1.2 g dry soil). For BC treatments, 81.7 and 163.4 g dry BC were added per pot and thoroughly mixed with the sandy soil, equivalent to 100 and 200 t BC ha−1 and 20 cm ploughing depth, respectively. Thus, the soil surface in the biochar-amended pots was 2–5 cm higher than in the control pots. The pots were made from commercial polyethylene pipes capped at the bottom with five draining holes per cap. The biochar (particle size <2 mm) had been purchased from EPRIDA, Athens, USA, where it had been produced from peanut hull residues at 498°C and 26269 Pa (0.263 bar), with a biomass feed rate of 16.3 kg hr−1, a steam temperature of 550.7°C and a steam flow rate of 10.2 kg hr−1 (Fig. 1a). The total C and N contents were 71.6% and 1.84%, determined following ISO 10694 and 13878, respectively. The pHCaCl2 and pHH2O were 7.6 and 8.1, respectively. The macro nutrient contents (in g kg−1 biochar) were: K 18.7, Ca 5.41, Mg 2.83, P 2.13, S 0.83 (all: analysis according to ISO 11885) and CaCO3 <10 (ISO 10693); Gaskin et al. 2010 report quite similar values for a peanut hull biochar also produced by Eprida at 400°C. The micro-nutrients or heavy metal contents (in mg kg−1 biochar) were as follows: Al 2900, As 0.545, Cd 0.05, Cu 21.6, Fe 1190, Hg <0.01; Ni 3.75, Pb 1.58 and Zn 42.1 (all: analysis according to ISO 11885). Thus, with 100 t ha−1 biochar, 1,840 kg of N ha−1 and 1,870 kg K ha−1 were applied. Gaskin et al. (2010) conclude from their results that not much of the applied biochar-N must have become plant-available via mineralization, despite a rather low C/N ratio of 38 (here: 39) for a plant-residue based biochar. Potassium, on the other hand, had in the first year contributed to higher tissue-K+ contents but not in the second year (Gaskin et al. 2010). Hence the peanut hull biochar was expected to improve cation nutrition but not to contribute significant labile nitrogen amounts over the course of this study. To avoid side effects due to either nutrient limitations, or probably due to an improved micro-nutrient supply with BC addition, a compound-fertilizer solution including micro-nutrients was applied in three doses (50, 25 and 25 kg N ha−1) with the regular watering on days 1, 25 and 29, respectively (day numbering: see below). The solution contained 8% N including 3.7% NH +4 , 2.3% NO −3 and 2% carbamid-N, 8% P2O5, 6% K2O, 0.01% B, 0.007% Cu, 0.001% Mo and 0.005% Zn.
a Scanning electron microscope (SEM) picture of the structural characteristics of the peanut hull biochar with narrow pores and gaps; b Sampling for greenhouse gas (GHG) flux measurements, figure is partially sliced. FC: foam coating; HS: headspace; PE: grey PE capping; PP: plant pot; RS: rubber septum; S: syringe for gas sampling (details not to scale)
The water holding capacity (WHC) of the soil-biochar mixtures was determined by submerging the entire pots for 24 h, and subsequent draining for another 24 h, and weighing (n = 8 per BC treatment) before the experiment started. Five seeds of Chenopodium quinoa Willd were sown into each pot and seedlings were counted per pot four times during germination. After 9 days (defined as day zero), when seedlings had about four leaves and were approximately 5 cm high, all but the largest seedling per pot were weeded. A WHC of 60% of the control was chosen as “sufficient water supply” treatment, i.e. pots were watered regularly to a target weight set to 60% of the WHC of the control soils without BC. For a “low water supply” treatment, pots were allowed to further dry out during seedling growth in the first 3 weeks of the culture. The low-water supply pots first dried down to 15% WHC. However, since plants showed severe wilting symptoms, the “low water” treatment was adjusted to 20% of the WHC of the control (also in case of the BC treatments) from day 27 on. Afterwards, the pots were weighed every 1 to 2 days, the respective water loss was recorded, and water was applied to achieve the desired target WHC's of 60% and 20% of the control. Since the WHC of the BC-amended soils was significantly larger this treatment ensured that a potential benefit from simply “more water per pot in the BC-amended soils” was excluded.
On day 28 and 29, two plants were accidentally broken during respiration measurements: The respective pots 17 and 22, a BC-200-wet and a BC-200-dry treatment were therefore not included in subsequent analyses.
Measurements performed during plant growth
Greenhouse gas (GHG: CO2, N2O, CH4) flux measurements on the entire plant–soil system as well as gas exchange measurements on the green plant parts were performed during the study. For the GHG fluxes, pots were covered tightly by grey PE chambers constructed from pipes, closed with a lid, and fitted with a rubber septum to allow gas sampling via syringe (Fig. 1b). Chambers or chamber extensions of 10–20 cm length were fitted gas-tight to the pots via elastic rubber seals or a rubber gasket. During chamber closure, pots were set onto soft, water-proof sealing foam to close the pot bottom. Gas samples (50 ml) were withdrawn with 60 ml PE syringes (Pastipak®, Medica, Germany) three times after 0, 2 and 4 h of closure; temperature and air pressure were recorded. Samples were analyzed within 24 h on a gas chromatograph fitted with an electron capture detector (ECD) and a flame ionization detector (FID: Shimadzu GC-14B; analytical scheme after Mosier and Mack 1980) and an automated sample-injection unit for syringes (Loftfield et al. 1997). The exact chamber headspace was calculated from the volume of the chamber itself, the respective extension tube, and the pot space between pot brim and soil surface (V = π * r² * h; h determined as the mean of five measurements per pot surface). Fluxes were calculated according to Hutchinson and Mosier (1981) by linear regression of the concentration increase inside the chambers and related to the total soil weight (respective the soil surface) of the pots. Methane uptake rates were near-zero at the detection limit and hence not reported.
To measure the total aboveground plant respiration, a LI-8100 soil CO2 efflux chamber (LICOR, Nebraska, USA; with the small chamber for 10.2 cm diameter soil frames) was employed 1 day prior to harvest. Immediately before measurement the soil surface around the Quinoa shoot was covered by a flexible diffusion-tight foam disc with a fitting slit. Next, an extension tube was fitted air-tight with a rubber gasket to the pot and the LI-8100 10-cm diameter survey chamber was set on top above the plant. The offset (height) of the entire pot-extension-tube system was entered into the LI-8100-driving software for correct flux calculations. Measurement time and observation delay were set to 120 s and 40 s, respectively, to allow sufficient chamber-volume mixing time. CO2-concentration increase always showed a linear slope with R² >0.9. The respiration rate was expressed per m² (of pot area, automatic function of the LI-8100), but also per g of aboveground dry biomass, alternatively per m² of total leaf area, i.e. based on values that were obtained 1 day later at the final harvest.
The relative chlorophyll content was measured four times on day 23, 31, 37 and 49, respectively, with five replicated measurements on three leaves (youngest fully expanded leaf) per plant using a SPAD 502 device (Minolta, USA).
Plant H2O/CO2 gas exchange measurements at the leaf level
Plant gas exchange characteristics were measured exemplarily on one plant leaf per treatment. Fully expanded upper leaves (10–15 days old) were inserted into a hermetically sealed compact minicuvette system (Waltz, Effeltrich, Germany) with a 500 ml cuvette, type GK-022. Leaf surfaces were positioned horizontally towards a polychromatic lamp, type LA-4, within a distance of 0.05 m. Photosynthetically active photon flux density (PPFD) on the lamina was stepwise increased by light filters NG3 (28 μmol m−2 s−1), NG4 (162 μmol m−2 s−1), NG5 (555 μmol m−2 s−1), NG11 (1,031 μmol m−2 s−1) up to full light (2,050 μmol m−2 s−1). The cuvette was covered with a non-transparent black rag for dark conditions. CO2 concentration in the cuvette was held constant at 380 ± 5 μl l−1 by purifying ambient air via potassium permanganate and afterwards adding CO2. CO2 and H2O concentrations were detected by infrared absorption using a dual comparison analyzer (Binos 100, and CO2 analyzer, type Binos4P, Rosemount, Hanau, Germany). Data acquisition was carried out at the steady state H2O concentration. Apparent photosynthesis efficiency (A), stomatal conductance (cond.) and transpiration rate (transp.) were calculated (based on Fick´s law of diffusion) using data of CO2/H2O concentration variation, ambient air pressure, leaf temperature and leaf area. Leaf water use efficiency was calculated as the ratio of transpiration rate to apparent photosynthesis efficiency. Calculation of the leaf respiration rate (Rleaf) and maximum photosynthesis efficiency (Amax) were done by curve fitting (SigmaPlot, Sysstat Software Inc., Richmond, USA) using the asymptotic saturation function described by Schulte and Brooks (2003).
Quantification of LSU-RuBisCO
Juvenile leaves (one sample per treatment) were ground in liquid nitrogen. A micro spoon of polyvinyl-polypyrrolidon (PVPP) was added. Subsequently, protein digestion was performed according to Granier (1988) in cold (0°C) extraction buffer (100 mM Imidazol and 1.25 mM EDTA; pH 7.8). Next, 75 μg Bovine serum albumin (BSA) was added as an internal standard. Low range proteins (14.3 to 220 kDa) were separated using sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to Laemmli (1970), containing a 6% acrylamide stacking gel and 12.5% acrylamide separation gel. An internal SDS-PAGE molecular weight standard (BIO-RAD Laboratories GmbH, Munich, GER) was used for calibration. Protein staining was done with Coomassie brilliant blue R-250 (B2025-1EA Sigma-Aldrich, St. Louis, USA) for 45 min. Gels were de-stained in 10% acetic acid and digitalized with a flat bed scanner after 1 day. After detection of the molecular weight quantification took place by integrating the signal strength of all impulses of a band with the image processing and analysis program ImageJ (National Institute of Health, Maryland, USA). The large subunit of Ribulose-1,5-bisphosphate-carboxylase/-oxygenase (LSU) was identified as a band in the range of 53 kDa (Ishida et al. 1997). The calculated total amount of LSU-RuBisCO was corrected with the BSA recovery rate (internal standard, s. a.).
Quantification of proline
Proline content was determined according to Bates et al. (1973) 1 day after harvest. Proline was extracted from approximately 50 mg (exact weight noted) of leaf dry mass by homogenizing in 10 ml of 3% (v/v) sulfosalicylic acid using a liquid nitrogen-chilled mortar and pestle. After filtration 1 ml of filtrate was added to 1 ml glacial acetic acid and 1 ml reaction dilution (0.63 g Ninhydrin dissolved in 15 ml glacial acetic acid, 10 ml 6 M phosphoric acid). After agitation and incubation in a water bath at 100°C, the reaction was terminated in ice. The formed colour complex was extracted with 2 ml of toluene. After vortexing absorbance of the toluene extract was recorded at 546 nm (Beckman photometer, Beckman Coulter inc., Fullerton, USA) and final proline concentration was calculated on basis of a standard curve (proline buffered and solved in 3% (v/v) sulfosalicylic acid).
Harvest
Total aboveground plant biomass was harvested on day 50 post germination. Plants had not yet reached full maturity, where they become senescent and dry, but had already begun to produce seeds. Fresh weight and dry weight (at 105°C to weight constancy) were determined of each plant separated into leaves (including stem), shoots, tap roots and seeds. Stem heights were measured, the total leaf number per plant was counted, and the total leaf area per plant was determined with a planimeter (type LI-3000A, Licor, Nebraska, USA). The dry leaf biomass was ground to powder with a Retsch type MM ball mill (Retsch, Düsseldorf, Germany). For the quantification of carbon and nitrogen, approximately 50 mg dry powder was analyzed by combustion with a macro CNS analyzer (type Vario MAX, Elementar Analysensysteme GmbH, Hanau, Germany). Leaves that were removed for subsequent analyses of RuBisCO, proline and osmotic potential were weighed, their area was measured, and the weights added to the final harvest results. The leaf nitrogen use efficiency of productivity NUEProd, i.e. the net primary production (NPP) per unit of nitrogen absorbed (Golluscio 2007) was calculated as aboveground dry matter produced per mg leaf-N.
Osmotic active substances were measured in the press-sap of approx. 1 g fresh leaf material with a cryo-osmometer (Osmomat 030, Gonotec GmbH, Berlin, Germany). Subsequently, cationic macronutrients such as K, Ca, Mg, and Na were measured in diluted solutions (1:10 or 1:100 v/v) with a flame atomic absorption spectrophotometer (PE2100, Perkin Elmer, USA). Anionic macronutrients (such as nitrate, phosphate and sulfate) were determined by ion-exchange chromatography (Metrohm 690 ion chromatograph, Metrohm, SUI).
Statistical analysis
Effects of the two water supply treatments and three levels of BC addition on all fully replicated measurements were tested via two-way analysis of variance (ANOVA). Significance of differences among treatment groups was determined with the Tukey test. Data were log- or root-transformed to normality if necessary. Differences at the P < 0.05 level are reported as significant and P < 0.1 results are reported as trends. All statistical tests were carried out with SigmaPlot 11.0 (Systat Software, Inc., Richmond, USA).
Results
Plant and soil variables at the harvest date
The water holding capacities were 0.223, 0.276 and 0.304 g H2O g−1 soil (mixture) dry weight (dwt) in 0, 100 and 200 t ha−1 soil-BC mixtures, respectively. The BC application significantly increased the WHC by 23.9% and 36.1% compared to the control (one-way ANOVA, P < 0.001, n = 8). After the target value of WHC had been reached (see methods), the total or average daily water consumption of the plants over the experimental duration was significantly lower in all 20% WHC compared to the 60% WHC treatments. In addition, there was a small, but non-significant reduction in water consumption with BC application (Table 1).
As expected, the reduced water availability (WHC 20%) significantly affected nearly all measured variables (Table 1; Figs. 2a, b, 3a), with the exception of the leaf mass to area ratio (LMA, g m−2, Table 1) and the leaf proline concentration (Fig. 3b).
a Mean water use efficiency of productivity (aboveground yield per H2O consumed) and b leaf-nitrogen use efficiency (aboveground dry matter produced per mg leaf-N) plus one standard deviation of the mean of quinoa plants grown under high and low water availability, and with biochar application rates of zero, 100 and 200 t biochar ha−1, respectively; different letters indicate significant differences between treatments
a Mean leaf N concentration, and b mean leaf proline concentration per g leaf dry matter plus one standard deviation of quinoa plants grown under high and low water availability, and with biochar application rates of zero, 100 and 200 t biochar ha−1 respectively; different letters indicate significant differences between treatments
Biochar application significantly increased the total leaf area and leaf biomass per plant in both water treatments (Table 1), while the total number of leaves per plant tended to increase with BC application (P = 0.090, Table 1). Although the mean area per leaf was larger with BC application, the increase was not high enough to become significant. With reduced water supply the average area per leaf tended to be reduced (P = 0.062, Table 1). The biomass of all plant parts and also of the total biomass (Table 1) were significantly increased by BC application (P values between 0.022 and <0.001). Tap root biomass also increased significantly (compared to the respective controls) and, in contrast to other parameters, linearly (P < 0.001) with increasing BC application rates under both water treatments, i.e. by 88% and 191%, or by 63% and 133% at WHC’s of 60% or 20%, respectively. Plant height was significantly lower with reduced water supply, but unchanged due to BC treatment, although the leaf area per plant was significantly larger with BC application (Table 1). H2O content in leaves tended to be higher at 20% WHC, and tended to be lower with BC application, in particular at 60% WHC. Water use efficiency of productivity (WUEProd), i.e. the total amount of aboveground biomass (without tap roots) produced per H2O consumed, generally increased with reduced water supply (F = 228.09, P < 0.001; Fig. 2a). Biochar application further significantly increased (in comparison to the respective controls) WUEProd (F = 63.72, P < 0.001) by +54% and +62%, and by +65% and +52% in well-watered and reduced-watered BC-100 and BC-200 treatments, respectively (Fig. 2a). The highest absolute WUEProd value was reached at 100 t ha−1 BC application and reduced water supply (Fig. 2a: 300% of the well-watered control). Lower water supply reduced leaf nitrogen use efficiency (NUEProd, aboveground dry matter produced per mg leaf-N) compared to high water supply (F = 51.43, P < 0.001), however, BC application significantly increased NUEProd in both water treatments (F = 28.02, P < 0.001), with a significant water × BC interaction (F = 5.415, P = 0.016).
Leaf N concentration, leaf proline concentration (Fig. 3a, b) and the relative chlorophyll content in leaves (Table 2) were all significantly reduced with BC application. However, the total amount of N that taken up into the leaves was nearly identical in all treatments; it was neither effected by BC- nor by water supply-treatments (BC: F = 1.051, P = 0.372; WS: F = 0.179, P = 0.678; over-all mean: 48.6 ± 6.2 mg leaf-N plant−1). Biochar hence significantly widened C to N ratios in plant leaves (P < 0.001), mirroring the reduction of leaf N concentration; the leaf C concentration was unchanged (not shown).
Treatment effects on the plant gas exchange
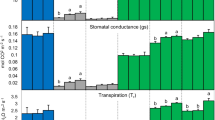
Plant gas exchange measurements at the leaf level were performed with one plant per treatment (Table 2), i.e. are unreplicated. However, each given variable is logically connected to one or more repeated variables (e.g. transpiration with water consumption and leaf area, Amax with N concentration and SPAD etc.). The stomatal conductance (cond.) was high at good soil water availability (Table 2). Low soil water availability (20% WHC) generally decreased the stomatal conductance, resulting in a decrease of transpiration rates, increased the maximum apparent photosynthesis (Amax) at light saturation, and decreased the leaf-level respiration (RLeaf) compared to the higher water availablility. Within both soil water availability levels, BC seemed to decrease transpiration and also Amax compared to the un-amended control, while RLeaf was visibly reduced only with the highest BC application, respectively. Without BC addition the RuBisCo concentration (per g fresh weight) was higher at 20% than 60% WHC, respectively. The relative change of RuBisCo concentration between zero-BC- and highest BC-treatment was much higher than the change of Amax suggesting an increased RuBisCo content.
Leaf osmotic active compounds
Low water supply significantly increased the osmotic value (Fig. 4a; WS: F = 62.22, P < 0.001), which further increased when BC was present, in particular at reduced water supply (BC: F = 11.48, P = 0.002; WS × BC: P = 0.067). Improved osmotic values in leaves with BC application were mainly caused by higher potassium concentrations (Fig. 4b, F = 65.35, P < 0.001) and sucrose. Here, the all-over trends were the same as for K+ (significant WS effect, F = 27.68, P < 0.001), but BC treatment resulted in no significant effects (F = 2.488, P = 0.133). Sulfate concentrations also followed the same increasing trend as sucrose (WS: P < 0.001, BC: P = 0.064). Most other osmotic active substances were in tendency or significantly increased by the lower water supply, but significantly decreased by BC application (Cl−: P = 0.007; Na+: P < 0.001; Ca2+: P < 0.001; Mg2+: P < 0.003). Phosphate tends to be reduced at low-water treatment (WS × BC: P = 0.081). Nitrate was unchanged.
a mean osmotic value in the leaf press-sap and b concentrations of the main osmotic substances potassium (left, open bars) and sucrose (right, hatched bars) per leaf fresh weight plus one standard deviation of quinoa plants grown under high and low water availability, and with biochar application rates of zero, 100 and 200 t biochar ha−1, respectively; different letters (a–d; or x–z) indicate significant differences between treatments
Treatment effects on plant–soil CO2 efflux and soil N2O emissions
At the beginning of the experiment (day 15), BC significantly increased plant–soil CO2 efflux (Rplant+soil, P = 0.013), however this was due to the100-kg BC treatment at 60% WHC which had significantly larger CO2 efflux rates than all other treatments (Table 3). After application of the fertilizer, the effect of BC vanished and remained non-significant in later measurements (Table 3). Rather, the BC treatments tended to have lower soil respiration rates (Rsoil, P = 0.077) expressed on a soil weight basis after harvest. After the targeted low water supply treatment of 20% WHC of the control had been fully established, Rplant+soil or Rsoil efflux rates at 20% WHC were significantly lower (P < 0.001) compared to the 60% WHC treatments. The effect of the water supply (20% vs. 60% WHC) on the aboveground plant respiration was significant, based on the ground area (larger plants). It was much less pronounced or absent, when based on the total plant–leaf area or the aboveground biomass, respectively (Table 3). BC application significantly increased aboveground plant respiration with sufficient water supply, but not with limiting water supply (related to the ground area, Table 3). Respiration was also calculated based on the aboveground biomassFootnote 1 or on the total leaf area1, respectively. This revealed that biomass-based Rplant was significantly reduced in the BC-grown plants compared to respective controls (Table 3). Based on total leaf area, Rplant was significantly reduced with BC application in the 20% WHC treatment but not in the 60% WHC treatment, as indicated by a significant water × BC interaction (Table 3).
N2O emissions were significantly lower (and often near the detection limit) with reduced water supply (Table 4). In the beginning of the study and directly after the first N-fertilizer application, BC-application did not reduce N2O emissions. However, BC reduced N2O emissions significantly during the later part of the study (repeated measures ANOVA, Table 4).
Discussion
The plant response patterns and growth-increasing mechanisms observed in this study with BC amendments were surprising and did not match all initial hypotheses. As intended, the reduced water supply treatment significantly impacted several measured parameters without generating severe water stress. This is indicated by the lack of a significant change of the proline concentrations (Ibarra-Caballer et al. 1988) but increases in the osmotic value.
In accordance with hypothesis #1 (Blackwell et al. 2009), application of BC did increase aboveground biomass of quinoa by 10–61%. However, the largest relative increase occurred at 60%- rather than at 20%-WHC, contradicting hypothesis #1. In two greenhouse studies with radish grown in a hard-setting Chromosol, Chan et al. (2007, 2008) observed biomass improvements and a significantly improved N-fertilizer use efficiency. Rondon et al. (2007) reported increased N2 fixation, legume-biomass and bean yields on BC-amended soils. Lower water consumption and larger biomass resulted in 160% up to 300% greater water use efficiencies with BC under good and low water supply, respectively. Hence, hypothesis #1 was not fulfilled in terms of absolute biomass increases, but in terms of improved relative water use efficiency.
Several mechanisms might contribute to the larger amount of quinoa biomass per water consumption with BC amendments. The water balance of the plants was improved, likely due to several mechanisms: Biochar significantly increased the water holding capacity of the sandy soil due to its porous nature (Fig. 1a; Cheng et al. 2006, Downie et al. 2009, Glaser et al. 2002). However, in this study control-WHCs (60% and 20%) were used as target values. As a consequence, the absolute water amounts in the BC-amended soils were not larger than in the control soils. In line with the findings of Gaskin et al. (2010), BC-addition also increased the overall accumulation of osmotic active substrances such as K+ in the plant tissues, likely due to its large cation content, leading to an improved plant water uptake. Moreover, BC stimulated tap-root growth (i.e. likely also fine root mass) and thus water uptake from fine BC pores. Improved plant water status with BC was also reflected by lower proline concentrations and higher osmotic values of the leaves, indicating a higher tolerance to potential water stress conditions (Barker et al. 1993; Gonzalez et al. 2009). Increased potassium concentrations in leaves were either due to an improved K+ nutrition via the BC as a nutrient carrier, to an increase of the osmolarity in the soil solution due to BC application or to better binding and access of the K+ that was repeatedly supplied in equal amounts to all treatments during the study. However, most of the K+ initially introduced with the BC will likely have been lost when the soil mixture pots were flooded and drained (washed) during initial the WHC determination.
A second reason for BC-mediated higher plant growth might be a reduced transpiration (Table 2). Together with increased osmolarity this might induce an improved drought tolerance. Biochar-plants used slightly less water despite larger leaf areas. The higher total-biomass-WUE was in line with a higher WUE of photosynthesis (WUEP).
Significant larger leaf areas of BC-grown plants allowed higher C gain. They were based on non-significantly larger mean leaf areas and leaf numbers, respectively.
The tap root biomass increased strongly with BC, with a shift towards a more pronounced belowground stimulation: the tap-root-to-shoot ratio showed a highly significant effect of BC application (P < 0.001), but no effect of the different water addition levels (P = 0.287; without interactions). Larger root systems or root biomass of BC-grown plants have also been reported by e.g. Major et al. (2010).
A further contribution could be a more efficient leaf-nitrogen use. The nitrogen concentration of 3.3% and 4% in the control leaves indicate that the applied N-fertilization was sufficient. However, the BC plants seemingly diminished the N-pool by stronger growth, which leads to lower leaf-N concentrations. The latter fits with the significantly reduced proline-, chlorophyll- and RuBisCo-concentrations and slightly reduced Amax rates in the BC plants. Hence, our results were in contrast to hypothesis #2 that these parameters remain unchanged.
The reduced dark respiration rates per g of dry mass were also in good accordance to the lower leaf-N concentrations (Reich et al. 2006). Therefore, reduced leaf-tissue N concentrations could have employed another growth-stimulating mechanism: reduced respiratory carbon losses per unit of carbon gain, i.e. a greater efficiency in the use of assimilated carbon. The soil respiration tended to decrease in presence of BC. This was surprising and in contrast to our expectation of increased soil respiration with larger plants (hypothesis #3). In summary, BC application improved quinoa growth via several interconnected mechanisms such as the plant water status and its water-, nitrogen- and respiratory carbon-use efficiency.
Many of the measured parameters were surprisingly similar to the stimulating effects that elevated atmospheric CO2 concentrations can have on plant growth (Owensby et al. 1999; Nösberger et al. 2006; Nowak et al. 2004). Elevated CO2 improves the WUE of plants which could even lead to soil moisture increases (Morgan et al. 2004). Analogous to BC, elevated CO2 usually decreases plant tissue N concentrations (e.g. Cotrufo et al. 1998), independent of the N supply, and subsequently protein concentrations such as RuBisCO (Stitt and Krapp 1999). However, the analogy does not include CO2 gas exchange. While photosynthesis, plant dark respiration and soil respiration usually increase under elevated CO2 (Long et al. 2006; Ainsworth and Rogers 2007), the opposite seems to occur with BC, contradicting hypothesis #4. Hence elevated CO2 and BC effects have many symptomatic similarities, but likely different mechanisms, which warrant further study. A mechanism involved in the stimulatory effect of BC on plant growth may have been the production of the phytohormone ethylene from either the added BC itself, or microbially mediated from the BC-amended soils, a finding recently reported by Spokas et al. (2010). This would be in line with the linear stimulation response of the tap root biomass with increasing char application rates where the biomass in the 100 t ha−1 BC application was double, and that of the 200 t ha−1 BC application was three times that of the control biomass, respectively.
We anticipated that soil-derived CO2 effluxes would be initially stimulated (hypothesis #3) due to priming (Wardle et al. 2008) or initial oxidation (Cheng et al. 2006). However, we found evidence against such mechanism, since larger BC application of 200 t BC ha−1 yielded in significantly lower CO2 effluxes than 100 t ha−1. In a recent 3.2 year lab study, the 14C-labelled BC lost less than <0.5% per year indicating that BC was quite stable against degradation (Kuzyakov et al. 2009). In our study, priming of pre-existing soil organic carbon (SOC) by BC application must have been too small, too short-lived, or simply non-existent to be detectable in the respiration rates (compare Lehmann et al. 2009; Liang et al. 2010). In agreement with our findings, significantly reduced soil CO2 respiration were reported by Kuzyakov et al. (2009), Spokas et al. (2009) or Novak et al. (2010). However, Kolb et al. (2009) observed increasing basal respiration rates, microbial biomass and activity with increasing rates of charcoal application in four different Wisconsin soils. The authors attribute this to accelerated old SOC mineralization after charcoal application. In a study in acidic tropical plantation soils, Steiner et al. (2007) reported increased basal respiration values but rising microbial efficiency (less respiratory CO2 loss per unit of microbial biomass-C). Hence it is not clear yet, if a general soil-respiration response pattern to BC addition exists or not. Our findings suggest that the plant–soil system loses less C via respiration per g C of produced plant biomass, when it is BC-amended. Although, one of the most crucial, but to date unanswered, questions is, whether reduced soil respiration after BC application indicates a decrease in soil fertility, i.e. if it is beneficial or not. Hence, long-term field studies are required.
As assumed (hypotheses #3 and #4), N2O emissions were not immediately reduced by the presence of BC, although they decreased when plants became larger (day 28 and day 51 of the study). A reduction in N2O emissions is in agreement with recent reports (e.g. Yanai et al. 2007; Lehmann 2007a; Spokas et al. 2009; van Zwieten et al. 2009; Taghizadeh-Toosi et al. 2011). Reduced water supply strongly reduced N2O emissions, indicating that denitrification was the dominant N2O-generating process (Granli and Bøckmann 1994). The observed reduction in N2O emissions in the presence of BC could have had several reasons: (a) a better soil aeration by the porous BC which reduces denitrification (Groffman and Tiedje, 1991), (b) adsorption of ammonium nitrogen (NH +4 ) to the charged BC surface in a way that probably reduced nitrification (and hence subsequent denitrification of NO −3 ), and (c) the increasing plant (root) biomass that likely outcompeted microbes for mineral N species (Smith and Tiedje 1979). We argue that soil aeration (a) and increasing plant biomass (c) are the most likely mechanisms. Since WHC was set to 60% or 20% of the controls, BC-amended soils which could hold more water than the controls were physiologically drier i.e. better aerated. More effort is clearly needed to understand the mechanisms of N2O-emission reduction in presence of BC (Clough and Condron 2010).
We expected that a very large BC application doses (200 t ha−1) would have negative effects (hypothesis #5). We found no evidence for this, only a slight improvement compared to the 100 t ha−1 rate. This is in line with findings of Chan et al. (2007, 2008) who also observed that the positive BC effect exhibited a kind of saturation curve. It may be necessary to investigate dose–response relationships prior to field trials to roughly assess the most (cost-) effective BC application rate which will strongly depend on biochar as well as soil type and agricultural management practices.
Taken together, BC application reduced the efflux of two potent greenhouse gases from the plant–soil system, while more biomass was produced and carbon sequestered. We conclude that for quinoa grown in a sandy soil application of BC can be beneficial and may thus warrant subsequent field trials.
Notes
Calculated with the total dry matter and leaf area at the harvest date, but without the few leaves that were harvested earlier for proline- Rubisco- and osmotic potential analyses.
Abbreviations
- BC:
-
Biochar
- WUE:
-
Water use efficiency
- NUE:
-
Nitrogen use efficiency
- WHC:
-
Water holding capacity
- SOC:
-
Soil organic carbon
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270. doi:10.1111/j.1365-3040.2007.01641.x
Amonette JE, Joseph S (2009) Characteristics of biochar: microchemical properties. In: Lehmann J, Joseph S (eds) Biochar for environmental management—science and technology. Earthscan, London, pp 33–52
Barker DJ, Sullivan CY, Moser LE (1993) Water deficit effects on osmotic potential, cell wall elasticity, and proline in five forage grasses. Agron J 85:270–275
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Blackwell P, Riethmuller G, Collins M (2009) Biochar application to soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 207–226
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2007) Agronomic values of greenwaste biochar as a soil amendment. Aust J Soil Res 45:629–634. doi:10.1071/SR07109
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Aust J Soil Res 46:437–444. doi:10.1071/SR08036
Chan KY, Xu Z (2009) Biochar: nutrient properties and their enhancement. In: Lehmann J, Joseph S (eds) Biochar for environmental management: science and technology. Earthscan, London, pp 67–84
Cheng C-H, Lehmann J, Thies JE, Burton AJ, Engelhard M (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37:1477–1488
Cheng C-H, Lehmann J, Thies JE, Burton SD (2008) Stability of black carbon in soils across a climatic gradient. J Geophys Res 113: doi:10.1029/2007JG000642
Clough TJ, Condron LM (2010) Biochar and the nitrogen cycle: Introduction. J Environ Qual 39:1218–1223.
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Global Change Biol 4:43–54
Downie A, Crosky A, Munroe P (2009) Physical properties of biochar. In: Lehmann J, Joseph S (eds) Biochar for environmental management—science and technology. Earthscan, London, pp 13–32
Elad Y, David DR, Harel YM, Borenshtein M, Kalifa HB, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology 100:913–921
Galwey NW (1989) Quinoa. Biologist 36:5
Gaskin JW, Speir RA, Harris K, Das KC, Lee RD, Morris LA, Fisher DS (2010) Effect of peanut hull and pine chip biochar on soil nutrients, corn nutrient status, and yield. Agron J 102:623–633
Gaunt JL, Lehmann J (2008) Energy balance and emissions associated with biochar sequestration and pyrolysis bioenergy production. Environ Sci Technol 42:4152–4158
Glaser B (2007) Prehistorically modified soils of central Amazonia: a model for sustainable agriculture in the twenty-first century. Phil Trans R Soc London B 362:187–196
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The ‘Terra Preta’ phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41
Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—a review. Biol Fertil Soils 35:219–230
Golluscio RA (2007) On the link between nitrogen productivity and residence time: two opposite nitrogen use strategies? J Arid Environ 68:165–169
Gonzalez JA, Gallardo M, Hila LM, Rosa M, Prado FE (2009) Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and waterlogging stresses: dry matter partitioning. Bot Stud 50:35–42
Graber ER, Harel YM, Kolton M, Cytryn E, Silber A, David DR, Tsechansky L, Borenshtein M, Elad Y (2010) Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant and Soil 337:481–496
Granier F (1988) Extraction of plant proteins for two-dimensional electrophoresis. Electrophoresis 9:112–718
Granli T, Bøckmann OC (1994) Nitrous oxide from agriculture. Norweg J Agr Sci Supp 12:1–128
Groffman PM, Tiedje JM (1991) Relationships between denitrification, CO2 production and air-filled porosity in soils of different texture and drainage. Soil Biol Biochem 23(3):299–302
Hansen J, Sato M, Kharecha P, Beerling D, Berner R, Masson-Delmotte V, Pagani M, Raymo M, Royer DL, Zachos JC (2008) Target atmospheric CO2: where should humanity aim? Open Atm Sci J 2:217–231
Hutchinson GL, Mosier AR (1981) Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci Soc Am J 45:311–316
Ibarra-Caballero J, Villanueva-Verduz C, Molina-Galan J, Sanchez-de-Jimenez E (1988) Proline accumulation as a symptom of drought stress in maize: a tissue differentiation requirement. J Exp Bot 39:889–897
IPCC (2007a) Climate change 2007: Climate change impacts, adaptation and vulnerability. Working Group II Contribution to the Intergovernmental Panel on Climate Change Fourth Assessment Report—Summary for Policymakers. IPCC, Bern
IPCC (2007b) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Ishida H, Nishimori Y, Sugisawa M, Makino A, Mae T (1997) The large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase is fragmented. Plant Cell Physiol 38:471–479
Jacobsen S-E, Stølen O (1993) Quinoa—morphology and phenology and prospects for its production as a new crop in Europe. Eur J Agron 2:19–29
Jensen CR, Jacobsen S-E, Andersen MN, Núñez N, Andersen SD, Rasmussen L, Mogensen VO (2000) Leaf gas exchange and water relation characteristics of field quinoa (Chenopodium quinoa Willd.) during soil drying. Eur J Agron 13:11–25
Kim J-S, Sparovek G, Longo RM, De Melo WJ, Crowley D (2007) Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol Biochem 39:684–690
Kimetu JM, Lehmann J, Kinyangi JM, Cheng CH, Thies J, Mugendi DN, Pell A (2009) Soil organic C stabilization and thresholds in C saturation. Soil Biol Biochem 41:2100–2104
Kolb SE, Fermanich KJ, Dornbush ME (2009) Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci Soc Am J 73:1173–1181. doi:10.2136/sssaj2008.0232
Kuzyakov Y, Subbotina I, Chen H, Bogomolova I, Xu X (2009) Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol Biochem 41:210–219. doi:10.1016/j.soilbio.2008.10.016
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 27:680–685
Laird DA, Brown RC, Amonette JE, Lehmann J (2009) Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod Biorefin 3:547–562
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627. doi:10.1126/science.1097396
Lal R (2009) Challenges and opportunities in soil organic matter research. Eur J Soil Sci 60:158–169
Lehmann J (2006) Bio-char sequestration in terrestrial ecosystems—a review. Mitigat Adaptat Strat Glob Chan 11:403–427. doi:10.1007/s11027-005-9006-5
Lehmann J (2007a) Bio-energy in the black. Front Ecol Environ 5:381–387
Lehmann J (2007b) A handful of carbon. Nature 447:143–144
Lehmann J, Czimczik C, Laird D, Sohi S (2009) Stability of biochar in soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management—science and technology. Earthscan, London, pp 183–205
Liang B, Lehmann J, Sohi SP, Thies JE, O'Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizão FJ (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem. doi:10.1016/j.orggeochem.2009.09.007
Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'Neill B, Skjemstad JO, Thies J, Luizao FJ, Petersen J, Neves EG (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730. doi:10.2136/sssaj2005.0383
Loftfield N, Flessa H, Augustin J, Beese F (1997) Automated gas chromatographic system for rapid analysis of the atmospheric trace gases methane, carbon dioxide, and nitrous oxide. J Environ Qual 26:560–564
Long SP, Zhu X-G, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29:315–330
Major J, Lehmann J, Rondon M, Goodale C (2010) Fate of soil-applied black carbon: downward migration, leaching and soil respiration. Global Change Biol 16:1366–1379. doi:10.1111/j.1365-2486.2009.02044.x
Marris E (2006) Putting the carbon back: black is the new green. Nature 442:624–626
Mathews JA (2008) How carbon credits could drive the emergence of renewable energies. Energ Pol 36:3633–3639
McHenry MP (2009) Agricultural bio-char production, renewable energy generation and farm carbon sequestration in Western Australia: certainty, uncertainty and risk. Agric Ecosys Environ 129:1–7
Morgan JA, Pataki DE, Körner C, Clark H, Del Grosso SJ, Grünzweig JM, Knapp AK, Mosier AR, Newton PCD, Niklaus PA, Nippert JB, Nowak RS, Parton WJ, Polley HW, Shaw MR (2004) Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140:11–25
Mosier AR, Mack L (1980) Gas chromatographic system for precise, rapid analysis of nitrous oxide. Soil Sci Soc Am J 44:1121–1123
Nösberger J, Long SP, Norby RJ, Stitt M, Hendrey G, Blum H (2006) Managed ecosystems and CO2: case studies, processes, and perspectives, vol 187. Ecological studies. Springer, Berlin, pp 457
Novak JM, Busscher WJ, Watts DW, Laird DA, Ahmedna MA, Niandou MAS (2010) Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 154:281–288
Nowak RS, Ellsworth DS, Smith SD (2004) Functional responses of plants to elevated atmospheric CO2—do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol 162:253–280
Owensby CE, Ham JM, Knapp AK, Auen LM (1999) Biomass production and species composition change in a tallgrass prairie ecosystem after long-term exposure to elevated atmospheric CO2. Global Change Biol 5:497–506
Reich PB, Tjoelker MG, Machado J-L, Oleksyn J (2006) Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439:457–461
Rondon M, Lehmann J, Ramírez J, Hurtado M (2007) Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol Fertil Soils 43:699–708
Schulte PJ, Brooks JR (2003) Branch junctions and the flow of water through xylem in Douglas-fir and ponderosa pine stems. J Exp Bot 54:1597–1605. doi:10.1093/jxb/erg169
Smith MS, Tiedje JM (1979) The effect of roots on soil denitrification. Soil Sci Soc Am J 43:951–955. doi:10.2136/sssaj1979.03615995004300050027x
Sperry JS, Hacke UG (2002) Desert shrub water relations with respect to soil characteristics and plant functional type. Funct Ecol 16:367–378
Spokas KA, Koskinen WC, Baker JM, Reicosky DC (2009) Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 77:574–581
Spokas KA, Baker JM, Reicosky DC (2010) Ethylene: potential key for biochar amendment impacts. Plant Soil 333:443–452
Steiner C, Glaser B, Teixeira WG, Lehmann J, Blum WEH, Zech W (2008) Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171:893–899. doi:10.1002/jpln.200625199
Steiner C, Teixeira M, Zech W (2007) Soil respiration curves as soil fertility indicators in perennial central Amazonian plantations treated with charcoal, and mineral or organic fertilisers. Trop Sci 47:218–230. doi:10.1002/ts.216
Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22:583–621
Taghizadeh-Toosi A, Clough TJ, Condron LM, Sherlock RR, Anderson CR, Craigie RA (2011) Biochar incorporation into pasture soil suppresses in situ nitrous oxide emissions from ruminant urine patches. J Environ Qual, in press (open access) https://www.soils.org/publications/jeq/view/40-42/q10-0419.pdf
van Zwieten L, Singh B, Joseph S, Kimber S, Cowie A, Chan KY (2009) Biochar and emissions of non-CO2 greenhouse gases from soil. In: Lehmann J, Joseph S (eds) Biochar for environmental management—science and technology. Earthscan, London, pp 227–249
van Zwieten L, Kimber S, Morris S, Downie A, Berger E, Rust J, Scheer C (2010) Influence of biochars on flux of N2O and CO2 from Ferrosol. Aust J Soil Res 48:555–568
Wardle DA, Nilsson M-C, Zackrisson O (2008) Fire-derived charcoal causes loss of forest humus. Science 320:629. doi:10.1126/science.1154960
Yanai Y, Toyota K, Okazaki M (2007) Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 53:181–188
Acknowledgements
The authors want to thank Christoph Forreiter for critical reading of the manuscript and Judy Libra for proof reading. The authors acknowledge the technical assistance of Nicol Strasilla and Gerlinde Lehr with proline and RuBisCO extractions and greenhouse gas analyses and Gerhard Mayer for his assistance at the ion-chromatograph. Thanks to Johanna Kreiling for technical assistance, and to the Department of Applied Microbiology, in particular to Stefan Ratering, for help with the GC analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johannes Lehmann.
Rights and permissions
About this article
Cite this article
Kammann, C.I., Linsel, S., Gößling, J.W. et al. Influence of biochar on drought tolerance of Chenopodium quinoa Willd and on soil–plant relations. Plant Soil 345, 195–210 (2011). https://doi.org/10.1007/s11104-011-0771-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-011-0771-5