Abstract
Panax quinquefolium (American ginseng) is a perennial understory herb that has been widely used as a medicinal plant in China and other countries. Autotoxicity has been reported to be one of the major problems hindering the consecutive cultivation of American ginseng. However, the potential autotoxic compounds produced by the root of American ginseng are less well known. Here, we report the isolation and characterization of five groups of autotoxic compounds from aqueous extracts of the fibrous roots of American ginseng. Ether extracts of the water-soluble compounds were further analyzed and separated into seven fractions. Among them, the most autotoxic fraction (Fraction V) was subjected to GC/MS analysis, and 44 compounds were identified. Based on literature information, 14 individual compounds were selected and their autotoxic effects on seedling growth were further tested. The results revealed that, of these 14 compounds, 9 phenolic compounds significantly reduced the growth of seedlings in a concentration-dependent manner, while 5 aliphatic compounds showed modest inhibition at all three concentrations tested. Furthermore, we verified the existence of the autotoxic compounds in the plow layer soil of commercially cultivated American ginseng fields, and the concentration of these compounds as determined by HPLC analysis was in line with the concentration determined to be bioactive. Taken together, our study established a functional link between the compounds produced by American ginseng and their autotoxic effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
American ginseng, Panax quinquefolium L. Araliaceae, originating from North America, has a long history of medicinal uses throughout the world. It has been used in Chinese herbal medicine for almost 200 years. However, commercial cultivation in China has only caught on in the past few decades. One of the major problems is that replantation in old fields usually fails, and it takes up to 10–15 years for previously cultivated fields to recover. The following factors have been suggested to contribute to the problem: (1) soil nutrient element depletion, (2) plant diseases (soil sickness), and (3) autotoxicity. It has been suggested that the autotoxicity is the most frequent cause of reseeding failure (Zhao et al. 2005a, 2005b).
Autotoxicity refers to a phenomenon whereby mature plants inhibit the growth of seedlings of their own species through the release of autotoxic chemicals into the environment. It has been suggested that autotoxicity plays a major role in population density control in natural systems (Mahall and Callaway 1992; Perry et al. 2005). The prevalence of autotoxicity has been reported in a number of plant species including crops and weeds in agroecosystems and wastelands (Blum and Gerig 2005; Ervin and Wetzel 2000; Singh et al. 1999). In agricultural practices, autotoxicity often leads to reductions in crop yields, as well as difficulties in reestablishing plants in old fields due to low seed germination and poor seedling growth. Several groups of compounds, such as terpenoids and steroids, phenols, coumarins, flavonoids, tannins, alkaloids, and cyanogenic glycosides, have been implicated in producing autotoxicity. Phenolic compounds in particular have been extensively studied with regard to their phytotoxicity (Canals et al. 2005; Rice 1984).
Autotoxicity in American ginseng has been the subject of many investigations during the past 10 years (Zhao et al. 2005a); however, few studies have attempted to identify the autotoxic compounds involved. In this study, we aimed to identify substances that contribute directly to the autotoxicity of American ginseng. A number of potentially autotoxic compounds from the fibrous roots of American ginseng were isolated and characterized. The inhibitory effect of these compounds on American ginseng seedling growth was determined. Furthermore, we determined the concentration of these bioactive compounds in the plow layer soil collected from 4-year-old American ginseng fields.
Materials and methods
Extraction, isolation, and identification of autotoxic chemicals
Fibrous roots of 4-year-old American ginseng plants at the harvest stage of growth were collected from commercially cultivated fields in Huai-rou County (116°62’E, 40°32’N), Beijing, in September 2006. Fibrous roots were air-dried and then soaked in water for 24 h at room temperature. This was repeated three times. An aqueous extract was concentrated under vacuum (60°C) to approximately one-third of the original volume. This was then partitioned with ethyl ether. The ether extracts were concentrated under vacuum and freeze dried under N2 atmosphere. Approximately 7.5 g dried material was obtained from 4 kg air-dried fibrous roots.
The ether extracts were subjected to a silica gel column, and eluted with a gradient of CHC13–methanol (MeOH) (10:0 to 0:10, v/v) to yield seven fractions. The solvents were evaporated under vacuum and freeze dried to yield seven fractions: fractions I (65 mg), II (65 mg), III (60 mg), IV (400 mg), V (1,020 mg), VI (1,080 mg), and VII (3,100 mg). Of the seven fractions, Fraction V exhibited the greatest inhibitory effect on radical growth of young seedlings of American ginseng (see below). Thus, Fraction V was further purified using reversed phase silica gel column chromatography (ODS-A S-50 μm, YMC, Kyoto, Japan) and eluted with a gradient of CH3OH–H2O (3:7 to 10:0, v/v) to yield the following five compounds: C1–C5. The structure of compounds C1–C5 was determined using Nuclear magnetic resonance spectroscopy (NMR) (500 MHz; Bruker Biospin, Fällanden, Switzerland) and MS (Autospec-UltimaETOF mass spectroscopy; Micromass, Manchester, UK) analyses.
The identity and spectral characteristics of compounds C1–C5 are as follows:
-
C1: vanillic acid, colorless crystal, mp 197–199°C, molecular formula: C8H8O4; EI-MS m/z: 168 [M+], 153, 151, 125, 97; 1H NMR (CD3OD): 12.41 (1H, brs, COOH), 9.70 (1H, brs, OH-4), 7.50 (1H, d, J = 2.0 Hz, H-2), 7.48 (1H, dd, J = 8.0 Hz and 2.0 Hz, H-6), 6.76 (1H, d, J = 8.0 Hz, H-5), 3.82 (3H, s, 3-OCH3); 13C NMR (CD3OD): 167.5 (-COOH), 150.8 (C-3), 146.8 (C-4), 123.6 (C-6), 121.3 (C-1), 115.2 (C-5), 112.7 (C-2), 55.9 (-OCH3).
-
C2: (P)-coumaric acid, pale yellow crystal, mp 208–210°C, molecular formula: C9H8O3; EI-MS m/z: 164 [M+], 147, 119, 107, 91; 1H NMR (CD3OD): 7.53 (1H, d, J = 16.0 Hz, H-7), 7.38 (2H, d, J = 8.5 Hz, H-2 and H-6), 6.74 (2H, d, J = 8.5 Hz, H-3 and H-5), 6.23 (1H, d, J = 16.0 Hz, H-8); 13C NMR (CD3OD): 170.5 (C-9), 160.9 (C-4), 146.8 (C-7), 130.6 (C-2 and C-6), 126.7 (C-1), 116.1 (C-3 and C-5), 114.9 (C-8).
-
C3: azelaic acid, pale yellow crystal, mp 102–104°C, molecular formula: C9H16O4; EI-MS m/z: 152 [M+], 124, 111, 98, 84, 69, 55; 1H NMR (CD3OD): 2.22 (4H, t, J = 7.0 Hz, H-2 and H-8), 1.54 (4H, m, J = 7.0 Hz, H-3 and H-7), 1.29 (6H, s, H-4, H-5 and H-6).
-
C4: palmitic acid, white solid, mp 61–64°C, molecular formula: C16H32O2; EI-MS m/z: 256 [M], 213, 129, 98, 87, 83, 73, 69, 55; 1H NMR (CDCl3): 2.23 (2H, t, J = 8.0 Hz, H-2), 1.54 (2H, m, H-3), 1.26 (24H, m, H-4 ∼ H-15), 0.84 (3H, s, H-16).
-
C5: (9Z,12Z)-linoleic acid, pale yellow oil, molecular formula: C18H32O2; EI-MS m/z: 280 [M+], 256, 213, 199, 185, 171, 157, 149, 143, 129, 115, 101, 87; 1H NMR (CD3OD): 5.25 ∼ 5.32 (4H, m, H-9. H-10, H-12 and H-13), 2.72 (2H, t, J = 6.5 Hz, H-11), 2.21 (2H, t, J = 7.0 Hz, H-2), 1.96 ∼ 2.03 (4H, m, H-8 and H-14), 1.54 (2H, m, H-3), 1.23 ∼ 1.28 (15H, m, H-8 and H-14), 0.84 (3H, m, H-18).
GC-MS analysis
The bioactive Fraction V was analyzed using GC-MS to determine the composition of all compounds. Analysis was performed according to the method of Porter et al. (1985) using a Thermo-Trace GC ultra 2000 (coupled to a Thermo-Trace DSQ Mass Selective Detector) equipped with a DB-1MS capillary column (30 mm × 0.25 mm × 0.25 μm, Agilent Technologies, Santa Clara, CA). Fraction V was dissolved in 2 mL redistilled MeOH. A 1 μL aliquot of this solution was evaporated to dryness under a stream of helium to remove residual water. A mixture of 10 μL redistilled MeOH and 10 μL N, O-Bis (trimethylsilyl) trifluoro acetamide (BSTFA) + 1% trimethylchlorosilane (TMCS) was added to the residue to produce trimethylsilyl derivatives by treating at 100°C for 1 h. The solution was filtered through 0.45-μm filters (Whatman, Buckinghamshire, UK) before being injected into the GC-MS system. The injection volume was 1 μL. The purge flow rate was set to 50 mL/min. Helium (99.995%) was used as the carrier gas. Initial head pressure was maintained at 8.80 psi and a constant flow rate of 0.8 mL/min was set throughout the run. Oven temperature was initially programmed at 50°C and ramped to 270°C at a rate of 5°C /min, where it remained for 10 min. Injector temperature and mass selective detector (MSD) transfer line heaters were kept constant at 250°C and 280°C, respectively. The mass spectrometer parameters for electron ionization (EI) mode were: ion source, 200°C; MS quadrupole, 150°C; electron energy, 70eV; Electron Multiplier Energy, 1,100–1,200 V. Data were acquired in scan mode from 50 to 650 amu.
Extraction of soil samples
Soil samples were collected during September 2006 at two sites at Huai-rou County, Beijing, China. The samples were collected from the plow layer soil of commercially cultivated fields that have been growing American ginseng for 4 years. The fields were cultivated as monoculture under artificial shade to mimic the natural ginseng growing environment. A total of five samples were collected (see Table 4). Samples 1–3 were from different quadrants of the same field in Nianfeng village. Sample 4 was from rhizosphere soil in Nianfeng village, and sample 5 was collected in Sijitun village. The control sample was from uncultivated soil in Nianfeng village.
The soil samples were sieved (4-mm mesh), air-dried, and stored at room temperature in the laboratory according to the method of Dalton et al. (1987). The extraction method was a modification of that of Whitehead et al. (1981; 1983) and Dalton (1999). Briefly, 20 g soil was added to 150 mL 2 mol/L NaOH solution and agitated for 24 h with a reciprocal shaker (WR-1, Shanghai S.R.D Scientific Instruments, Shanghai, PR China). The soil suspension was centrifuged at 5,000 g for 15 min (LG16-W Beijing Jingli Centrifuge, Beijing, PR China), and the supernatant was filtered through filter paper (Whatman No. 1). The pH of the filtrate was adjusted to 2.5 with 5 mol/L HCl, and extracted five times with ethyl acetate. The resulting extracts were pooled and evaporated to dryness under N2 atmosphere at 40°C. The residue was dissolved in 5 mL 80% MeOH and kept in the dark at 4°C. Each soil sample was processed in duplicate, and the final extracts were analyzed using high-performance liquid chromatography (HPLC).
HPLC analysis
The concentration of autotoxic compounds in soil samples was determined using a Waters HPLC system (Waters, Milford, MA) equipped with a Waters 600 pump, a Waters 600 system controller, a 2996 UV-DAD detector and model 7752 injector equipped with 20-μL sample loop. Nine phenolic compound standards were purchased from Sigma (St. Louis, MO). All separations were performed with a YMC-Pack ODS-A column (25 cm × 4.6 mm i.d., 5 μm), and the flow rate was kept constant at 0.8 mL/min. Detection was performed at 280 nm. The injection volume was 20 μL and the column temperature was maintained at 25°C. The chromatographic data were recorded and processed with a Waters empower workstation. The concentrations of each compound in the soil samples were obtained based on peak areas using external standards and are expressed as milligrams per kilogram of dry soil.
HPLC separations were conducted using the following mobile phase solutions: mobile phase A was methanol, and mobile phase B consisted of 0.01 mol/L potassium dihydrogen phosphate in water (regulated to pH 2.8 with phosphate). The linear gradient system ranged from 20% B to 100% B over a period of 60 min (Banwart et al. 1985). The rate of recovery for each of the nine phenolic compounds found in the soil samples (see Table 4) was determined using the corresponding standards. The separation procedures were repeated six times for each standard compound, and data represent mean ± SD. The specific recovery rates are (%): cinnamic acid, 94.9 ± 4.7; p-coumaric acid, 99.3 ± 4.8; ferulic acid, 89.5 ± 6.1; vanillic acid, 90.5 ± 4.3; vanillin, 90.1 ± 6.1; salicylic acid, 89.1 ± 2.6; syringic acid, 90.9 ± 5.6; benzoic acid, 90.4 ± 4.5; and p-hydroxybenzoic acid, 94.9 ± 5.4.
Bioassays
To examine the functional effect of potential autotoxic compounds identified in American ginseng, bioassays were performed according to methods described previously by Chon and Kim (2002) and Zhao et al. (2005a). American ginseng seeds were sterilized with distilled water:Clorox (95:5) for 10 min, and rinsed 4 times with distilled water. Ten American ginseng seeds with radicals of 1 mm length were separately placed into 9-cm Petri dishes containing double-layered filter paper (Whatman No. 1). The ether-extracted fractions I-VII were diluted with distilled water into 0.5, 1 and 2 mg/mL, respectively. Each diluted solution of 5 mL was transferred to the Petri dishes. Individual compounds including five aliphatic and nine phenolics as identified in fraction V were dissolved in a small volume of methanol and transferred onto double-layered filter paper. The solvents were evaporated in a draft chamber for 1 h. The filter paper containing these compounds was moistened with 5 mL distilled water. The final concentrations of the compounds in water were 0.1, 1, and 10 mmol/L; distilled water was used as a control. The Petri dishes were covered and wrapped with Parafilm, and cultivated in a growth chamber at 24°C in dark. The experiments were repeated three times at each concentration. Radical lengths were measured on day 10 following treatment.
Statistical analysis
All treatments were arranged in a completely randomized design. Data were analyzed with SPSS (version 10.0) (http://www.spss.com/) using Duncan’s test.
Results
Effect of root extracts on growth of American ginseng seedlings
To determine whether aqueous extracts of American ginseng root contain autotoxic chemicals, we performed bioassays with each of the ether fractions (Fractions I–VII) to examine their ability to inhibit the growth of American ginseng seedlings. There was a 97–100% reduction in the length of the seedlings in the presence of Fraction V (P < 0.01; Table 1), indicating that chemical compounds residing in Fraction V have a major auto-inhibitory effect on seedling growth of American ginseng. Thus, Fraction V was considered a good candidate for further identification of potential autotoxic compounds.
Purification and characterization of compounds in bioactive fraction V
Since Fraction V contains potential autotoxic compounds, we performed a GS/MS analysis to determine the composition of this fraction. By comparing with gas chromatography and mass spectrometry (GC/MS) user-library spectra of pure reference compounds, we identified a total of 44 compounds in Fraction V (Table 2). Interestingly, several aliphatic acids (e.g. stearic acid, oleic acid, and dodecanoic acid) and phenolic acids (e.g. salicylic acid, syringic acid, and ferulic acid) identified here have previously been reported to have phytotoxic properties (Blum and Gerig 2005; Machrafi et al. 2006; Rice 1984). To confirm the results obtained by GC/MS analysis, we further purified five compounds from Fraction V using reverse-phase silica gel chromatography. These compounds were identified to be vanillic acid, (P)-coumaric acid, azelaic acid, palmitic acid, and (9Z,12Z)-linoleic acid (C1–C5, respectively) using NMR and MS analyses. All five compounds purified are among the compounds identified by GC/MS analysis. The specific characteristics of these compounds are listed in the Materials and methods section.
Potential autotoxic effect of compounds identified in bioactive Fraction V
Previous studies have suggested that several of the chemicals found in the bioactive Fraction V are potential allelopathic compounds secreted by other crops (Bertin et al. 2003; Kalinova et al. 2007; Seal et al. 2004). Based on these published results and the availability of the chemicals in Fraction V, we chose nine phenolic compounds and five aliphatic acids to test their functional effect on the growth of American ginseng seedlings. The compounds were numbered 1–14 for the sake of simplicity (Table 3). The first nine compounds belong to the phenolic acid family, while the last five compounds are aliphatic acids. Among the 14 compounds examined, the phenolic compounds showed various degrees of dose-dependent inhibition on American ginseng seedling growth. Specifically, seedling radical length was reduced significantly (by 41–95%) when treated with the phenolic compounds at a concentration of 10 mmol/L (P < 0.01). Salicylic acid and benzoic acid were the most potent compounds tested. Most of the compounds inhibited growth significantly at even lower concentration (e.g., compounds 1–8 at 1 mmol/L, P < 0.01). Similarly, most of the compounds tested in the aliphatic acid family exhibited dose-independent inhibitory effects on seedling growth. The extent of inhibition was around 40% for each compound (Table 3). Since the compounds in the aliphatic acid group all have very limited water solubility, it is likely that they reached saturation in aqueous solution even at the lowest concentration tested under the conditions of our experiments. Thus, no further inhibition was observed at higher concentrations for this group of compounds. Note that trans-cinnamic acid (instead of cinnamic acid as identified in Table 2) was used in this experiment. Since GC/MS analysis is not capable of distinguishing enantiomers of cinnamic acid, and trans-cinnamic acid was found in the soil samples (see Table 4), we decided to test the auto-inhibitory effect of this compound. Similarly, benzoic acid was used here as it was more readily detected in soil samples (see Table 4), and it is likely derived from other hydrolysable benzoic acids found in the root extracts. Taken together, our results demonstrated directly that the compounds isolated from the roots of American ginseng are potent inhibitors of seedling growth. In addition, compounds in both the phenolic acid and aliphatic acid groups may contribute to the autotoxicity of American ginseng.
Identification of autotoxic compounds in soil samples
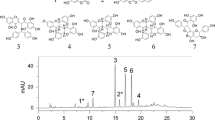
To address the question of whether the autotoxic compounds identified in American ginseng root extracts exist in soil samples, we extracted chemicals from the soil samples and determined the concentration of the nine phenolic compounds found to be autotoxic as shown above. A representative elution profile shows the detection of nine phenolic compounds from soil samples using HPLC analysis (Fig. 1). In the 4-year-old American ginseng soils, the nine phenolic compounds were measured at various concentrations (Table 4). Specifically, (P)-coumaric acid was the most abundant while trans-cinnamic acid was the least abundant. In the control soil samples, only six phenolic compounds were measured. The concentration of vanillic acid, salicylic acid, and trans-cinnamic acid were below the detection limit. Thus, the findings presented here indicate that the compounds identified in American ginseng roots can be found in relative abundance in soils previously cultivated with American ginseng, whereas they are either absent or present at much lower concentration in non-cultivated soils.
A representative HPLC-UV [diode array detector (DAD)] chromatogram of soil samples from the plow layer soil of 4-year-old American ginseng fields. Numbers above peaks correspond with the compound numbers as listed in Table 4. Compounds detected from soil samples (in the order of their appearance in the eluant): (P)-hydroxybenzoic acid (9), vanillic acid (4), syringic acid (7), vanillin (5), (P)-coumaric acid (2), ferulic acid (3), benzoic acid (8), salicylic acid (6), and trans-cinnamic acid (1)
Discussion
Our results demonstrate that the compounds isolated from ether-soluble extracts of fibrous roots of American ginseng have auto-inhibitory effects on the growth of American ginseng seedlings. Specifically, we identified 44 compounds including 17 aliphatic compounds, 20 phenolic compounds, 4 bases, and 3 other compounds, by GC/MS analysis from the most bioactive fraction. Of these, nine phenolic compounds were found to be the most potent components in the autotoxic fraction. More importantly, we were able to detect all nine bioactive phenolic compounds in the plow layer soil of American ginseng fields. Taken together, our study provides the first direct evidence that the autotoxic chemicals detected in soils of cultivated American ginseng fields can be traced back to the roots of American ginseng. During the soil sample collection process, we noticed that a large amount of fibrous root residue was left in the soil after harvest. It is likely that the autotoxic compounds found in soils are derived partly from root exudates or from plant tissue degradation. Once released into the soil and accumulated, these compounds may play a major role in mediating the alleged autotoxic effects that interfere with seedling growth of American ginseng.
Previous reports have suggested that the concentration of similar phenolic compounds ranging from 0.001 mmol/L to 1.0 mmol/L was potentially allelopathic to other crops (Chon et al. 2002; Sène et al. 2001). In this study, we found that the total content of all nine bioactive phenolic compounds varied from 0.90 to 1.10 mg/kg (dry weight) (around 7–8 mmol/L) in the soil samples. Thus, the highest concentration of the phenolics (10.0 mmol/L) tested in our study that demonstrated potent autotoxic effects on the growth of American ginseng seedlings was within the concentration range of the phenolic compounds found in cultivated fields. Note that the inhibitory effect of each compound was not as potent as bioactive Fraction V (containing a mixture of all compounds) when tested separately. Given the fact that we detected the co-existence of these compounds in soils, a potentially much stronger auto-inhibitory effect is expected as the compounds work synergistically in natural settings. Similar results have also been reported for other plant species (Einhellig 1996; Kong et al. 1999). Although a number of aliphatic acids were detected in the root extracts, the concentration of these compounds in soil samples was difficult to measure in our assays due to the limitation of our detection method (we used a UV detector connected to HPLC to determine the presence of different compounds in soil samples; however, aliphatic compounds do not have detectable absorption under UV). Our study here indicated that this group of compounds has a modest auto-inhibitory function. More studies are required to determine whether aliphatic compounds are present in soils and linked to autotoxicity directly.
Perennial American ginseng typically grows beneath mixed-hardwoods in moist, well-drained slopes and in particularly lush sites. In its native environment, autotoxicity of American ginseng is likely to be a protection mechanism for controlling competition from nearby seedlings to ensure the longevity of the existing plants. If the seeds are not dispersed far enough from the parent plants, the seedlings would be in a position to compete for resources, especially water. This self-regulatory effect of autotoxicity has been reported for Centaurea maculosa, where seeds that survive the auto-inhibitory pressure are likely to outlive and replace the parent plants eventually (Boggs and Story 1987; Perry et al. 2005). Furthermore, the autotoxic compounds identified in our study may have allelopathic effects to suppress the growth of other species as well (Singh et al. 1999). Since fields previously cultivated with ginseng are often used subsequently for growing other crops or vegetables in China, future research is needed to determine which crop species may be susceptible to the autotoxic compounds identified from American ginseng.
The difficulty of replanting American ginseng in previously cultivated fields is often referred to as ‘replant disease’. Replant disease is also known as ‘sick soil syndrome’, and is commonly observed in many types of fruit trees such as apples, pears, and plums. A wide range of tree pathogens, including bacteria, fungi, nematodes, and viruses, have been linked to replant disease in fruit trees. These pathogens can co-exist with mature trees but prevent the establishment of young trees in the same field (Weller et al. 2002). Similarly, it has been reported that the presence of fungal pathogens in soils contributes to replant disease of American ginseng (Mahfuzur and Zamir 2007; Zamir 1997). However, the results of the present study confirm that autotoxicity is another major cause of replant disease (Zhao et al. 2005a). The identification of the autotoxic compounds may help to provide future guidance for controlling replant disease of American ginseng.
References
Banwart WL, Porter PM, Granato TC, Hassett JJ (1985) HPLC separation and wavelength area ratios of more than 50 phenolic acids and flavonoids. J Chem Ecol 11:383–395 doi:10.1007/BF01411424
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83 doi:10.1023/A:1026290508166
Blum U, Gerig TM (2005) Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: nutrient culture studies. J Chem Ecol 31:1907–1932 doi:10.1007/s10886-005-5934-5
Boggs KW, Story JM (1987) The population age structure of spotted knapweed (Centaurea maculosa) in Montana. Weed Sci 35:194–198
Canals RM, Emeterio LS, Peralta J (2005) Autotoxicity in Lolium rigidum: analyzing the role of chemically mediated interactions in annual plant populations. J Theor Biol 235:402–407 doi:10.1016/j.jtbi.2005.01.020
Chon SU, Choi SK, Jung S, Jang HG, Pyo BS, Kim SM (2002) Effects of alfalfa leaf extracts and phenolic allelochemicals on early seedling growth and root morphology of alfalfa and barnyard grass. Crop Prot 21:1077–1082 doi:10.1016/S0261-2194(02)00092-3
Chon SU, Kim JD (2002) Biological activity and quantification of suspected allelochemicals from alfalfa plant parts. J Agron Crop Sci 188:281–285 doi:10.1046/j.1439-037X.2002.00574.x
Dalton BR (1999) The occurrence and behavior of plant phenolic acids in soil environment and their potential involvement in allelochemical interference interactions: methodological limitations in establishing conclusive proof of allelopathy. CRC, Boca Raton
Dalton BR, Weed SB, Blum U (1987) Plant phenolic acids in soils: a comparison of extraction procedures. Soil Sci Soc Am J 51:1515–1521
Einhellig FA (1996) Interactions involving allelopathy in cropping systems. Agron J 88:886–893
Ervin GN, Wetzel RG (2000) Allelochemical autotoxicity in the emergent wetland macrophyte Juncus effusus (Juncaceae). Am J Bot 87:853–860 doi:10.2307/2656893
Kalinova J, Vrchotova N, Triska J (2007) Exudation of allelopathic substances in buckwheat (Fagopyrum esculentum Moench). J Agric Food Chem 55:6453–6459 doi:10.1021/jf070795u
Kong C, Hu F, Xu T, Lu Y (1999) Allelopathic potential and chemical constituents of volatile oil from ageratum conyzoides. J Chem Ecol 25:2347–2356 doi:10.1023/A:1020882109682
Machrafi Y, Prevost D, Beauchamp CJ (2006) Toxicity of phenolic compounds extracted from bark residues of different ages. J Chem Ecol 32:2595–2615 doi:10.1007/s10886-006-9157-1
Mahall BE, Callaway RM (1992) Root communication mechanisms and intracommunity distributions of two mojave desert shrubs. Ecology 73:2145–2151 doi:10.2307/1941462
Mahfuzur R, Zamir KP (2007) Biological control of damping-off on American ginseng (Panax quinquefolius) by Clonostachys rosea f. catenulata (=Gliocladium catenulatum). Can J Plant Pathol 29:203–207
Perry LG, Thelen GC, Ridenour WM, Weir TL, Callaway RM, Paschke MW, Vivanco JM (2005) Dual role for an allelochemical: (+/−)-catechin fromCentaurea maculosa root exudates regulates conspecific seedling establishment. J Ecol 93:1126–1135 doi:10.1111/j.1365-2745.2005.01044.x
Porter PM, Banwart WL, Hassett JJ (1985) HPLC isolation and GC-MS identification of genistein, daidzein, and coumestrol from unhydrolyzed soybean root extracts. Environ Exp Bot 25:229–232 doi:10.1016/0098-8472(85)90006-1
Rice EL (1984) Allelopathy. Academic, Orlando
Seal AN, Pratley JE, Haig T, An M (2004) Identification and quantitation of compounds in a series of allelopathic and non-allelopathic rice root exudates. J Chem Ecol 30:1647–1662 doi:10.1023/B:JOEC.0000042074.96036.14
Sène M, Gallet C, Doré T (2001) Phenolic compounds in a sahelian sorghum (Sorghum bicolor) genotype (CE145–66) and associated soils. J Chem Ecol 27:81–92 doi:10.1023/A:1005620000835
Singh HP, Batish DR, Kohli RK (1999) Autotoxicity: concept, organisms, and ecological significance. Crit Rev Plant Sci 18:757–772 doi:10.1080/07352689991309478
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348 doi:10.1146/annurev.phyto.40.030402.110010
Whitehead DC, Dibb H, Hartley RD (1981) Extractant pH and the release of phenolic compounds from soils, plant roots and leaf litter. Soil Biol Biochem 13:343–348 doi:10.1016/0038-0717(81)90074-2
Whitehead DC, Dibb H, Hartley RD (1983) Bound phenolic compounds in water extracts of soils, plant roots and leaf litter. Soil Biol Biochem 15:133–136 doi:10.1016/0038-0717(83)90092-5
Zamir KP (1997) Fungal pathogens of American ginseng (Panax quinquefolium) in British Colombia. Can J Plant Pathol 19:301–306
Zhao YJ, Wang YP, Shao D, Yang JS, Liu D (2005a) Autotoxicity of Panax quinquefolium L. Allelophathy J 15:67–74
Zhao YJ, Wang YP, Yang JS, Liu D (2005b) A study on the rotation of crops among Panax quinquefolium, Perilla frutescens and Coix lacryma-jobi (in Chinese with English abstract). Zhongguo Zhong Yao Za Zhi 30:12–15
Acknowledgments
The authors are grateful to the staff of the Department of Medicinal Analysis, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, for performing EI-MS, NMR, and thank Dr. Xiang-ning Jiang, the College of Biological Sciences and Biotechnology, Beijing Forestry University, for analysis of GC-MS. This work was supported by the Beijing Natural Science Foundation (6072025), Beijing Science and technology project (D0206001040591) of China and the National Key Technology R&D Program (2006BA109B03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Petra Marschner.
Rights and permissions
About this article
Cite this article
He, C.N., Gao, W.W., Yang, J.X. et al. Identification of autotoxic compounds from fibrous roots of Panax quinquefolium L.. Plant Soil 318, 63–72 (2009). https://doi.org/10.1007/s11104-008-9817-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9817-8