Abstract
Since very young seedlings are sensitive to dehydration, soil desiccation is often responsible for seedling death in water-stressed environments. Roots play a major role in overcoming water stress and plant establishment, thus early root development in response to limited water availability becomes a strategy that may ensure seedling recruitment. We explored whether different water availabilities altered growth patterns of very young seedlings, focussing on root elongation, and hypothesized that seedling responses would depend on species-specific drought tolerance and seed size. We carried out a greenhouse experiment exposing 2-week-old seedlings of three Mediterranean shrubland species, the drought-tolerant and small-seeded Genista umbellata (L’Hér.) Dum. Cours. and Lycium intricatum Boiss., and the drought-sensitive, large-seeded Retama sphaerocarpa (L.) Boiss., to two watering quantities and monitored plant and root growth weekly in glass cases for 5 weeks. We found that at such early stages, reduced water quantity enhanced root growth in all three species, regardless of drought tolerance and seed size, although root plasticity was the highest in the small-seeded and drought-tolerant Genista. In contrast, shoot elongation and mass allocation, root-to-shoot mass (R:S) ratio, was unaffected by watering. Seedlings responded to lower water availability with faster root elongation rate and greater absorptive root surface, which can account for the enhanced relative growth rate (RGR) of the small-seeded Genista and Lycium under reduced watering. By contrast, a larger root absorptive surface did not lead to higher RGR in the large-seeded Retama probably because of its greater independence from external mineral resources. Our data evidence the importance of water availability on the initial developmental stages of these three species regardless of seed size and drought tolerance. Root growth can be interpreted as an adaptive strategy to deal with drying soils and decreasing soil moisture since larger roots enable to exploit unexplored soil areas of soil, which may ensure recruitment success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant communities are shaped by germination and recruitment processes (Donovan et al. 1993), which ultimately affect community composition and structure (Grubb 1977; Harper 1977). Plants do not actively choose the habitat they grow in (Bazzaz 1991); rather, habitat choice is first imposed on plants by seed dispersal, and then by environmental factors which constrain seed survival, germination, seedling establishment and growth (Schupp 1995). After seed dispersal, germination does give way to the most critical phase in the regeneration process, seedling establishment (Fenner and Kitajima 1999). Very young seedlings are susceptible to many hazards, such as extreme temperatures and radiation, competition, pathogens, herbivory or drought (Moles and Westoby 2004a), and as a result high mortality rates are often associated to this stage (Fenner 1987). An important determinant of successful seedling recruitment is the microsite where the seed is placed, often a safe site providing conditions and resources required for germination and establishment (i.e., the regeneration niche sensu Grubb 1977; Fenner 1987). However, seed–seedling conflicts may arise when environmental conditions promoting seed germination are not favorable for seedling survival and growth (Schupp 1995), e.g., conditions good enough for triggering germination may not be as good for seedling growth. Eventually, seedling’s fate and recruitment success will depend on the seedling’s ability to cope with limiting environmental conditions.
Because emerged seedlings are much more sensitive to dehydration than seeds or juvenile individuals (Evans and Etherington 1991), drought is often the main cause of seedling death in many environments (Moles and Westoby 2004a). This is particularly true in water-stressed Mediterranean ecosystems, where a dry, long summer season jeopardizes recruitment of seedlings emerged in winter and spring (Herrera 1992). In addition, seedlings in arid environments are exposed to highly variable rainfall, both in duration and amount, being characteristic the presence of dry periods interspersed between rain events (Lázaro et al. 2001). Establishment success in such areas greatly depends on seedling ability to overcome water shortage (Davis 1989), and root systems play a major role. Large biomass allocation to roots is often related to higher survival rates through improved water and nutrient uptake (Lloret et al. 1999; Pugnaire et al. 2006) linked to reaching moister soil layers and exploring larger soil volumes (Davis 1989; Donovan et al. 1993; Leishman and Westoby 1994a). Consequently, deep-rooted seedlings have a probability of surviving summer drought higher than shallow-rooted seedlings (Padilla and Pugnaire 2007). Species-specific drought tolerance, however, is a main factor for seedling survival in drying soils (Ackerly 2004), and Davis (1989) and Hasting et al. (1989) found in the California chaparral that seedlings of drought-tolerant species, usually shallow-rooted, survived water shortage better than seedlings of drought-avoider species, often deep-rooted, because of the greater tolerance to low soil water potentials of tolerant species. Seed size has also been related to successful recruitment in dry habitats (Leishman and Westoby 1994a; Moles and Westoby 2004b). Large-seeded species have storage reserves in cotyledons that sustain growth during unfavorable periods, and are more likely to have large seedlings and longer roots than small-seeded species (Buckley 1982; Jurado and Westoby 1992; Fenner and Kitajima 1999), traits shown to be related to a higher probability of survival by allowing access to soil moisture at deeper levels (Donovan et al. 1993).
Given the typically unpredictable and variable rainfall in arid environments and Mediterranean ecosystems, and the fact that climate change scenarios forecast for the western Mediterranean Basin a mean annual precipitation reduced by ∼30% and shifts in the frequency of rain events, i.e., greater, less frequent events followed by longer drought periods (IPCC 2001), understanding seedling responses to changes in water availability is important. Here, we explored whether differences in watering altered growth patterns of seedlings at the very early stages of development, with cotyledons still attached. We carried out an experiment in mini-rhizotrons, subjecting very young seedlings of three perennial woody species of Mediterranean shrubs to reduced watering, monitoring plant and root growth. We reduced the amount of water supplied and its frequency expecting that pulses of water of different magnitude have different effects on plants, even if the amount of water provided is kept constant. Research has shown that roots grow towards resource patches (Reader et al. 1993; Cahill and Casper 1999; Rajaniemi and Reynolds 2004; Eapen et al. 2005), showing an elongation response in low moisture (Evans and Etherington 1991). Furthermore, it is widely accepted that plants adjust to resource imbalance by allocating biomass to organs that acquire the limiting resource (Chapin et al. 1987). Therefore, we expected larger biomass allocation to roots relative to shoots and larger root elongation rates in response to drought as a means to overcome water shortage. We hypothesized that (1) seedling responses would depend on species’ water stress tolerance, so that drought-sensitive species would show stronger responses to drought than drought-tolerant species as a means to overcome their lower capacity of dealing with low water availability and, following Leishman and Westoby (1994a) (2) root growth would be positively associated to seed size, so that large-seeded species would show stronger responses to drought than small-seeded species because cotyledons allow plant to growth under unfavorable conditions.
Materials and methods
Species
Three perennial woody species co-occurring in open Mediterranean semiarid shrublands of southeast Spain were selected; Genista umbellata (L’Hér.) Dum. Cours., Lycium intricatum Boiss., and Retama sphaerocarpa (L.) Boiss. Hereafter we refer to these species by their generic names only. Two of the species were nearly leafless legumes with photosynthetic stems, the small shrub Genista and the large shrub Retama, whereas Lycium was a thorny shrub with drought-deciduous succulent leaves. Our species differed in drought-tolerance strategy based on rooting depth and minimum pre-dawn water potential (Ψ pd) measured in the field during the water shortage. Retama, a very deep-rooted species accessing stable water sources through the year (Haase et al. 1996), may be considered as drought–avoider given the usually high Ψ pd reported (≈−1.5 MPa, Haase et al. 1999). The other two species can be classified more properly as drought-tolerant. Lycium stands very low water potentials (≈−5 MPa, Tirado 2003) and its drought-deciduous habit evidences shallow rooting depth. There are no data available for G. umbellata, a shallow-rooted species (<0.75 m, pers. obs.), but a closely related species, G. hirsuta, showed high tolerance to Mediterranean stress, reaching Ψ pd under -6 MPa (Lansac et al. 1994). Species also differed in seed mass. Genista and Lycium are relatively small-seeded species, whereas Retama is a larger-seeded species with very heavy seed coat (up to 35 mg, Table 1).
Experimental design
Freshly collected seeds of the three species were sown separately in germination trays containing type III vermiculite (Verlite®, Vermiculita y Derivados SL, Gijón, Spain) in laboratory at room temperature and light on 22 March 2005. Seeds were collected in the field or provided by local nurseries. All seeds germinated within 2 weeks, and very young seedlings were transferred to glass cases on 13 April 2005, once that cotyledons had fully emerged from seed coats. Six randomly selected seedlings of every species were harvested before transplanting (Table 1). Four transparent glass cases, 129 cm length, 43 cm depth, 3 cm width set at a 30° angle from the vertical, were filled with vermiculite and placed in the greenhouse (Fig. 1). Because of the narrow design of the cases, we selected vermiculite because its lower compaction and greater oxygenation than other growing media. The case bottom was perforated to allow for water drainage. At transplant, individuals of each species were placed completely at random 8 cm from each other and near the lower side of each case. Given the small seedling size, the lack of lateral roots and the short monitoring period, this distance seemed enough to prevent competition. The lower side of the glass case was covered by a black canvas so that roots grew in darkness on this side and root growth could be monitored through the glass. The other side was left uncovered. Each individual was watered with 40 ml every 3 days during the first week following transplant. After acclimation, on 19 April 2005, seedlings were allocated to treatments following a factorial design with two factors and two levels each. Watering quantity included a control (20 ml every time) and a watering of 30% less than the control (14 ml). A second factor included frequency of watering, and comprised a ‘normal’ level (two waterings per week) and half the number of events (one per week). Each of the four combinations comprised five replicates per species. All waterings were done with a syringe to prevent flooding. Seedlings grew in a greenhouse sheltered from direct radiation for 5 weeks without fertilization and the cases position was rearranged weekly. The mean daily temperature in the sheltered area was 18.9 ± 0.3°C, and the mean maximum and minimum were 23.9 ± 0.4 and 13.7 ± 0.3°C, respectively.
Measurements
Shoot height and root length of each plant were measured weekly during the manipulation period. Shoot height was measured with a calliper and new root segments and trajectories were drawn on the glass surface using different colour markers. At the end of the experiment, root length marks on the glass were traced to acetate sheets and digitalized with a portable scanner (Epson GT7000, Seiko Epson Corp., Nagano, Japan) at 300 dpi. Root length was measured from digitalized traces using the macro RootMeasure v.1.80 (Kimura and Yamasaki 2003) implemented on the software Scion Image Beta v. 4.02 (Scion Corp., MD, USA). We calculated mean root and shoot elongation rates for each plant between the initial and final lengths. Growth curves were obtained by plotting cumulative root length data against time. Maximum rooting depth was recorded before harvesting. At harvest, on 24 May 2005, shoots of each species were clipped at surface level, stored in paper bags, dried at 71°C for at least 48 h in a ventilated oven and weighed. Glass cases were then emptied out gently so as not to break root systems and vermiculite particles attached to root hairs were removed by gently washing and brushing them out. Roots were labelled, placed into wet paper towels and kept cool in zip bags in a refrigerator until they were scanned. Root length and root area of each plant were digitalized and measured following the procedure described above for traced roots. Root biomass was obtained after drying samples as with shoots, and root-to-shoot mass ratio (R:S ratio) for each plant was calculated from these data. Specific root length (SRL, cm g−1) on the entire root system was computed from total root length and mass.
Growth analysis
Relative growth rate (RGR, mg g−1 day−1) during the monitoring period was calculated from data at harvest (W 2) and transplant (W 1) following:
where t2 - t1 was 41 days, using the Hunt et al. (2002) spreadsheet tool. We calculated water-use efficiency (WUE, mg l−1) as the ratio between biomass gained and water received during the experiment, taking into account averaged initial biomass at transplant (Kikvidze et al. 2006). From seedling root length in reduced and control water levels at harvest, we calculated for each species the relative interaction index (RII, Armas et al. 2004) as an index of root plasticity to reduced watering, expressed as:
where Rr and Rc were root length in reduced and control plants, respectively. Although this is not a specific plasticity index, its strong mathematical and statistical properties make it appropriate for comparisons between plants growing in two treatment groups, in this case control and reduced.
Statistics
Data were exploratory analyzed as a two-factor design (watering quantity and frequency), however, analyses showed no differences in any variable between normal watering and half the number of events in the frequency factor. Likely, pulses of water of different magnitude while keeping constant the amount of water provided did not affect soil moisture in our conditions. For this reasons we excluded the frequency factor from analyses to gain statistical power since some plants died after transplant, and those data were pooled either into corresponding control or reduced quantity level since the amount of water provided was kept constant within the frequency factor (i.e., plants in the control water quantity received 40 ml per week in one (half events) or two events (normal frequency), and similarly in the reduced water quantity (28 ml distributed in a single or two 14 ml events per week).
Data were then analyzed as a factorial design with two factors, species and water quantity. Differences in mean growth rate, total root length, root area, maximum rooting depth, biomass, SRL, R:S ratio and WUE were tested using two-way analysis of variance (ANOVA) for each variable followed by Tukey HSD post hoc comparison tests. For total root length analysis we used length of traced roots instead of length of scanned roots since the former data were more homoscedastic. Differences in root length measurements between the two procedures were not significant (paired t-test, P = 0.47). Because of the unequal sample size, we used type III sum of squares. Heteroscedastic variables were transformed to meet ANOVA assumptions. When variables were still heteroscedastic (as in WUE), we ran for each species separately the non-parametric Mann–Whitney U-test (M–W U). Comparisons in plasticity index (RII) among species were conducted from standard errors since all replicates belonging to a treatment were integrated in computation.
Since plotted data of cumulative root length against time showed a linear trend, growth curve analyses were conducted by fitting individual data to a linear function Y = mX + b, where Y was length (cm), X was time (days), m was the slope and b the y-intercept. Differences in growth curves between species and water treatment were tested by comparing regression slopes of each plant (m) through ANOVA. We could not perform repeated-measures and multivariate ANOVA to test growth responses because our data violated statistical assumptions (Von Ende 2001). Only those individuals whose roots could be seen through the glass case from the beginning of the experiment were included into root growth analysis.
All tests were conducted with Statistica v. 6.0 (Statsoft Inc, Tulsa, OK, USA) and differences were considered significant at P < 0.05. Data are presented as means ± one standard error.
Results
Cumulative root length over time was best adjusted to a linear function. Growth curves were statistically different between control and reduced water quantity in all species (P = 0.014), with roots under drought growing faster (Fig. 2). This was reflected in root elongation rate (ANOVAwater P = 0.013); plants subjected to lower watering elongated more than control plants (8.58 ± 0.74 vs. 6.74 ± 0.65 mm day−1), regardless of species identity (ANOVAspecies × water P = 0.99, Fig. 3a). We found significant differences in mean root elongation rate among species, with Lycium having the highest rate (10.57 ± 0.58 mm day-1), followed by Retama (6.66 ± 0.66) and Genista (4.67 ± 0.49, Table 2). As for root plasticity, all species responded to reduced watering by developing longer roots (as reflected by positive values of RII), though Genista showed the strongest response (0.142 ± 0.024), whereas in Lycium and Retama it was lower (0.078 ± 0.011 and 0.083 ± 0.017, respectively).
Root elongation curves. Cumulative root length over time in control (solid symbols) and reduced watering (white symbols), and fitted linear functions (lines) with r 2 and P-values of regression. Growth curves of control and reduced treatments are statistically different (ANOVAwater F 1,39 = 6.589, P = 0.014), regardless of species (ANOVAspecies × water F 2,39 = 0.062, P = 0.940)
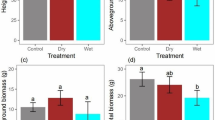
Plant growth at harvest. (a) Mean root elongation rate (mm day−1) in the control (solid bars) and reduced watering treatment (white bars), ANOVAwater P = 0.013, (b) root area (cm2) at harvest, ANOVAwater P = 0.007, and (c) water use efficiency (mg l−1), Mann–Whitney test. A cross indicates marginal differences between water quantities (P < 0.1) and asterisks significant differences (*P < 0.05; **P < 0.01). Values are means ± 1 SE. n = 6–9
Total root length and root area at harvest differed among species, decreasing Lyicum > Retama > Genista (Table 2, Fig. 3b). There were also significant differences in root length and root area between water treatments (ANOVAwater P < 0.01), regardless of species (ANOVAspecies × water P > 0.6, Figs. 2, 3b). When compared to control, plants supplied with reduced water quantity showed longer roots (28.65 ± 2.26 cm vs. 35.75 ± 2.57 cm) and greater root area (2.69 ± 0.26 cm2 vs. 4.02 ± 0.47 cm2). On the contrary, we only detected a tendency to root deeper in response to lower water availability (25.55 ± 2.03 cm for reduced vs. 22.45 ± 1.71 cm for control plants, ANOVAwater P = 0.13, Tables 2, 3). Roots of Lycium and Genista had higher SRL than Retama, although no significant adjustment in response to altered watering quantity was detected in any species (P water = 0.86, P species × water = 0.31). Root-to-shoot mass ratio was below 0.6 in all species (Table 3), ranging from 0.47 ± 0.05 in Genista and 0.41 ± 0.03 in Lycium to 0.28 ± 0.02 in Retama. We did not detected significant effects of water quantity on R:S ratio in any species (ANOVAwater P = 0.42).
We found differences among species in plant, shoot and root mass at harvest (ANOVAspecies P < 0.001, Table 2), in contrast, no differences were observed in mean shoot elongation in any species in response to drought (M–W U water P > 0.25). The effects of watering quantity on biomass depended on species, as revealed by the species × water interaction (ANOVA P < 0.03); plants supplied with lower water quantity tended to exhibit larger mass than those in control in Lycium and Genista, whereas Retama performed nearly the same both in control and reduced levels. The same pattern was observed if plant growth was considered with respect to initial plant size (i.e., RGR); RGR of total plant, shoot and root masses were higher under reduced water in Genista and Lycium, whereas differences in Retama were less patent (Table 3). This mirrored in water use efficiency of productivity. Plants supplied with lower water quantity produced significantly more biomass per water received than those in control in Lycium (M–W U water = 5, P < 0.01), and marginally in Genista (M–W U water = 8, P = 0.06). In Retama, however, biomass gain was independent of water provided (M–W U water = 27, P = 0.91, Fig. 3c).
Discussion
A small reduction in water supply enhanced root elongation in all our species at very early stages of development, when cotyledons were still attached. This could be an analogous response to etiolation of shoots under shaded conditions (Leishman and Westoby 1994b). Despite the contrast in seed mass and drought tolerance among Retama, Lycium and Genista, all three species, either drought-tolerant or sensitive, large or small-seeded, responded equally to reduced watering. These data evidence the importance of water availability for seedling development during such early stage. The increase in root length and area in plants under reduced watering can be interpreted as an adjustment of absorptive surfaces to find water resources (Hutchings and de Kroon 1994). By increasing root length, plants exploit a larger soil volume tapping otherwise unexplored areas and increase their resource uptake capacity, which depends on root surface area (Lambers et al. 1998b).
Our findings agree with reports showing root elongation in response to low soil moisture (Evans and Etherington 1991). Reader et al. (1993) found that rooting depth of seedlings of wild species increased in response to drought due to higher elongation rates, particularly in species that regenerate mainly from seeds after disturbance (seeders), suggesting that selective pressures favor plasticity in root growth, affecting traits that promote seedling survival. Although we do not report significant differences in rooting depth between control and reduced water (P = 0.13), most likely because of the short time period considered, our data are consistent with this explanation. Thus, early root growth shows an adaptive strategy to deal with water stress at the seedling stage (Fitter 1991). Root elongation and deeper rooting depth in response to water stress is presumably also an adaptation that allows exploitation of declining soil moisture (Lambers et al. 1998a) and in fact, the ability to develop roots accessing deep soil moisture has proved decisive for survival of seedlings during summer months in a Mediterranean semiarid environment (Padilla and Pugnaire 2007). Our hypothesis that root growth response would be stronger in the drought-sensitive and large-seeded Retama because of its sensitivity to dehydration and larger seed reserves could be rejected since a drought-tolerant and small-seeded species (Genista) showed a distinctly plastic response. Developing seedlings of large-seeded species acquire most resources from seed reserves (Fenner and Kitajima 1999), and then they are relatively more independent from external resources than small-seeded species. However, the weak response we found may not involve a disadvantage in the field, since germination timing and seedling size may offset low root growth capacity. Interestingly, there are reports of greater root elongation rate in drought-tolerant turfgrass (Huang 1999) and phreatophyte seedlings (Horton and Clark 2001), and in seedlings of species restricted to dry sites (Evans and Etherington 1991) when subjected to lower water availability. It is clear that root plasticity is under genetic control (Sydes and Grime 1984; Sharp et al. 2004) and species do not show the same ability to elongate; however, whether root plasticity is linked to the species’ drought tolerance, and the underlying mechanisms, still remains unclear.
Surprisingly, we found larger shoot mass and higher RGR in Lycium and Genista seedlings supplied with less water, whereas differences were negligible in Retama. It is improbable that this was due to greater root biomass allocation or root length exploiting potentially more soil volume of Retama, since it allocated the least to roots (lowest R:S ratio) and showed one of the shortest root lengths at transplant. Rather, seed size and cotyledon reserves can explain such response, since they strongly affect seedling growth (Leishman and Westoby 1994b; Cornelissen et al. 1996; Bonfil 1998; Hanley et al. 2004; Hanley and May 2006). Large-seeded species, indeed, have storage cotyledons characterized by a slow, prolonged mobilization of reserves (Kidson and Westoby 2000), relying to a greater extent on cotyledons than on soil resources and light (Milberg and Lamont 1997), whereas small-seeded species are more dependent on light and soil resources (Leishman and Westoby 1994b; Fenner and Kitajima 1999). In our experiment, all three species retained green cotyledons until harvest, but cotyledon reserves lasted longer in Retama than in Lycium and Genista because of its differences in seed size (up to two orders of magnitude) and cotyledon mass. All three species increased root absorptive surface with lower water availability as a strategy to maximize water uptake, allowing secondarily greater nutrient uptake; Retama, however, did not show changes in shoot growth due to its greater dependency on cotyledons. In this sense, Jurado and Westoby (1992) found that seedlings from large-seeded species thrived better under nutrient stress than small-seeded species, since their growth remained independent from external resources, and similar results were reported by Milberg and Lamont (1997). In conclusion, increased root absorptive surface caused by low water availability was a response of all three species to maximize water uptake, which also allowed for greater nutrient uptake. In fact, Wan et al. (2002) also found that drought induced root production and enabled droughted plants to produce above-ground biomass similar to that of plants receiving full watering. However, in our experiment, growth depended on cotyledon reserves. Shoot growth and RGR was higher under reduced watering in Genista and Lycium because of greater root exploitation and resource uptake, while Retama depended more on cotyledon reserves and shoot growth was relatively unaffected by nutrient uptake.
Having small-diameter roots (i.e., higher SRL) favors greater rates of water and nutrient uptake (Eissenstat 1992; Cornelissen et al. 2003), therefore larger SRL under reduced water availability could be expected as a strategy to maximize absorptive surfaces (Reich et al. 1998; Wright and Westoby 1999). All species showed increased root length under reduced water availability, evidencing changes in root morphology with water quantity, but SRL did not differ between watering treatments. This inconsistency can be due to the fact that we used the whole root systems to obtain this measurement, and Nicotra et al. (2002) showed that SRL of the entire root systems can differ from that measured on the main axis or secondary roots. Similarly, large biomass allocation to roots relative to shoots (i.e., higher R:S ratio) also favors water and nutrient uptake (Chapin et al. 1987; Lambers et al. 1998a), and therefore we expected larger R:S ratios under reduced water availability. However, plants did not respond to water stress by shifting allocation patterns, and the R:S ratio did not change. Although the allocation model is widely accepted (see e.g., Chapin et al. 1987; Kozlowski and Pallardy 2002), other factors do impact upon R:S partitioning. Evidence suggests that plasticity in R:S ratio may be highly species-specific (Joslin et al. 2000) and that in some species R:S ratio is remarkably stable (Klepper 1991) or subjected to developmental constraints (Gedroc et al. 1996; McConnaughay and Coleman 1999). Additionally, root demography and the ability to alter rates and place of root proliferation may have greater importance for plants than changes in mass allocation between roots and shoots (Reynolds and D’Antonio 1996).
Overall, we showed that very young seedlings responded to reduced water availability by elongating roots, whereas no significant changes in R:S ratio were detected. Greater absorptive root surface likely allowed seedlings to increase growth rate in the small-seeded species, whereas growth of the large-seeded species seemed independent from external resources. Root growth may be considered an important factor in early seedling development, since rapid extension of roots enables seedlings to tap water from previously unexplored areas of soil (Schütz et al. 2002). Regardless of seed size and drought tolerance strategy, root elongation in our three species is a common adaptive trait to cope with soil dryness at early stages. However, further research is needed to link root plasticity to species-specific drought tolerance.
References
Ackerly DD (2004) Functional strategies of Chaparral shrubs in relation to seasonal water deficit and disturbance. Ecol Monogr 74:25–44
Armas C, Ordiales R, Pugnaire FI (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Bazzaz FA (1991) Habitat selection in plants. Am Nat 137S:116–130
Bonfil C (1998) The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). Am J Bot 85:79–87
Buckley RC (1982) Seed size and seedling establishment in tropical arid dunecrest plants. Biotropica 14:314–315
Cahill JR, Casper BB (1999) Growth consequences of soil nutrient heterogeneity for two old-field herbs, Ambrosia artemisiifolia and Phytolacca americana, grown individually and in combination. Ann Bot 83:471–478
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37:49–57
Cornelissen JHC, Castro-Díez P, Hunt R (1996) Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. J Ecol 84:755–765
Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas J, Poorter H (2003) A handbook of protocols for standardised and easy measurements of plant functional traits worldwide. Aust J Bot 51:335–380
Davis SD (1989) Patterns in mixed chaparral stands: differential water status and seedling survival during summer drought. In: Keeley SC (ed) The California chaparral: paradigms re-examined. Natural History Museum of Los Angeles County, Los Angeles, pp 97–105
Donovan LA, Mausberg J, Ehleringer JR (1993) Seedling size and survival for Chrysothamnus nauseosus. Great Basin Nat 53:237–245
Eapen D, Barroso ML, Ponce G, Campos ME, Cassab GI (2005) Hydrotropism: root growth responses to water. Trends Plant Sci 10:44–50
Eissenstat DM (1992) Costs and benefits of constructing roots of small diameter. J Plant Nutr 15:763–782
Evans CE, Etherington JR (1991) The effect of soil water potential on seedling growth of some British plants. New Phytol 118:571–579
Fenner M (1987) Seedlings. New Phytol 106S:35–47
Fenner M, Kitajima K (1999) Seed and seedling ecology. In: Pugnaire FI, Valladares F (eds) Handbook of functional plant ecology. Marcel-Dekker, New York, pp 589–621
Fitter AH (1991) Characteristics and functions of root systems. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel-Dekker, New York, pp 3–25
Gedroc JJ, McConnaughay KMD, Coleman JS (1996) Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Funct Ecol 10:44–50
Grubb PJ (1977) Maintenance of species-richness in plant communities—importance of regeneration niche. Biol Rev Camb Philos Soc 52:107–145
Haase P, Pugnaire FI, Fernández EM, Puigdefábregas J, Clark SC, Incoll LD (1996) An investigation of rooting depth of the semiarid shrub Retama sphaerocarpa (L.) Boiss. by labelling of ground water with a chemical tracer. J Hydrol 177:23–31
Haase P, Pugnaire FI, Clark SC, Incoll LD (1999) Diurnal and seasonal changes in cladode photosynthetic rate in relation to canopy age structure in the leguminous shrub Retama sphaerocarpa. Funct Ecol 13:640–649
Hanley ME, May OC (2006) Cotyledon damage at the seedling stage affects growth and flowering potential in mature plants. New Phytol 169:243–250
Hanley ME, Fenner M, Whibley H, Darvill B (2004) Early plant growth: identifying the end point of the seedling phase. New Phytol 164:61–66
Harper JL (1977) Population biology of plants. Academic Press, New York
Hasting SJ, Oechel WC, Sionit N (1989) Water relations and photosynthesis of chaparral resprouts and seedlings following fire and hand clearing. In: Keeley SC (eds) The California Chaparral: paradigms re-examined. Natural History Museum of Los Angeles County, Los Angeles, pp 107–113
Herrera CM (1992) Historical effects and sorting processes as explanations for contemporary ecological patterns-character syndromes in Mediterranean woody-plants. Am Nat 140:421–446
Horton JL, Clark JL (2001) Water table decline alters growth and survival of Salix gooddingii and Tamarix chinensis seedlings. For Ecol Manage 140:239–247
Huang B (1999) Water relations and root activities of Buchloe dactyloides and Zoysia japonica in response to localized soil drying. Plant Soil 208:179–186
Hunt R, Causton DR, Shipley B, Askew P (2002) A modern tool for classical plant growth analysis. Ann Bot 90:485–488
Hutchings MJ, de Kroon H (1994) Foraging in plants—the role of morphological plasticity in resource acquisition. Adv Ecol Res 25:159–238
IPCC (2001) Third assessment report of the intergovernmental panel on climate change. Cambridge University Press, New York
Joslin JD, Wolfe MH, Hanson PJ (2000) Effects of altered water regimes on forest root systems. New Phytol 147:117–129
Jurado E, Westoby M (1992) Seedling growth in relation to seed size among species of arid Australia. J Ecol 80:407–416
Kidson R, Westoby M (2000) Seed mass and seedling dimensions in relation to seedling establishment. Oecologia 125:11–17
Kikvidze Z, Armas C, Pugnaire FI (2006) The effect of initial biomass in manipulative experiments on plants. Funct Ecol 20:1–3
Kimura K, Yamasaki S (2003) Accurate root length and diameter measurement using NIH image: use of Pythagorean distance for diameter estimation. Plant Soil 254:305–315
Klepper B (1991) Root-shoot relationships. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel-Dekker, New York, pp 265–286
Kozlowski TT, Pallardy SG (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68:270–334
Lambers H, Chapin FS, Pons TL (1998a) Growth and allocation. In: Plant physiological ecology. Springer, New York, pp 299–351
Lambers H, Chapin FS, Pons TL (1998b) Mineral nutrition. In: Plant physiological ecology. Springer, New York, pp 239–298
Lansac AR, Zaballos JP, Martin A (1994) Seasonal water potential changes and proline accumulation in Mediterranean shrubland species. Vegetation 113:141–154
Lázaro R, Rodrigo FS, Gutiérrez L, Domingo F, Puigdefábregas J (2001) Analysis of a 30-year rainfall record (1967–1997) in semi-arid SE Spain for implications on vegetation. J Arid Environ 48:373–395
Leishman MR, Westoby M (1994a) The role of seed size in seedling establishment in dry soil-conditions—experimental evidence from semiarid species. J Ecol 82:249–258
Leishman MR, Westoby M (1994b) The role of seed size in shaded conditions: experimental evidence. Funct Ecol 8:205–214
Lloret F, Casanovas C, Peñuelas J (1999) Seedling survival of Mediterranean shrubland species in relation to root:shoot ratio, seed size and water and nitrogen use. Funct Ecol 13:210–216
McConnaughay KDM, Coleman JS (1999) Biomass allocation in plants: ontogeny or optimality? A test along three resource gradients. Ecology 80:2581–2593
Milberg P, Lamont BB (1997) Seed/cotyledon size and nutrient content play a major role in early performance of species on nutrient-poor soils. New Phytol 137:665–672
Moles AT, Westoby M (2004a) What do seedlings die from and what are the implications for evolution of seed size? Oikos 106:193–199
Moles AT, Westoby M (2004b) Seedling survival and seed size: a synthesis of the literature. J Ecol 92:372–383
Nicotra AB, Babicka N, Westoby M (2002) Seedling root anatomy and morphology: an examination of ecological differentiation with rainfall using phylogenetically independent contrasts. Oecologia 130:136–145
Padilla FM, Pugnaire FI (2007) Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct Ecol 21:489–495
Pugnaire FI, Chapin FS, Harding MT (2006) Evolutionary changes in correlations among functional traits in Ceanothus in response to Mediterranean conditions. Web Ecol 6:17–26
Rajaniemi TK, Reynolds HL (2004) Root foraging for patchy resources in eight herbaceous plant species. Oecologia 141:519–525
Reader RJ, Jalili A, Grime JP, Spencer RE, Matthews NN (1993) A comparative-study of plasticity in seedling rooting depth in drying soil. J Ecol 81:543–550
Reich PB, Tjoelker MG, Walters MB, Vanderklein DW, Buschena C (1998) Close association of RGR, leaf and root morphology, seed mass and shade tolerance in seedlings of nine boreal tree species grown in high and low light. Funct Ecol 12:327–338
Reynolds HL, D’Antonio CM (1996) The ecological significance of plasticity in root weight ratio in response to nitrogen: opinion. Plant Soil 185:75–97
Schupp EW (1995) Seed seedling conflicts, habitat choice, and patterns of plant recruitment. Am J Bot 82:399–409
Schütz W, Milberg P, Lamont BB (2002) Germination requirements and seedling responses to water availability and soil type in four eucalypt species. Acta Oecol 23:23–30
Sharp RE, Poroyko V, Hejlek LG, Spollen WG, Springer GK, Bohnert HJ, Nguyen HT (2004) Root growth maintenance during water deficits: physiology to functional genomic. J Exp Bot 55:2343–2351
Sydes CL, Grime JP (1984) A comparative-study of root development using a simulated rock crevice. J Ecol 72:937–946
Tirado R (2003) Interacciones positivas entre plantas: mecanismos y consecuencias. Ph.D. Thesis, Seville University, Spain
Von Ende CN (2001) Repeated-measures analysis. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 134–157
Wan C, Yilmaz I, Sosebee RE (2002) Seasonal soil–water availability influences snakeweed root dynamics. J Arid Environ 51:255–264
Wright IJ, Westoby M (1999) Differences in seedling growth behaviour among species: trait correlations across species, and trait shifts along nutrient compared to rainfall gradients. J Ecol 87:85–97
Acknowledgments
We are grateful to Kazuhiko Kimura for helping with the root macro, Florentino Mostaza for root scanning and Consejería de Medio Ambiente (Junta de Andalucía) for seed donation. Two anonymous reviewers made valuable comments on an earlier draft. The Spanish Ministry of Education and Science funded this work (grant CGL2004-00090/CLI). FMP was supported by a predoctoral I3P fellowship (CSIC-European Social Fund).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tibor Kalapos.
Rights and permissions
About this article
Cite this article
Padilla, F.M., Miranda, J. & Pugnaire, F.I. Early root growth plasticity in seedlings of three Mediterranean woody species. Plant Soil 296, 103–113 (2007). https://doi.org/10.1007/s11104-007-9294-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9294-5