Abstract
We studied differences in nitrogen use efficiency (NUE) among six species [Calamagrostis epigejos (L.) Roth., Carex duriuscula C.A. Mey., Phragmites communis (L.) Trin., Salix gordejevii Y.L. Chang, Salix cheilophila Schneid., and Typha minima Funk.] growing in two contrasting habitat types, i.e., a riverine wetland with high water supply and a riparian zone with low water supply. The two sites were different in soil water supply, but not in nitrogen supply. Here, NUE was defined as the total net primary production per unit nitrogen absorbed. There was no significant difference in NUE between the species growing in the riverine wetland (Carex duriuscula, P. communis, S. cheilophila, T. minima) and the species growing in the river bank (Carex duriuscula, Calamagrostis epigejos, P. communis, S. gordejevii). We further analyzed NUE as the product of the nitrogen productivity (A, the rate of dry matter production per unit of nitrogen in the plant) and the mean residence time of nitrogen (MRT, the period of time a unit of nitrogen is present in the plant). The species growing in the riverine wetland had larger A but lower MRT than the species growing in the river bank. There was an inverse relationship between A and MRT. Consequently, NUE was similar among species and habitats. These results suggested that environmental factors, such as soil water supply, can influence N use by plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) availability is closely associated with plant productivity (Vermeer and Berendse 1983) and often limits plant N uptake in terrestrial ecosystems (Tilman 1985; Vitousek and Howarth 1991). To adapt to the N limitation, plants should utilize the limited N more efficiently. Therefore, it is important to study the efficiency with which plants use this resource for growth (Vitousek 1982; Aerts and Chapin 2000; Yuan et al. 2006). Nitrogen use efficiency (NUE), a measure of how a plant responds to soil nitrogen, is generally defined as the amount of organic matter lost or produced in a plant per amount of nitrogen used (Hirose 1971; Vitousek 1982). However, Berendse and Aerts (1987) proposed to redefine the concept of NUE as the product of nitrogen productivity (A, the rate of dry matter production per unit of nitrogen in the plant) and the mean residence time of the nitrogen in the plant (MRT, the period of time a unit of nitrogen is present in the plant), i.e., NUE = A × MRT.
The NUE in relation to habitat fertility has been studied in many different species (e.g., Aerts and De Caluwe 1994; Vázquez de Aldana and Berendse 1997; Nakamura et al. 2002). However, other factors, such as water, could influence plant N use too. Soil water supply affects nutrient transport, uptake, and transformation. It may be the underlying cause of variation in habitat fertility. Plants with different soil water supply can have widely different traits such as photosynthetic capacity, growth rate leaf N content (Lambers et al.1998), and then N use strategies. To our knowledge, there are few studies based on the NUE index of Berendse and Aerts (1987), and the effect of soil water supply on NUE and its two components, i.e., A and MRT, has not been studied. Furthermore, most of these studies according to the NUE index of Berendse and Aerts (1987) were carried out under controlled conditions (e.g., Aerts and de Caluwe 1994; Vázquez de Aldana and Berendse 1997; Yuan et al. 2007). Also, the relationship between A and MRT is still under debate: some studies suggest a negative relationship (Yasumura et al. 2002; Silla and Escudero 2004), while others have found no relationship (Aerts and de Caluwe 1994; Nakamura et al. 2002; Yuan et al. 2004). If the trade-off between A and MRT exists among plants growing in their natural habitats, is this trade-off related to soil water supply?
In this paper, we used the NUE index defined by Berendse and Aerts (1987) to study the N economy of six broadly distributed species growing in two contrasting habitats differing in soil water supply. For woody shrubs, we measured A, MRT, and NUE at the leaf level. Because leaf production is a major component of primary production and most N is invested in leaves, NUE at leaf level could be expected to be closely related to that at whole-plant level, and thus differences in whole-plant NUE could be explained largely by leaf-level attributes (Escudero et al. 1992; Garnier et al. 1995). More recently, Yasumura et al.(2002) studied how the difference in irradiance influenced the leaf-level NUE of canopy and understory species in a beech forest. They demonstrated that aboveground NUE of three shrub species showed the same trend as the leaf-level NUE, suggesting that aboveground NUE and its components could be approximated by leaf-level values in these species. For perennial herbs, we measured A, MRT, and NUE at the aboveground level.
The purpose of this study was to address whether NUE and its two components change among species with different water supply. As the amount of soil water supplied to the plant is reduced, the rate of photosynthesis will decline (Lambers et al.1998). Therefore, we hypothesized that plant nitrogen economy, particularly A and MRT, was different when growing in habitats with different water supply. Compared with species with low water supply, species with high water supply would have higher A and thus higher NUE. MRT, however, is expected to be similar among the species because leaf lifespan and N resorption efficiency (R EFF, the percentage of N recovered from senescing leaves), both of which strongly affect MRT, do not vary appreciably among these species (Eckstein et al. 1999; Yasumura et al. 2002). In the present study, we tested this hypothesis by studying the nitrogen economy of six species growing in two distinct natural habitats with different water supply.
Materials and methods
Study site and species
The study was carried out in Duolun County, which is located on the southern edge of the Hunshandake Sandland in the central part of Inner Mongolia Autonomous Region, China. This area belongs to a typical agropastoral ecotone. The climate belongs to the semiarid monsoon climate of a moderate temperature zone. Mean annual precipitation is around 386 mm and mean annual temperature is 1.6°C, with mean monthly temperature ranging from −18.3°C in January to 18.5°C in July. The study site was situated in a riparian zone along the Xiaohezi River, 2 km south of Duolun Town (42°11′N, 116°48′E). We selected two habitat types: riverine wetland and river bank. The river bank was situated in the riparian zone, 5–10 m away from the riverine wetland. Table 1 contains data on site characteristics of the two habitats. During the growing season (from June to October), we selected five replicates, 50–100 m away from each other, for each habitat. At each replicate, three core soil samples, for analysis, were taken at 0–20 cm depth from the riverine wetland and the river bank each month. Soil organic matter was estimated from total organic carbon, determined by dry combustion (Nelson and Sommers 1996), total N by the semimicro Kjeldahl technique (Bremner and Mulvaney 1982), and available P according to Bray and Kurtz (1945). Soil texture was determined by the pipette method (Gee and Bauder 1986). Soil bulk density was determined using a soil core (stainless steel cylinders with a volume of 100 cm3). Soil moisture was determined gravimetrically by oven-drying the samples at 105°C for 24 h. Water holding capacity (WHC) was determined as the weight difference between water-saturated soil and soil dried at 100°C. The pH was measured in an aqueous soil extract (1:2.5 soil:water ratio) by an electrode pH meter. Inorganic nitrogen (\({\text{NH}}_4^ + \) and \({\text{NO}}_4^ - + {\text{NO}}_2^ - \)) was measured in the soil samples for each site. Approximately 20 g of moist sample were extracted with 50 ml of 1 M KCl for 1 h, centrifuged, filtered, and frozen for later analysis. The \({\text{NH}}_4^ + \) and \({\text{NO}}_3^ - + {\text{NO}}_2^ - \) concentrations were measured colorimetrically in these extracts using a continuous flow analyzer (RFA-300, Alpkem Corp., Clackamas, OR, USA, and Scan Plus analyzer, Skalar Analytical B.V., Breda, the Netherlands). \({\text{NH}}_4^ + \) and \({\text{NO}}_3^ - + {\text{NO}}_2^ - \) concentrations were expressed per unit of soil dry mass (μg g−1). The data in Table 1 were averages of five replicates and eight sampling dates. The results showed that the soils underlying the study site consisted of coarse sand with low soil N availability (Table 1). There was no significant difference in soil N availability between the two habitats. Soil moisture contents at both habitats were lower than their maximum WHC. The riverine wetland and the river bank sites differed significantly with respect to water supply (P < 0.01). Average soil moisture was higher at the riverine wetland site than at the river bank site during the study period. The vegetation of the river bank was dominated by shrubs such as Salix spp. and Hedysarum leave Maxim., several graminoid species such as Calamagrostis epigejos (L.) Roth., and Carex species. The vegetation of the riverine wetland was occupied by Salix cheilophila Schneid., Phragmites communis (L.) Trin., Typha minima Funk., and several Carex species.
In total, six species of vascular plants of two life forms (woody shrubs vs perennial herbs) were included in this study. S. gordejevii Y.L. Chang, P. communis, Carex duriuscula C.A. Mey., and Calamagrostis epigejos were selected as the dominant species in the river bank sites and S. cheilophila, P. communis, Carex duriuscula, and T. minima as the species in the riverine wetland sites. S. gordejevii and S. cheilophila are woody shrubs and the others are perennial herbs. In particular, P. communis and Carex duriuscula were present at both habitats, allowing us to compare variation in N use traits at inter- and intraspecific levels. Further, we selected two Salix shrubs and thus we could also compare N use traits between the congener species.
Calamagrostis epigejos is a perennial rhizomatous grass widely distributed in the semiarid region of the Inner Mongolia Plateau. Carex duriuscula, small to middle sized, occurs in dry to somewhat moist habitats. S. gordejevii, which grows on the sand dunes, is a perennial shrub, widely distributed in the study area. It survives in the shifting sand dunes as pioneer species because of its high drought tolerance. S. cheilophila is usually more abundant along the streamside margins. This shrub grows to 2–5 m tall. The worldwide distribution of P. communis and T. minima reflects their ability to adapt to a wide range of environments.
Plant biomass and nitrogen analyses
For woody shrub species, aboveground litter was collected with litter traps during the growing season in 2003. Litter traps (n = 5 for each species) were placed around each plant so as not to damage or shade the plant. Litter was collected twice a month from mid-June to later October, until leaf fall was completed. In the laboratory, litter samples were sorted into leaf and other parts, and weighed after oven-drying at 70°C for at least 72 h. Total N concentration was measured with an NC analyzer (KDY-9820, Tongrun Ltd., China). Green leaves of shrub species were sampled twice a month until leaf fall was completed. Senesced leaves were sampled on 5 November 2003 by random collection from the ground. The samples were dried and weighed, and then total N concentration was determined as described above.
For perennial herb species, 100 healthy looking, roughly equal sized aboveground shoots of each species were tagged at the beginning of the growing season. The selected shoots (n = 10 for each species) were harvested twice a month. At each harvest, the plants were clipped at the soil surface and immediately brought to the laboratory. Plant materials were carefully separated into live and senesced tissues and into leaves, axes (including leaf sheath), and reproductive parts (including the axis carrying the flowers). All plant parts were dried, weighed, and then total N concentration was determined as described above.
Definition and calculations
We calculated A, MRT, and NUE as follows:
where NPP, NPOOL, and NLOSS are net primary productivity, mean N content in a growing season, and N loss, respectively. For woody shrub species, NPP was the total dry mass of leaf litter. NPOOL was the average N content of green leaves, and NLOSS was the total amount of N in leaf litter collected over the growing season. Therefore, the leaf-level NUE of shrub species can be measured as the inverse of N concentration of leaf litter and the aboveground NUE of shrub species can be measured as the inverse of N concentration of aboveground litter (Vitousek 1982). For perennial herb species, we calculated NPP as the aboveground biomass of dead shoots in October and total dry mass of litter, because the populations would be stable and approach their maximum biomass. N pool sizes of live and dead tissue were calculated by the product of the mean N concentration and the mean dry mass, respectively. The average annual N pool was estimated as an average of N pool at each sampling time. N loss was estimated as the total N found in the plant at the October harvest and in the litter because the aboveground parts of these species had died by this time. Since rainfall is negligible in the region when leaves senesce, leaching of N from leaves was assumed to be minimal and was not taken into account. When leaves senesce, part of leaf N is resorbed into storage organs. The fraction of the leaf N pool annually resorbed prior to leaf fall was then calculated as resorption efficiency (R EFF) (Yasumura et al. 2002; Escudero and Mediavilla 2003; Yuan et al. 2005):
where max NPOOL denotes NPOOL at the seasonal maximum and total N in leaf litter represents the total N in leaf litter collected throughout the experiment. N concentration in senesced leaves is considered a direct indicator of N resorption proficiency (R PROF), which is defined as the absolute level to which species reduce N in senescent leaves (Killingbeck 1996), reported here for N as concentration (mg N per g dry weight). Consequently, plants with a lower N concentration in freshly fallen leaves are more proficient than plants with a higher concentration in senescing leaves.
Statistical analyses
Statistical tests were performed using SPSS version 10.0 (SPSS Ins., Chicago, IL, USA). The effects of species, habitat, and life form on leaf N concentration, NPP, N pool, N loss, A, MRT, NUE, R EFF, and R PROF were tested by three-way analysis of variance (ANOVA). Differences between two variables were tested by Student’s t-test, and differences among three or more variables were tested by the Tukey–Kramer test. The Spearman correlation was employed to assess the relationship between A and MRT.
Results
Plant N status
For all species, N concentration in biomass (leaves, shoots, and inflorescences) declined during the growing season (Fig. 1). N concentration of green leaves differed significantly among species and between life forms (woody shrubs vs perennial herbs), but not between habitats (Table 2). There was no significant variation in N concentration of senesced leaves among species, habitats, or life forms. In all species, green leaves had significantly higher N concentration than the senesced leaves (P < 0.001) (Fig. 1). Aboveground net productivity (NPP) varied significantly among species, habitats, and life forms (Table 2). NPP was lowest in Typha minima while highest in Salix gordejevii. The species growing in riverine wetland had higher NPP than those in river bank. Also, NPP was higher in the woody shrubs than in perennial herbs. The six species showed relatively similar N pool patterns characterized by a distinct peak (Fig. 2). Average N pool varied significantly among species, habitats, and life forms (Table 2, Fig. 2), with the lowest value in Carex duriuscula (1.8 mg green leaf N per plant) and the highest value in Salix gordejevii (112 mg green leaf N per plant). The species growing in riverine wetland had a higher N pool (107 mg green leaf N per plant on average) than those in river bank (88 mg green leaf N per plant on average). Also, there was significant variation in N loss among species and between life forms, but not between habitats (Table 2).
N resorption
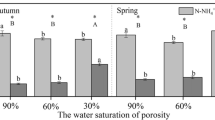
There was a significant difference in N resorption efficiency (R EFF) across species, habitats, and life forms (Fig. 3, Table 2). R EFF was highest in S. gordejevii and lowest in T. minima. The species growing in the riverine wetland had lower R EFF (48 ± 6%) than those growing in the river bank (61 ± 4%). Woody shrubs also had higher R EFF than perennial herbs. N resorption proficiency (R PROF), which is inversely related to the N concentration of senesced leaves, did not differ significantly across species, habitats, or life forms (Fig. 3, Table 2).
N resorption efficiency (R EFF, percentage reduction of nitrogen between green and senescing tissues) and N resorption proficiency (R PROF, absolute reduction of nitrogen in senescing tissues, unit is mg N per g DM). Means with SE. Differences between all pairs refer to differences among species for R EFF and R PROF and were tested using the Tukey–Kramer test. Different letters indicate a statistical difference at P = 0.05. n = 5 for woody shrubs and n = 10 for perennial herbs. Symbols: □ R EFF, ■ R PROF
Nitrogen productivity (A), mean residence time (MRT), and nitrogen use efficiency (NUE)
Both A and MRT varied significantly across species, habitats, and life forms (Table 2, Fig. 4). A was lowest in S. gordejevii (95 ± 9 g g−1 yr−1) and highest in T. minima (227 ± 22 g g−1 year−1). The species growing in the riverine wetland had higher A than those growing in the riparian zone (P < 0.05) (Table 2). A was higher in perennial herb species than in woody shrub species (P < 0.05). In contrast to A, MRT was longer in S. gordejevii than in other species. The species growing in the riverine wetland had lower MRT (average: 2.0 ± 0.2 years) than those growing in the riparian zone (average: 2.7 ± 0.3 years). NUE, the product of A and MRT, was on average 364 g g−1 N, with the highest value of 414 g g−1 N in P. communis growing in the riparian zone and the lowest value of 301 g g−1 N in S. cheilophila growing in the riverine wetland. Despite a 25% variation, NUE was not significantly different across species (F = 1.612, P = 0.254), habitats (F = 2.111, P = 0.154), or life forms (F = 1.755, P = 0.218).
Relationship between N productivity (A, the rate of dry matter production per unit of nitrogen in the plant) and mean residence time of nitrogen (MRT, the period of time a unit of nitrogen is present in the plant, estimated from N pool and loss); means with SE. Symbols: ● P. communis growing in the riverine wetland, ○ P. communis growing in the river bank, ■ Carex duriuscula growing in the riverine wetland, □ Carex duriuscula rowing in the river bank, ▲ S. cheilophila, △ S. gordejevii, ◆ T. minima, * Calamagrostis epigejos
NUE can differ depending on the tissue that is evaluated (Garnier and Aronson 1998; Aerts and Chapin 2000). We calculated aboveground NUE of the two shrub species (as the inverse of N concentration of aboveground litter including senesced leaf and stem; Vitousek 1982) and compared these to the leaf-level NUE values. In S. gordejevii, aboveground NUE (371 ± 26 g g−1 N) was slightly higher than leaf-level NUE (332 ± 15 g g−1 N) due to low N concentrations of branches and twigs (P = 0.048). Aboveground NUE for S. cheilophila (320 ± 23 g g−1 N) was almost the same as leaf-level NUE (310 ± 18 g g−1 N) (F = 1.928, P = 0.214). There was no significant difference in aboveground NUE between S. gordejevii and S. cheilophila. Therefore, aboveground NUE showed the same pattern as the leaf-level NUE, indicating that aboveground NUE and its components can be approximated by leaf-level values in these shrub species.
Discussion
In this study, we studied the N economy of six species growing in two habitats with different water supply. The hypothesis that NUE and A are higher in the riverine wetland than in the river bank habitats, and that MRT is similar in the two habitats, was only partly supported. The species growing in the riverine wetland with adequate water supply had a significantly higher A but a lower MRT than the species growing in the riparian zone (Fig. 4). NUE, the product of A and MRT, did not differ significantly between the two habitats with different water supply (Table 2).
The NUE values of the six species in this study (364 g g−1 N on average) were fairly high when compared with the range reported for other species (60–231 g g−1 N in Vázquez de Aldana and Berendse 1997; 121–282 g g−1 N in Nakamura et al.2002; 209–296 g g−1 N in Silla and Escudero 2006). The perennial herb species growing in the riparian zone, in particular, had relatively high NUE. NUE values of the two Salix species (334 g g−1 N for S. gordejevii and 310 g g−1 N S. cheilophila), though low, were still higher than other Salix species (180 g g−1 N in Adegbidi et al. 2001); and NUE values of the three herb species (average: 375 g g−1 N) growing in the riverine wetland were also higher than that reported for other herb species (121–282 g g−1 N in Nakamura et al. 2002). A high NUE is generally thought to be an adaptation to N-poor habitats (Chapin 1980; Chapin and Shaver 1989). The soils underlying the study site had low soil N contents compared to those in the above studies. Therefore, the high NUE we measured very likely reflects the infertile soils at the study site and the adaptation of the species to those infertile soils.
Variations in NUE values within shrubs or herbs showed the same pattern among all species. There was a significant difference in A and MRT between the two congener shrubs (S. gordejevii, S. cheilophila) and between the same species (Phragmites communis, Carex duriuscula) growing in different habitats, but not in NUE. For example, P. communis growing in the riverine wetland had higher A but lower MRT than that growing in the river bank, thus similar NUE (Table 2, Fig. 4). The same pattern was found in Carex duriuscula presented at both habitats. Also, the two Salix species (S. gordejevii, S. cheilophila) growing in the riverine wetland and the river bank had similar NUE but different A and MRT. This suggested that the soil water supply might strongly influence plant N use traits among and within species.
In our paper, the species growing in the riverine wetland had higher A than those growing in the river bank (Fig. 4). The soil water content in the riverine wetland was about fourfold higher than that in the river bank (Table 1, P < 0.01). However, there was no significant difference in soil inorganic nitrogen between the two habitats (F = 4.58, P = 0.06), although soil available P differed from two habitats. However, the effect of soil P on plant N uptake and use could be indirect. Because soil water supply influences plant productivity and then A (Eq. 1; Lambers et al. 1998), therefore most of the difference in A could be attributed to differences in soil water supply between the riverine wetland and the river bank. However, the lack of a positive association between A and habitat fertility needs further study to clarify it. These results suggest that water supply could influence plant N productivity (A) and then N use strategies.
Previous studies show that variations in MRT, another component of NUE, are affected by leaf lifespan (Escudero et al. 1992; Golluscio 2007), as well as by N resorption (Eckstein et al. 1999; Aerts and Chapin 2000). All of the six species selected here are deciduous species and have similar leaf lifespan. Therefore, the significant differences in MRT among six species should not be ascribed to leaf lifespan. In particular, the two congener Salix species had different MRT (F = 67.28, P < 0.01), with the higher value in the river bank habitats. MRT values also differed for P. communis (F = 14.87, P < 0.05) and Carex duriuscula (F = 14.87, P < 0.05) growing in two contrasting habitats. Because MRT is the function of leaf lifespan and N resorption (Eckstein et al. 1999; Aerts and Chapin 2000; Golluscio 2007), the difference in their MRT should be attributed to N resorption. In this paper, N resorption efficiency (R EFF) was significantly lower in the species growing in the riverine wetland than in the species growing in the river bank, while N resorption proficiency (R PROF) was not significantly different among species (Fig. 3). Thus, the difference in MRT could be explained by the difference in R EFF. High R EFF contributes to high MRT and hence high NUE, but leads to low litter N concentrations and vice versa (Eckstein et al. 1999; Aerts and Chapin 2000). The difference in two measures of N resorption indicates that it is also important to distinguish them when assessing plant N resorption.
To the best of our knowledge, only a few experiments have directly tested the relationship between A and MRT and literature reports remain inconclusive. In some studies A was found to be negatively correlated with MRT (Yasumura et al. 2002; Silla and Escudero 2004); in others no trade-off was found (Aerts and de Caluwe 1994; Nakamura et al. 2002; Yuan et al. 2004). In this study, we found that A was significantly correlated with MRT within the six species growing in two contrasting habitats (Fig. 4, r = −0.910, P < 0.001). However, we still do not know whether this trade-off represents ecophysiological links between plant traits or a simple problem of autocorrelation of the data, as discussed by Knops et al. (1997) and Vitousek (1997). Further study is needed to make it clear.
We did not estimate the effect of Salix bark, wood, and reproductive parts on NUE. However, non-leaf litter production is a minor component of primary production and a large portion of N is invested in leaves, and thus we ignored Salix bark, wood, and reproductive parts. Also, the allocation of N to roots could differ between habitats. In the present study, the calculation of NUE was based only on data of aboveground primary production and N loss. The amount and loss of N in roots were not evaluated. This is an important drawback because an NUE based on only aboveground data might differ from NUE based on whole plant due to (1) differences in rhizome and root production and storage and (2) differences in N concentrations in rhizome/root compared with concentrations in shoots (Berendse and Aerts 1987; Aerts and Chapin 2000). Unfortunately, we had no root data in this field study owing to the difficulty of measuring belowground N dynamics in natural ecosystems. Furthermore, some species included in this study have clonal growth habits; hence, it is difficult to exactly match the belowground parts with sampled aboveground units. However, the results of root turnover studies support the hypothesis that root longevity is greater in dry habitats (Eissenstat and Yanai 1997; López et al. 1998). These facts indicate that species with low water supply have a low root turnover, and we assume that the species ranking in our results would not be greatly altered if whole plants were considered.
In this paper, we investigated the N use traits of six species growing in two contrasting habitats with different water supply by studying NUE and its components A and MRT. Although the species growing in the riverine wetland had higher A by virtue of high water supply, NUE did not differ among the species. The species growing in the riverine wetland, due to lower N resorption, had shorter MRT than the species growing in the river bank. The results supported the hypothesis that plant N use strategies are influenced by soil water supply. Although NUE can be a very useful index, there are a number of environmental factors, including soil fertility and moisture, temperature, light etc., not all of which are independent. It is those fundamental components that merit attention if we are to understand plant nutrient economy.
References
Adegbidi HG, Volk TA, White EH, Abrahamson LP, Briggs RD, Bickelhaupt DH (2001) Biomass and nutrient removal by willow clones in experimental bioenergy plantations in New York State. Biomass Bioenerg 20:399–411
Aerts R, Chapin FS III (2000) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res 30:1–67
Aerts R, De Caluwe H (1994) Nitrogen-use efficiency of Carex species in relation to nitrogen supply. Ecology 75:2362–2372
Berendse F, Aerts R (1987) Nitrogen-use-efficiency: a biologically meaningful definition? Funct Ecol 1:293–296
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorous in soil. Soil Sci 59:39–45
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2: chemical and microbiological properties. American Society of Agronomy, Madison, WI, USA, pp 595–624
Chapin FS III (1980) The mineral nutrition of wild plants. Ann Rev Ecol Syst 11:233–260
Chapin FS III, Shaver GR (1989) Differences in growth and nutrient use among arctic plant growth forms. Funct Ecol 3:73–80
Eckstein RL, Karlsson PS, Weih M (1999) Leaf life span and nutrient resorption as determinants of plant nutrient conservation in temperate-arctic regions. New Phytol 143:177–189
Eissenstat DM, Yanai R (1997) Ecology of root lifespan. Adv Ecol Res 27:1–60
Escudero A, Mediavilla S (2003) Decline in photosynthetic nitrogen-use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J Ecol 91:880–889
Escudero A, del Arco JM, Sanz IC, Ayala J (1992) Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia 90:80–87
Garnier E, Aronson J (1998) Nitrogen-use efficiency from leaf to stand level: clarifying the concept. In: Lambers H, Poorter H, Van Vuuren MMU (eds) Inherent variation in plant growth. Physiological mechanisms and ecological consequences. Backhuys, Leiden, the Netherlands, pp 515–538
Garnier E, Gobin O, Poorter H (1995) Nitrogen productivity depends on photosynthetic nitrogen use efficiency and on nitrogen allocation within the plant. Ann Bot 76:667–672
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis. Part 1, Agron. Monogr. 9, 2nd edn. ASA and SSSA, Madison, WI, USA, pp 383–411
Golluscio RA (2007) On the link between nitrogen productivity and residence time: two opposite nitrogen use strategies? J Arid Environ 68:165–169
Hirose T (1971) Nitrogen turnover and dry matter production of a Solidago altissima population. Jpn J Ecol 21:18–32
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77:1716–1727
Knops JMH, Koenig WD, Nash TH III (1997) On the relationship between nutrient use efficiency and fertility in forest ecosystems. Oecologia 110:550–556
Lambers H, Chapin FS III, Pons TL (1998) Plant physiological ecology. Springer, New York
López B, Sabaté S, Gracia CD (1998) Fine root dynamics in a Mediterranean forest: effects of drought and stem density. Tree Physiol 18:601–606
Nakamura T, Uemura S, Yabe K (2002) Variation in nitrogen use traits within and between five Carex species growing in the lowland mires of northern Japan. Funct Ecol 16:67–72
Nelson DW, Sommers LE (1996) Total carbon, organic carbon and organic matter. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnson CT, Sumner ME (eds) Methods of soil analysis. Part 3, chemical methods. Soil Sci. Soc. Am. Book Ser. 5. SSSA, Madison, WI, USA, pp. 961–1010
Silla F, Escudero A (2004) Nitrogen-use efficiency: trade-offs between N productivity and mean residence time at organ, plant and population levels. Funct Ecol 18:511–521
Silla F, Escudero A (2006) Coupling N cycling and N productivity in relation to seasonal stress in Quercus pyrenaica Willd. saplings. Plant Soil 282:301–312
Tilman D (1985) The resource-ratio hypothesis of plant succession. Am Nat 125:827–852
Vázquez de Aldana BR, Berendse F (1997) Nitrogen-use-efficiency in six perennial grasses from contrasting habitats. Funct Ecol 11:619–626
Vermeer JG, Berendse F (1983) The relationship between nutrient availability, shoot biomass and species richness in grassland and wetland communities. Vegetatio 53:121–126
Vitousek PM (1982) Nutrient cycling and nitrogen use efficiency. Am Nat 119:553–572
Vitousek PM (1997) On regression and residuals: response to Knops et al. (1997). Oecologia 110:557–559
Vitousek PM, Howarth RH (1991) Nitrogen limitation on land and in the sea, how can it occur? Biogeochemistry 13:87–115
Yasumura Y, Hikosaka K, Matsui K, Hirose T (2002) Leaf-level nitrogen-use efficiency of canopy and understorey species in a beech forest. Funct Ecol 16:826–834
Yuan ZY, Li LH, Han XG, Jiang FH, Zhao MX, Lin GH (2004) Plant sizes in relation to nitrogen use efficiencies of competing individuals in a dense stand of Helianthus annuus. Acta Bot Sin 46:889–895
Yuan ZY, Li LH, Han XG, Huang JH, Jiang GM, Wan SQ (2005) Soil characteristics and nitrogen resorption in Stipa krylovii native to northern China. Plant Soil 273:257–268
Yuan ZY, Li LH, Han XG, Chen SP, Wang ZW, Chen QS, Bai WM (2006) Nitrogen response efficiency increased monotonically with decreasing soil resource availability: a case study from a semiarid grassland in northern China. Oecologia 148:564–572
Yuan ZY, Liu WX, Niu SL, Wan SQ (2007) Plant nitrogen dynamics and nitrogen-use strategies under altered nitrogen seasonality and competition. Ann Bot 100:821–830
Acknowledgements
We gratefully acknowledge the assistance of X.R. Shi, L.F. Ren, Z.Q. Yan, M.W. Dong, F.T. Qiu, and H.Y. Wang in the field and laboratory work. We wish to thank the director and the staff of the Grassland Station of Duolun County for their kindness and cooperation during this study. Stephen Hart kindly corrected the English language of the manuscript. This study was partly supported by the Natural Science Foundation of China (30600076), the State Science & Technology Promotion Program (2006bad16b01), and an innovative group research grant (No. 30521002) from the National Natural Science foundation of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Angela Hodge
Rights and permissions
About this article
Cite this article
Yuan, ZY., Li, LH. Soil water status influences plant nitrogen use: a case study. Plant Soil 301, 303–313 (2007). https://doi.org/10.1007/s11104-007-9450-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9450-y




 senesced leaf,
senesced leaf,  shoot,
shoot,  inflorescence
inflorescence 

