Abstract
Functional genomics through transgenesis has provided faster and more reliable methods for identifying, characterizing, and utilizing genes or quantitative trait loci linked to agronomic traits to target yield. The present study explored the role of Big Grain1 (BG1) gene of rice (Oryza sativa L.) in yield improvement of crop plants. We aimed to identify the genetic variation of OsBG1 in various indica rice cultivars by studying the allelic polymorphism of the gene, while also investigating the gene’s potential to increase crop yield through the transgenic approach. Our study reports the presence of an extra 393 bp sequence having two 6 bp enhancer elements in the 3′ regulatory sequence of OsBG1 in the large-grain cultivar IR64 but not in the small-grain cultivar Badshahbhog. A single copy of the OsBG1 gene in both the cultivars and a 4.1-fold higher expression of OsBG1 in IR64 than in Badshahbhog imply that the grain size is positively correlated with the level of OsBG1 expression in rice. The ectopic expression of OsBG1 under the endosperm-specific glutelin C promoter in Badshahbhog enhanced the flag leaf length, panicle weight, and panicle length by an average of 33.2%, 33.7%, and 30.5%, respectively. The length of anthers, spikelet fertility, and grain yield per plant increased in transgenic rice lines by an average of 27.5%, 8.3%, and 54.4%, respectively. Heterologous expression of OsBG1 under the constitutive 2xCaMV35S promoter improved the number of seed pods per plant and seed yield per plant in transgenic tobacco lines by an average of 2.2-fold and 2.6-fold, respectively. Improving crop yield is crucial to ensure food security and socio-economic stability, and identifying suitable genetic factor is the essential step towards this endeavor. Our findings suggest that the OsBG1 gene is a promising candidate for improving the grain yield of monocot and dicot plant systems by molecular breeding and genetic engineering.
Key message
Rice Big Grain1 increases grain yield in rice by improving panicle architecture and spikelet fertility rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice provides more than 70% of the energy supply in the Asian continent and accounts for nearly half of the food expenses of people experiencing poverty (Wassmann et al. 2010). Rice yield is essential to ensure food security, economic growth, and stability (Yadav and Kumar 2019). The strategies targeting consistent crop productivity include reducing crop losses and advances in crop yield using marker-assisted breeding approaches, spatiotemporal regulation of genes through genetic engineering, targeted genome editing, agronomic management, etc. (Bailey-Serres et al. 2019). Green Revolution utilizing semidwarf cultivars since the 1960s improved the yield of rice crops, but productivity in recent years has plateaued. The genomic era provides scope for further improvement in crop productivity for sustainable agriculture (Datta 2004). With the rice (Oryza sativa L.) genome sequenced, many genes linked to agronomically important traits have been identified, cloned, and characterized (Jena and Kim 2020). Several studies have utilized the tools of genetic engineering to identify and characterize the genes regulating primary components of yield like grain weight, grain number, number of panicles, and percentage of filled grains. For example, Gn1a– a major QTL (quantitative trait loci) governing grain number in rice encoding cytokine oxidase/dehydrogenase has been identified. Transgenic rice plants with RNAi-silenced Gn1a (OsCKX2) developed more grains (Ashikari et al. 2005). The overexpression of AtCKX2, the Arabidopsis ortholog of rice OsCKX2, resulted in fewer flowers in transgenic Arabidopsis (Werner et al. 2003). Other studies identifying and characterizing rice QTLs - GS3 and GW2 - realized their potential for grain size and yield improvement (Fan et al. 2006; Song et al. 2007).
Rice Big grain1 (OsBG1) in japonica rice has recently been reported as a positive regulator for grain size (Liu et al. 2015). The study reported an Agrobacterium T-DNA insertion rice mutant big grain1-D with a bigger grain phenotype and recorded an increase in the 1000-grain weight, length, and breadth of the grains of the mutant line. The mutant also exhibited increased height and larger panicles than the untransformed control. The site-finding PCR revealed that the T-DNA was inserted at 0.8 kb upstream of the gene Os03g0175800. The expression analysis revealed a 10-fold higher expression of Os03g0175800, hinting that the overexpression of Os03g0175800 is responsible for BG1-D mutant phenotypes. The OsBG1-overexpression under the ACTIN1 promoter led to phenotypes similar to the mutant line, and the phenotypes were correlated with the level of OsBG1 expression (Liu et al. 2015). The OsBG1 silencing line (BG1-Ri) showed decreased plant height, grain size and weight, and panicle length. The study reported that the overexpression of OsBG1 in Arabidopsis led to increased plant growth and grain size, highlighting OsBG1’s potential to improve plant biomass in different plant species through heterologous expression (Liu et al. 2015). In a previous study, we explored the functional prospects of OsBG1 through the structure-based function prediction approach. In silico structure analyses revealed OsBG1 as a DNA- and phytohormone-binding protein, whereas its heterologous expression in a rhizospheric yeast enhanced the cell density, specific growth rate, and biomass, suggesting OsBG1’s potential to enhance the growth and biomass of organisms other than plant (Ekta et al. 2023). Lo et al. (2020) reported another grain yield regulating gene, Rice Big Grain 1 (RBG1) (accession number: NM_001189625), which is different from Oryza sativa Big Grain1 (OsBG1) (accession number: XM_015773860.2), the gene of interest in the present study.
Utilizing the genes of agronomical importance through various biotechnological interventions like molecular breeding, marker-assisted genomic selection, recombinant DNA technology/transgenic approach, CRISPR/Cas9, etc., has immense scope in yield improvement of crops (Hussain et al. 2022). Molecular-assisted breeding and genomic selection, which are part of the new breeding techniques, can significantly accelerate the breeding programs aimed at improving the agronomic traits of crops. To improve the selection efficiency in marker-assisted selection (MAS), use of DNA markers is widespread for rice yield improvement (Hussain et al. 2022). Molecular markers are a robust and reliable tool in breeding as they reduce the selection cycle and are not influenced by environmental factors (Budak et al. 2015). Determining variation in the genomic regions and their trait association are the main components of MAS. Studying the natural genetic variations in genes related to agronomic traits can lead to the development of phenotypes-associated molecular markers that can be utilized in breeding programs to improve yield. In this study, we investigated the genetic variation in OsBG1 and its regulatory regions in indica rice cultivars differing in grain size to explore its utility as the phenotype-associated genetic marker.
Liu et al. (2015) reported an insignificant change in grain yield per plant of transgenic lines due to a reduced percentage of filled grains caused by the expression of OsBG1 under a strong constitutive promoter ACTIN1. Another study involving the ectopic expression of the ortholog of OsBG1 under rice Actin promoter in wheat reported no significant change in yield (Milner et al. 2021). Constitutive promoters for transgene expression often lead to developmental abnormalities, lowered yield, expression in undesired tissues and developmental stages of host plants, and seasonal effects on the promoter’s strength (Pandey et al. 2019). Studies conducted have highlighted the advantages of inducible or tissue-specific promoters for transgene constructs over constitutive promoters for reasons like the precise and confined expression of the genes of interest, easy harvesting of the recombinant proteins, avoiding unnecessary depletion of plant nutrients and energy, etc. (Hussain et al. 2018; Liu 2009). Guo et al. (2022) reported that the constitutive overexpression of TaCYP78A5 in wheat resulted in increased seed size and weight, but no change in yield occurred due to the undesirable pleiotropic phenotypes.
The glutelin genes’ promoters have been reported as endosperm-specific promoters (Qu et al. 2008). Promoter plays a pivotal role in determining any gene’s spatiotemporal expression pattern and level. Constitutive high gene expression under strong promoters harms the host organisms and causes pleiotropic effects (Cheon et al. 2004). Moreover, the endosperm-specific promoter-driven expression of recombinant genes has been reported to be more stable than the expression under constitutive promoter (Choi et al. 2003, 2009). The promoter of one of the glutelin genes, GluC, is an endosperm-specific promoter with high and even expression covering the entire endosperm. A study by Qu et al. (2008) suggested that the activity of the glutelin gene’s promoter was not detected in the root, leaf, and stems of the transgenic lines expressing the GUS gene under glutelin gene promoters. The GluC promoter has been utilized earlier to drive the seed-specific transgene expressions in indica rice (Das et al. 2017, 2018).
With the available literature, we hypothesized that spatiotemporally-regulated expression of OsBG1 might improve the grain yield of the poor-yielding indigenous aromatic rice cultivar while avoiding the pleiotropic effects and undesirable phenotypes caused by constitutive expression of the transgene. For this, we designed the transgene construct for the regulated expression of the OsBG1 gene under the promoter of the glutelin C gene, GluC. Badshahbhog is an indigenous aromatic small-grain indica rice cultivar that offers a diverse and unique genetic pool for several valuable traits like stress resistance, nutritional quality, and aroma but is challenged with poor yield and lodging due to its low tillering and tall stature (Bisne and Sarawgi 2008; Singh et al. 2012; Verma and Srivastav 2017). The present study investigates the allelic variation of OsBG1 gene in different small-grain and large-grain indica rice cultivars, while exploring its potentiality in improving the yield components in a monocot rice cultivar, Badshahbhog and a model dicot plant, tobacco (Nicotiana tobaccum).
Materials and methods
Bacterial strains, plant cultivars, and oligonucleotide primers used in the study
Escherichia coli DH10B cells were used for gene cloning. Agrobacterium tumefaciens strain EHA105 was used for rice calli transformation, while strain LBA4404 was used for the tobacco leaf disc transformation.
Indica cultivars of rice (Oryza sativa L.) differing in grain size received from Bidhan Chandra Krishi Viswavidyalaya (BCKV), West Bengal, were used in the study (Fig. S1 and Table S1). The tobacco (Nicotiana tobaccum L.) cultivar Siri was included in the study as a model dicot plant system. The primers used for various polymerase chain reactions (PCRs) in the study are listed in Table S2.
PCR-based study of the genetic variation in OsBG1
To study the genetic variation of OsBG1, the primers were designed to amplify the coding DNA sequence (CDS) and the flanking 5’ and 3’ regulatory sequences of the gene based on the reference sequence (NCBI Reference ID - XM_015773860.2) from the Nipponbare cultivar of rice (Oryza sativa L. ssp. japonica). The primer pairs used for the purpose are listed in Table S2. PCR was performed to amplify the fragments from the genomic DNA isolated from different rice varieties using Taq polymerase (New England Biolabs, Ipswich, MA, USA) (Fig. S2 and 1 A). The amplicons from Badshahbhog and IR64 were sequenced, and the obtained sequences were aligned with the reference gene (XM_015773860.1) (Fig. S3, S4, and S5).
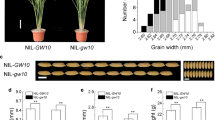
Detection of DNA polymorphism in 3’ regulatory sequence of OsBG1 in rice cultivars differing in grain size and examination of transcript expression of the single copy OsBG1 gene in young panicles of two selected cultivars. A Picture of a 1% agarose gel showing the DNA fragments of 3’ regulatory sequences of OsBG1, which are PCR amplified from the genomic DNA (gDNA) of various indica rice cultivars differing in grain size. Lane 1 to 5: Amplified PCR product from the gDNA of indica rice cultivars Khanika, Tulaipanji, Radhunipagol, Kabirajsail, and Hatipajar, respectively. Lane 6: 100 bp DNA ladder. Lane 7 to 11: Amplified PCR product from the gDNA of indica rice cultivars Chini Atap, Latisail, Patnai, Jaladhi, and Radhatilak, respectively. Lane 12: 100 bp ladder DNA. Lane 13 to 16: Amplified PCR product from the gDNA of indica rice cultivars Dudheswar, Dehradun Basmati, IR64, and Badshahbhog. Lane 17 and 18: 100 bp ladder DNA. Lane 19: Amplified PCR product from the gDNA of indica rice cultivar Gobindobhog. B Schematics representing the aligned sequences of OsBG1 CDS and its 3’ regulatory sequence depicting the presence of extra 393 nucleaotides in the 3’ regulatory sequence of OsBG1 in large-grain rice cultivar IR64. The red-colored stars in the 3’ regulatory sequence of the aligned sequences represent the presence of enhancer elements ‘CAATTG’ and ‘CACGTG’ in the large-grain indica cultivar IR64 but absent in small-grain indica cultivar Badshahbhog and japonica cultivar Nipponbare. C Graph representing the relative abundance of OsBG1 transcript in young panicles of rice cultivars Badshahbhog (small-grain) and IR64 (large-grain). The rice Actin 1 gene expression was taken as an endogenous housekeeping reference for normalization of OsBG1 expression. The comparative fold-change in the expression of OsBG1 in IR64 was calculated by taking the expression of OsBG1 in Badshahbhog as unity. The standard bar represents the deviation from the average. ‘**’ represents the statistical significance calculated using the student’s t-test with p-value < 0.01. D Southern blot picture depicting a single copy of OsBG1 present in the genomes of Badshabhog (small-grain) and IR64 (large-grain) indica rice cultivars. Lane 1: DIG-labelled marker III (Roche, Basel, Switzerland). Lane 2: eluted OsBG1 DNA fragment of 270 bp as a positive control. Lane 3 and 4: gDNA of IR64 and Badshahbhog digested with PstI and SacI showing bands of ~ 4.4 kb developed after hybridization with DIG-labeled probe specific for OsBG1. Lane 5: DIG-labelled marker III (Roche, Basel, Switzerland). E Schematic representation of the restriction digestion pattern of the OsBG1 gene locus (CDS, and 5’ regulatory sequences) using the PstI and SacI restriction endonucleases
Southern hybridization for copy number detection of OsBG1
Genomic DNA samples isolated from Badshahbhog and IR64 rice cultivars digested with restriction endonucleases were fractionated and transferred to a nylon membrane using a Biometra vacuum blot system (Analytik Jena, Jena, Germany) and fixed to the membrane using a UV-cross linker (Stratagene, San Diego, California, USA). The nylon blot was then hybridized with the OsBG1-specific DIG-labeled probe prepared from the PstI and SacI digested 270 bp DNA fragment of OsBG1. After hybridization, the probes were immunodetected using anti-digoxigenin-AP fab fragments, followed by the development of the blot using NBT/BCIP color substrate (Roche, Basel, Switzerland) as per the manufacturer’s instruction manual.
Isolation, cloning, and sequencing of OsBG1
Full-length CDS of OsBG1 (933 bp) was isolated from the genomic DNA of an indica rice cultivar Badshahbhog (Fig. S6A), using the primers BG1 New For and BG1 New Rev (Table S2). The PCR amplicon from Badshahbhog was ligated to the pCRTM-BLUNT vector (Zero Blunt™ PCR cloning kit) (Thermo Fisher Scientific, Waltham, MA, USA), and the resulting recombinant plasmid was used to transform chemically competent E. coli DH10B. The recombinant clones (pBLUNT-OsBG1) were confirmed by restriction digestion (Fig. S6B) and sequencing (Fig. S6C).
Preparation of the genetic construct for ectopic expression of OsBG1 using GluC promoter in indica rice cultivar Badshahbhog
Plasmid pBLUNT-OsBG1 was digested using PstI and BamHI enzymes. The digested and purified OsBG1 DNA fragment was ligated to HindIII and BamHI digested pCAMBIA1300-NOS vector and HindIII and PstI digested glutelin gene GluC promoter (Das et al. 2017) in a tripartite ligation using T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). The resulting recombinant plasmid (Fig. S7A) was used to transform chemically competent cells of E. coli, and the positive clones were confirmed by restriction digestion (Fig. S7B). Next, the competent cells of Agrobacterium tumefaciens strain EHA105 were transformed with the recombinant plasmid pCAM-GluC-OsBG1-NOS and selected on YEP (yeast extract peptone) medium (Himedia, Mumbai, India) plates with rifampicin (20 mg/L) and kanamycin (50 mg/L).
Preparation of the genetic construct for constitutive expression of OsBG1 in heterologous host tobacco
OsBG1 was PCR amplified using BG1 Bam For and BG1 Kpn Rev primer pair (Table S2) and pBLUNT-OsBG1 plasmid as a template. The amplified PCR product was digested with BamHI and KpnI and ligated to the pUC19 vector digested with the same set of restriction endonucleases. The ligated plasmid pUC19-OsBG1 was used to transform the competent cells of E. coli using the heat shock method of transformation. The clones were confirmed with restriction digestion (Fig. S8A). The BamHI and KpnI digested OsBG1 DNA fragment from pUC19-OsBG1 was eluted, purified, and ligated to the pCAM2300-2xCaMV35S-NOS digested with the same set of restriction endonucleases. The ligated product pCAM-2xCaMV35S-OsBG1-NOS was used to transform the competent cells of E. coli, and the clone was confirmed with restriction enzyme digestion (Fig. S8B). After confirmation, the recombinant plasmid pCAM-2xCaMV35S-OsBG1-NOS (Fig. S8C) was used to transform the competent cells of Agrobacterium tumefaciens strain LBA4404, followed by a selection of clones on YEP agar plates supplemented with suitable antibiotics.
Agrobacterium-mediated transformation, regeneration, and screening of transformed rice and tobacco plants
The selected two recombinant A. tumefaciens strains were used to transform embryogenic rice calli and tobacco leaf discs following protocols described by Bhattacharya et al. 2014. The regenerated plantlets (T0 generation) were selected in a hormone-free (-H) liquid medium (Table S3) supplemented with 50 mg/L hygromycin (rice plantlets) and 50 mg/L kanamycin (tobacco plantlets) before moving them to paper cups with SOIL-RITE (Keltech, Bangalore, India). After hardening, the plantlets were potted in a potting mix (1:2 SOIL-RITE and soil) and transferred to the net house facility (Fig. S7C and S8D).
PCR screening was performed using the genomic DNA (gDNA) isolated from the transformed plants (T1 generation). The primer pair hptII For and hptII Rev and primer pair KanS 5’ and KanS 3’ (Table S2) were used to screen the transgenic rice and tobacco plants, respectively (Fig. S7D and S8E).
Southern hybridization was carried out to check the integration of the transgene expression cassette into the genomes of the transformed plants. The gDNA isolated from the T2 generation of transgenic rice plants was digested, transferred to nylon blot, and hybridized with the DIG-labeled probe designed from the XhoI digested hptII DNA fragment of pCAM-GluC-OsBG1-NOS. Similarly, the DIG-labeled XhoI digested nptII DNA fragment of pCAM-2xCaMV35S-OsBG1-NOS was used for the Southern hybridization of the gDNA isolated from the transgenic tobacco plants. Blots were developed, and bands were observed for transgenic rice plants (Fig. S7E) and tobacco lines (Fig. S8F).
In silico analysis
RiceXPro (https://ricexpro.dna.affrc.go.jp/) was used for the in silico spatiotemporal expression analysis of the genes OsBG1 and glutelin (Fig. S9A). The expression potential of OsBG1 in various developmental stages (vegetative and reproductive) of rice plants was analyzed using Genevestigator (https://plants.genevestigator.com/local_plants/index.jsp) (Fig. S9B). The protein-protein interaction network of the OsBG1 was constructed using the Search Tool for Retrieval of Interacting Genes (STRING) database (https://string-db.org/cgi/input?sessionId=bUSsXdjrvfpA&input_page_active_form=single_sequence) that helps predict the target protein’s function by functional enrichment of the proteins in the network (Fig. S10).
Scanning electron microscopy for the observation of the anthers
To study the epidermal cuticle of the anthers from transgenic rice and untransformed control, the anthers from the spikelets before anthesis were collected. The samples were prepared following the method described previously (Vaikuntapu et al. 2014) and were imaged using Carl Zeiss (Merlin) scanning electron microscope (FE-SEM), Merlin® (Zeiss, Oberkochen, Germany).
Quantitative reverse-transcription PCR for relative expression study of OsBG1
For the expression analysis of OsBG1 in small-grain Badshahbhog, long-grain IR64, and the transgenic lines, quantitative reverse-transcription PCR (qRT-PCR) was performed. Total RNA isolated from 100 mg of fresh tissue from the young panicles of rice plants was treated with DNase I (Sigma-Aldrich, Saint Louis, MO, USA). The complementary DNA (cDNA) synthesized from DNase-treated RNA was taken as a template for the qRT-PCR reactions. Primer pairs for the qRT-PCR reactions for OsBG1 and Actin are listed in Table S2. qRT-PCR was carried out using the SYBR green-based relative quantification method in a StepOnePlus™ real-time PCR system (Applied Biosystems, Waltham, MA, USA). The 2(−∆∆CT) method was applied to analyze the relative change in gene expression in the target sample to the reference sample (Winer et al. 1999). The CT value of the target gene was normalized against the CT value of the internal housekeeping control Actin.
Analysis of the cooking quality of rice seeds
Analysis of the cooking quality of the rice seeds from the transgenic lines and the untransformed Badshahbhog (control) was performed following the method described previously (Faruq et al. 2015).
Proportionate change (PC) was calculated using the formula:
In the equation, LF and BF are the average length and breadth of the kernel after cooking, and L0 and B0 are the average length and breadth of the rice kernels before cooking, respectively.
The kernel elongation ratio (ER) was calculated as follows:
ER = Length of cooked rice kernels / Length of raw rice kernels.
Water uptake (WU) was calculated using the formula:
Where WC is the average weight of cooked rice kernels, WUC is the average weight of uncooked rice kernels.
Assessment of the nutritional content of rice seeds
To assess the nutritional content of rice seeds from the transgenic lines and the untransformed Badshahbhog (control), the methods were followed as described previously (Choudhury et al. 2020). The Bradford method determined the rice seeds’ total soluble protein. For the quantification of the total carbohydrate phenol-sulfuric method was applied (Zhang et al. 2020). To estimate lipids in rice seeds, the classic method of chloroform/methanol extraction and gravimetric determination of lipids was used, as described previously (Yoshida et al. 2011).
Statistical analyses
Data are presented as the mean values ± standard deviation of three sets of data from three independent experiments. Statistical significance in the data sets was evaluated by performing the Student’s t-test.
Results
Large-grain indica rice cultivars have extra nucleotides in the 3’ regulatory sequence of OsBG1 and exhibit higher relative gene expression
Fifteen rice cultivars (Fig. S1) were categorized for grain size and type (Table S1) and were screened for the genetic variation of the OsBG1, a grain size regulatory gene, if any, present in their genotypes. The full-length CDS of OsBG1 gene and its 5’ and 3’ regulatory sequences were amplified by PCR. The DNA fragments of PCR-amplified OsBG1 CDS (933 bp) from different genotypes showed no allelic length polymorphism, as observed in the 1% agarose gel (Fig. S2A). Similarly, no length polymorphism was detected for the 5’ regulatory sequence (~ 1 kb) of the gene amplified from the genotypes of the rice cultivars tested (Fig. S2B). Sequence verification through alignment with the reference sequence (NCBI reference sequence XM_015773860.2) confirmed the absence of any nucleotide polymorphism in these DNA fragments (Fig. S3 and S4). However, the PCR amplified 3’ regulatory sequence of the OsBG1 (~ 1.4 kb) showed length polymorphism as observed in 1% agarose gel (Fig. 1A and B).
A pattern associated with the grain size phenotype and the length polymorphism of the amplified DNA fragments from the 3’ regulatory sequences of OsBG1 in different rice cultivars was observed. The expected amplicon size of ~ 1 kb was seen for all the small-grain rice cultivars, whereas, the amplicon size of ~ 1.4 kb was observed for all the large-grain rice cultivars tested (Fig. 1A). The amplified DNA fragments from the 3’ regulatory sequences of OsBG1 from two representative indica rice cultivars - Badshahbhog (small-grain) and IR64 (large-grain) were sequenced. The obtained sequences of the 3’ regulatory sequences of OsBG1 from Badshabhog and IR64 were aligned with the 3’ regulatory sequences of the reference sequence (NCBI Reference sequence XM_015773860.2) from Oryza sativa japonica. The sequence alignment disclosed the presence of an extra 393 nucleotides in the 3’ regulatory sequence of OsBG1 in IR64 (Fig. S5). Inspection of the aligned 3’ regulatory sequences of OsBG1 from Badshahbhog, IR64, and the reference sequence XM_015773860.2 revealed the presence of two enhancer elements ‘CAATTG’ and ‘CACGTG’ in the extra 393 nucleotides present in the 3’ regulatory sequence of OsBG1 from the large-grain indica rice cultivar IR64. In contrast, the abovementioned 393 nucleotides sequence containing the two enhancer elements was missing in the 3’ regulatory sequence of OsBG1 from the Badshahbhog (small-grain indica rice cultivar) and the reference sequence XM_015773860.2 from the Nipponbare (small-grain japonica rice cultivar) (Fig. S5 and 1B).
Presuming the enhancer elements present in the 3’ regulatory sequences of OsBG1 might upregulate the expression of the gene in large-grain indica rice cultivars; we intended to examine the relative abundance of OsBG1 in both the large-grain rice cultivar (IR64) and the small-grain rice cultivar (Badshahbhog). A priori, in silico study of the spatiotemporal expression pattern of OsBG1 suggested a high expression of the gene in stem and inflorescence (Fig. S9). Therefore, as we were interested in grain-specific traits, young panicles were selected to inspect the relative expression of OsBG1. The qRT-PCR analysis revealed a 4.1-fold higher expression of OsBG1 in the young panicles of the large-grain indica rice cultivar IR64 than in the small-grain indica rice cultivar Badshahbhog (Fig. 1C).
Southern hybridization performed to detect the gene copy number of OsBG1 in indica rice cultivars Badshahbhog (small-grain cultivar) and IR64 (large-grain cultivar) using the OsBG1-specific DIG-labeled probe confirmed the presence of a single copy of the gene in each of their genomes (Fig. 1D).
Ectopic expression of OsBG1 causes shorter plant height and longer flag leaves in tall indica rice cultivar Badshahbhog
Ectopic expression of OsBG1 under endosperm-specific glutelin C (GluC) promoter in indica rice tall cultivar Badshahbhog led to a slight reduction in plant height by an average of 4.6% ± 2 (significant at p-value < 0.05) in the T2 transgenic rice lines (TL1, TL2. TL3, and TL4) compared to the untransformed control (Fig. 2A and B). However, no substantial change in the effective number of tillers was observed between the transgenic rice lines and the untransformed control (Fig. 2C). The chlorophyll content of the flag leaves in transgenic rice lines was significantly higher (p-value < 0.01) by an average of 33.7% ± 3 compared to the control (Fig. 2D). The length of flag leaves (reproductive stage) in transgenic rice lines significantly increased (p-value < 0.01) by an average of 33.2% ± 5 compared to the untransformed control (Fig. 2E and F).
Comparison of the morphological features between the transgenic rice lines (TL1, TL2, TL3, and TL4) ectopically expressing OsBG1 and the untransformed Badshahbhog (Control). A Images depicting a reduction in plant height of the transgenic lines with respect to the control. B, C, D and E Graphs representing the measured plant height, effective tillers, chlorophyll content (SPAD value) of flag leaves, and flag leaf length of the transgenic lines and control, respectively. F Images representing the length of the flag leaves from different transgenic lines and control. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘*’ and ‘**’ represent statistical significance at p-value < 0.05 and p-value < 0.01, respectively
Expression of OsBG1 improves the panicle architecture of the transgenic rice lines
OsBG1’s protein interaction network was constructed in silico using the STRING web-based tool that helps predict the target protein’s function by functional enrichment of the proteins in the network. The analysis identified potential functions of OsBG1 in relation to plant architecture, panicle organization, and grain size (Fig. SS10). Several parameters related to panicle architecture (Fig. S11) were examined in the transgenic rice lines ectopically expressing OsBG1 and compared with the untransformed control. Panicle weight and length significantly increased (p-value < 0.01) by an average of 33.7% ± 9 and 30.5% ± 9, respectively, in transgenic lines compared to the control (Fig. 3A). However, no significant change in primary branch length was detected in transgenic lines compared to the control. The rachis length, exsertion length, and node length significantly increased (p-value < 0.01) in the transgenic lines by an average of 40.9% ± 14, 4.42-fold, and 2.04-fold, respectively, compared to the control (Fig. 3B). Some representative pictures of panicles are shown to indicate the variations in the panicle size (Fig. 3C) and panicle architecture (Fig. 3D) between the control and the transgenic rice lines.
Comparison of the panicle architecture between the transgenic rice lines (TL1, TL2, TL3, and TL4) and the untransformed Badshahbhog (Control). A and B Graphs representing the various parameters of panicle architecture– A panicle weight, panicle length, primary branch length, B Rachis length (RL), Exsertion length (EL), and Node length (NL) of the panicles from the transgenic lines and control. C Pictures of the panicle of transgenic lines with the control to showcase the difference in the panicle size. D Pictures of the panicle of transgenic lines with the control to showcase the difference in the panicle architecture. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘**’ represents statistical significance at p-value < 0.01. In panel D of the figure, the same panicle ‘Control’ is taken for comparison with the panicles from transgenic lines
Expression of OsBG1 increases anther length, spikelet fertility, and grain yield in the transgenic rice lines
The length of anthers in spikelets of transgenic rice lines with ectopic expression of OsBG1 significantly increased (p-value < 0.05) by 27.5%; however, no significant change was observed for the breadth of the anthers (Fig. 4A-E). Observation of outer epidermal cells of anthers under a scanning electron micrograph revealed a loose knitted arrangement of the epidermal cuticle of the anthers of the transgenic lines compared to the untransformed control (Fig. 4F). The ectopic expression of OsBG1 led to a significant increase in spikelet fertility and grain yield per plant (Fig. 5A). The increase in the spikelet fertility rate was significant (p-value < 0.05) in transgenic lines by an average of 8.3% compared to the control. The increase in total and filled grains per panicle was highly significant (p-value < 0.01) by an average of 54.8% and 67.8%, respectively. Consequently, a highly significant (p-value < 0.01) increase in grain yield per plant of 54.4% was recorded for the transgenic lines compared to the untransformed control (Fig. 5A). The qRT-PCR analysis revealed the expression of OsBG1 in young panicles (before anthesis) and endosperm of the developing rice seeds (14 DAF) increased significantly (p-value < 0.01) by 3.8- and 14.8-fold, respectively, in transgenic lines compared to the untransformed control (Fig. 5B and C).
Comparison of the spikelet features between the transgenic rice line (TL) and the untransformed Badshahbhog (Control). A Picture representing the spikelets of the transgenic line and control at anthesis. B Microscopic images representing the anthers of the transgenic line and control inside the spikelet before anthesis. C Microscopic images representing the anther length of the isolated anther from transgenic rice line and control. D and E Graphs representing the anther length and anther breadth of the transgenic rice line and the control, respectively. Values are represented as the average of three biological replicates. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘*’ represents statistical significance at p-value < 0.05. F Scanning electron micrograph (SEM) images of the epidermal cuticle of anthers from the transgenic rice line and control. The SEM images from both samples were taken at 150 x, 5 K x, 10 K x, and 20 K x magnifications
Comparison of the grain yield parameters between the transgenic rice lines (TL1, TL2, TL3, and TL4) and the untransformed Badshahbhog (Control). A Graphs representing the different parameters related to grain yield– spikelet fertility rate, total grains per panicle, filled grains per panicle, and grain yield per plant of the transgenic lines and control. B and C Graphs representing the relative abundance of OsBG1 transcript in young panicles (before anthesis) and endosperm of developing rice seeds (14 DAF) of the transgenic lines and control, respectively. The rice Actin 1 gene was taken as an endogenous housekeeping reference for the normalization of OsBG1 expression. The comparative fold-change in the expression of OsBG1 in IR64 was calculated by taking the expression of OsBG1 in Badshahbhog as unity. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘*’ and ‘**’ represent statistical significance at p-value < 0.05 and p-value < 0.01, respectively
Ectopic expression of OsBG1 increased grain length by an average of 14.2% in transgenic lines compared to the untransformed control (p-value < 0.05); whereas, no significant change in the breadth of the rice grains was observed respectively between the transgenic lines and the control (Fig. 6). The length/breadth (L/B) ratio and the thickness of the grains from the transgenic lines were found to be significantly increased by an average of 17.4% (p-value < 0.01) and 14.2% (p-value < 0.05), respectively, compared to the control (Fig. 6B). A substantial increase of 5.1% (p-value < 0.01) in the 1000-grain weight of the transgenic rice lines compared to the untransformed control was recorded (Fig. 6B).
Comparison of the grain morphology between the transgenic rice lines (TL1, TL2, TL3, and TL4) and the untransformed Badshahbhog (Control). A The image showing the grain length of different transgenic lines and control. B Graphs representing the grain length, grain breadth, length/breadth (L/B) ratio, grain thickness, and 1000-grain weight of the grains from the transgenic lines and control. Values are represented as the average. The standard bar represents the deviation from the average values. Student’s t-test was performed to calculate statistical significance. ‘*’ and ‘**’ represent statistical significance at p-value < 0.05 and p-value < 0.01, respectively
Ectopic expression of OsBG1 enhances rice grain size without altering the nutritional content of the grain in Badshahbhog
The nutrient content of the rice grains was evaluated by measuring the total protein, total carbohydrate, and total fat percentages of the transgenic and untransformed control rice grains. No significant difference in the nutritional content of the rice grains between the control and transgenic lines was observed (Fig. 7A). The cooking quality of the seeds obtained from the T2 (2nd generation) transgenic rice lines was also evaluated. The proportionate change (PC) in the length and breadth of the cooked transgenic rice grains was highly significant (p-value < 0.01) with an average increase of 2.51-fold compared to the untransformed control (Fig. 7B). An increase in the elongation ratio (ER) by an average of 3.4% (p-value < 0.01) was observed for the transgenic rice grains compared to the control. The apparent water uptake of the transgenic rice grains was significantly higher (p-value < 0.01) than the control rice seeds by an average of 2.4-fold (Fig. 7B).
Comparison of the proximate nutritional content and cooking quality of the grains obtained from transgenic rice lines (TL1, TL2, TL3, and TL4) with the untransformed Badshahbhog (Control). A Graphs representing the change in nutritional content (carbohydrate, protein, lipid) of the transgenic lines and control. B Graphs representing the cooking quality parameters, like proportionate change (PC), elongation ratio (ER), apparent water uptake (%) of the transgenic lines and control. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘**’ represents statistical significance at p-value < 0.01
Heterologous expression of OsBG1 with 2xCaMV35S promoter results in increased seed yield in the tobacco plant
Heterologous expression of rice OsBG1 with a constitutive promoter, 2xCaMV35S, led to enhanced plant growth in tobacco. The T2 transgenic lines grown in greenhouse conditions (25 °C, 14 h light:10 h dark cycle, and 60% humidity) showed a significant increase in plant height compared to the untransformed control tobacco plants under the same conditions. A significant average increase of 28.1% (p-value < 0.01) in plant height was observed for transgenic tobacco plants expressing OsBG1 compared to the control (Fig. 8A and B). The total number of flowers (seed pods) per plant significantly increased (p-value < 0.01) in transgenic tobacco lines by an average of 2.2-fold compared to the control (Fig. 8C and D).
Comparison of the morphological features between the transgenic tobacco lines (TL1, TL2, and TL3) constitutively expressing OsBG1 and the untransformed tobacco cultivar Siri (Control). A Image depicting an increase in plant height of the transgenic lines compared to the control. B Graph representing the plant height of the transgenic lines and control. C Images depicting an increase in flower numbers (seed pods) of the transgenic lines compared to the control. D Graph representing the number of pods (flowers) per plant of the transgenic lines and control. E Image depicting an increase in leaf length and breadth of the transgenic line compared to the control. F, G and H Graphs representing the leaf length, leaf breadth, and chlorophyll content (SPAD value) of the transgenic lines and control, respectively. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘**’ represents statistical significance at p-value < 0.01
The length and breadth of leaves of transgenic tobacco lines at maturity significantly increased by 22% (p-value < 0.01) and 55% (p-value < 0.01), respectively, compared to the untransformed control (Fig. 8E-G). The chlorophyll content of the leaves of tobacco plants measured using the SPAD meter showed a significant increase by an average of 37.2% (p-value < 0.01) in transgenic lines compared to the untransformed control (Fig. 8H).
The average pod length, pod breadth, and seed weight in transgenic tobacco lines were significantly reduced compared to the untransformed control by an average of 12.1% (p-value < 0.01), 16.7% (p-value < 0.05), and 5.1% (p-value < 0.01), respectively (Fig. 9A-C). However, an average of 30.1% significant increase (p-value < 0.01) in the number of seeds per pod and a 2.6-fold increase (p-value < 0.01) in the total seeds per plant (yield) of the transgenic tobacco lines were recorded compared to the untransformed control (Fig. 9D and E).
Comparison of the yield-related parameters of the transgenic tobacco lines (TL1, TL2, and TL3) and the untransformed tobacco cultivar Siri (Control). A Image depicting a decrease in pod size of the transgenic lines compared to the control. B Graph representing the pod length and breadth of the transgenic lines and control. C Graph representing the seed weight (per 100 seeds) of the transgenic and control. D Graph representing the number of seeds per pod of the transgenic lines and control. E Graph representing the seed yield per plant of the transgenic lines and control. Values are represented as the average. The standard bar represents the deviation from the average. Student’s t-test was performed to calculate statistical significance. ‘*’ and ‘**’ represent statistical significance at p-value < 0.05 and p-value < 0.01, respectively
Discussion
The development of molecular markers and their use for detecting DNA polymorphism in gene loci have a significant impact on molecular genetics (Semagn et al. 2006). The presence of extra nucleotides in the 3’ regulatory sequence of OsBG1 gene in all the tested indica rice cultivars with the large-grain phenotype (Fig. 1A and S5) suggests that the trait-associated genetic variation of OsBG1 might be utilized as a molecular marker for marker-assisted selection of existing genotypes or hybrid progeny for the desired grain size. Several studies have recorded upregulation of the expression of genes by transcriptional regulators through enhancers - cis-regulatory DNA elements (Li et al. 2022; Pennacchio et al. 2013; Tone et al. 2014; Zou et al. 2005). The large-grain rice cultivar IR64 has a relatively higher expression (4.1-fold) of OsBG1 compared to the small-grain rice cultivar Badshahbhog (Fig. 1C), possibly due to the presence of the two enhancer sequences in the 3’ regulatory region of the gene upregulating its expression (Fig. 1B and S5). The results suggest that the grain size phenotype in indica rice cultivars is positively correlated with the OsBG1 gene’s expression level. Furthermore, the presence of a single copy of the OsBG1 gene in both the large-grain and small-grain rice cultivars, as detected by Southern hybridization (Fig. 1D), confirmed that the difference in the level of OsBG1 expression is not attributed to any difference in the copy number of the gene.
In this work, we aimed to evaluate the potential of OsBG1 in yield improvement of rice through regulated expression. Our study documents a reduction in plant height of the transgenic indica rice tall cultivar Badshahbhog with ectopic expression of OsBG1 driven by the GluC promoter (Fig. 2), contrary to the increased plant height in transgenic lines constitutively overexpressing OsBG1 driven by ACTIN1 promoter in a small-grain japonica cultivar Nipponbare (Oryza sativa L. ssp. japonica) reported by Liu et al. (2015). Interestingly, a highly significant average increase of 54.4% ± 8 in grain yield of transgenic Badshahbhog lines was observed (Fig. 5), which is much higher than the yield increases of 6.6% and 16.6% of the two transgenic Nipponbare lines overexpressing OsBG1 reported by Liu et al. (2015). Several studies have documented a reduction in plant height to compensate for increased grain yield (Rebetzke et al. 2011; Rebetzke and Richards 2000; Spielmeyer et al. 2002; Xing et al. 2015). Yield in seed crops is the fruition of the source and sink strengths. The flag leaf of the rice plant plays a crucial role in photosynthesis and mobilization of photoassimilates during grain filling (Sperotto et al. 2013; Wang et al. 2011). A significant increase in flag leaf length of the transgenic rice lines (Fig. 2E and F) justifies the enhanced grain yield observed in the present study. Therefore, our findings suggest that the spatiotemporal regulation of OsBG1’s ectopic expression under the endosperm-specific GluC promoter in transgenic rice lines might have led to the increased mobilization of photosynthetic assimilates from the longer flag leaves to the rice seeds, leading to a significant increase in grain size and yield compensated by a reduction in plant height.
The STRING analysis of OsBG1 hints at its functional and biological role in panicle development (Fig. S10). We observed a highly significant increase of 30.5% in the panicle length of transgenic lines (Fig. 3). The in silico observation and the relative expression study of OsBG1 in young panicles (before anthesis) of the untransformed control and transgenic lines (Fig. 5B) strongly suggest that the higher expression of OsBG1 driven by the GluC promoter leads to highly significant improvement in the panicle architecture of the transgenic rice lines.
The length and size of the anthers have been reported to positively correlate with the number of pollen grains per anther, as well as pollen viability, thereby influencing spikelet fertility (Farrell et al. 2006; Nguyen et al. 2015; Nishiyama 1982). In our study, the transgenic rice lines exhibited a significant increase in the anther length (Fig. 4). Moreover, the scanning electron micrographs of the anthers showed a comparative loose knitted pattern of the epidermal cuticle of the anther of the transgenic line (Fig. 4F). A study by Hu et al. (2021) suggested that the compact knitted cuticle structure of the outer epidermis of anther led to low anther dehiscence rate, low pollen shedding, and hence, low spikelet fertility in the rice varieties tested. These observations strongly suggest that the increase in the anther length and the less compact cuticle of the anther aid in increasing the spikelet fertility rate and the number of filled grains of the transgenic rice lines with ectopic expression of OsBG1.
Our previous study, which sought to gain functional insight into OsBG1, revealed its role in growth promotion and biomass accumulation through cell-cycle regulation. We showed that OsBG1’s expression boosted the G2/M progression of the cell cycle through the upregulation of cyclin B1 in a heterologous unicellular rhizospheric yeast, leading to increased specific growth rate, cell number, and biomass in transgenic lines compared to the control (Ekta et al. 2023). Studies have shown that the grain weight, size, and yield are directly influenced by the increased cell proliferation and expansion in grain tissues of rice and wheat (Chen et al. 2021; Jiang et al. 2022; Ye et al. 2023). Keeping these observations in mind, we reasonably argue that an observed 14.8-fold higher expression of OsBG1 in the endosperm of developing seeds (14 DAF) (Fig. 5C) due to spatiotemporally-regulated expression of the gene might have increased the number of filled grains (Fig. 5A) per panicle and 1000-grain weight (Fig. 6B). Consequently, a significant 54.4% increase in grain yield of the transgenic rice lines with ectopic expression of OsBG1 was achieved (Fig. 5A). Liu et al. (2015) documented an insignificant change in grain yield per plant due to a lower percentage of filled grains in transgenic lines constitutively overexpressing OsBG1. Our results signify that the choice of promoter influences the impact of OsBG1’s expression on grain yield. The success of targeting agronomic interests of economically important crops through biotechnological approaches depends on the expression of the gene of interest in specific tissue at a given stage, which is why the choice of promoter is crucial (Basso et al. 2020). The promoter specificity and the promoter strength (level of activity) in the target tissue are essential criteria for controlling the expression of the gene of interest. The present study’s findings help us conclude that the ectopic expression of OsBG1 using the GluC promoter can significantly improve the grain size and yield in rice plants.
Next, we aimed to evaluate the quality of transgenic rice grains. The parameters for grain quality evaluation are hard to define as they depend heavily on regional consumers’ preferences and market demands (Khush 2000). The widely accepted consensus of rice grain quality evaluation involves observation of biochemical composition, cooking, sensory, and nutritional properties (Butardo et al. 2019). The evaluation of grain cooking quality revealed a significant increase in the transgenic seeds’ proportionate change, elongation ratio, and apparent water uptake (Fig. 7). These parameters are reported to positively correlate with the length/breadth (L/B) ratio, length, and size of uncooked kernel (Chauhan et al. 1995; Mohapatra and Bal 2006; Singh et al. 2005), suggesting that an increase in kernel length, thickness, and L/B ratio (Fig. 6) due to the ectopic expression of OsBG1 led to a significant increase in the cooking quality of transgenic rice grains compared to the untransformed control (Fig. 7B) without altering the proximate nutritional content (Fig. 7A).
Our study involving the heterologous expression of the rice OsBG1 with a constitutive promoter 2xCaMV35S in tobacco showed a significant increase in the leaf area and chlorophyll content compared to the untransformed control (Fig. 8). Several studies on oil palm, maize, tobacco, cassava, rice, wheat, etc. have reported a positive correlation between the leaf area, chlorophyll meters, and yield (Hardon et al. 1969; Haripriya Anand and Byju 2008; Muhammad et al. 2019; Simkin et al. 2015; Wood et al. 1993). These studies and our experimental observations suggest that the significant increase in the leaf area (length and breadth) (Fig. 8E-G) and the leaf chlorophyll content (Fig. 8H) during the vegetative stage of the transgenic tobacco plants constitutively expressing OsBG1 might play an essential role in the highly significant yield increase of the transgenic lines compared to the untransformed control (Fig. 9E). Even though the size of the pods (Fig. 9A and B) and seed weight (Fig. 9C) were reduced considerably in the transgenic tobacco lines compared to the untransformed control, the seed yield (g) per plant was significantly higher (Fig. 9E) due to the increased total number of pods per plant (Fig. 8D) and the number of seeds per pod (Fig. 9D) in transgenic tobacco plants compared to the control.
Hence, our study documents that OsBG1 is a potential candidate for improving plant growth and yield in dicot plant tobacco as well. Furthermore, we have recently documented that OsBG1 could be a DNA and phytohormone interacting transcription factor that enhances the biomass in a transformed yeast, Candida tropicalis, by upregulating the cell division (Ekta et al. 2023). Therefore, the studies on OsBG1 so far underscore its physiological implication in biomass and yield improvement in various organisms. In the future, detailed insights into OsBG1’s function can be explored through transcriptomic analyses of transgenic rice and tobacco plants. Chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis can identify the binding sites of OsBG1– a potential transcription factor. Techniques like co-immunoprecipitation (Co-IP), protein microarrays, pull-down assay, etc., may reveal the functional partners of OsBG1 that can enhance the understanding of the gene’s regulatory mechanism.
In conclusion, the present study establishes that OsBG1 holds promise for boosting grain yield in monocot and dicot plants and can be utilized through various biotechnological interventions to improve crop productivity. However, its deployment in agricultural settings requires careful consideration of several factors. Field trials across diverse geographical conditions are necessary to assess adaptability. Studies involving multiple genetic backgrounds are crucial for understanding genotype-specific responses. Overall, a comprehensive approach is needed to realize the full potential of OsBG1 in improving agricultural productivity sustainably.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information file.
References
Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309:741–745. https://doi.org/10.1126/science.1113373
Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GED, Schroeder JI (2019) Genetic strategies for improving crop yields. Nature 575:109–118. https://doi.org/10.1038/s41586-019-1679-0
Basso MF, Arraes FBM, Grossi-de-Sa M, Moreira VJV, Alves-Ferreira M, Grossi-de-Sa MF (2020) Insights into genetic and molecular elements for transgenic crop development. Front Plant Sci 11:509. https://doi.org/10.3389/fpls.2020.00509
Bisne R, Sarawgi AK (2008) Agro-morphological and quality characterization of Badshah bhog group from aromatic rice germplasm of Chhattisgarh. Bangladesh J Agric Res 33:479–492. https://doi.org/10.3329/bjar.v33i3.1607
Budak H, Hussain B, Khan Z, Ozturk NZ, Ullah N (2015) From genetics to functional genomics: improvement in drought signaling and tolerance in wheat. Front Plant Sci 6:1012. https://doi.org/10.3389/fpls.2015.01012
Butardo VM, Sreenivasulu N, Juliano BO (2019) Improving rice grain quality: state-of-the-art and future prospects. In: Sreenivasulu N (ed) Rice grain quality: methods in molecular biology. Springer, Humana press, New York, NY, pp 19–55. https://doi.org/10.1007/978-1-4939-8914-0_2
Chauhan JS, Chauhan VS, Lodh SB (1995) Comparative analysis of variability and correlations between quality components in traditional rainfed upland and lowland rice. Indian J Genet Plant Breed 55:6–12
Chen K, Łyskowski A, Jaremko Ł, Jaremko M (2021) Genetic and molecular factors determining grain weight in rice. Front Plant Sci 12:605799. https://doi.org/10.3389/fpls.2021.605799
Cheon BY, Kim HJ, Oh KH, Bahn SC, Ahn JH, Choi JW, Ok SH, Bae JM, Shin JS (2004) Overexpression of human erythropoietin (EPO) affects plant morphologies: retarded vegetative growth in tobacco and male sterility in tobacco and Arabidopsis. Transgenic Res 13:541–549. https://doi.org/10.1007/s11248-004-2737-3
Choi HW, Lemaux PG, Cho MJ (2003) Long-term stability of transgene expression driven by barley endosperm-specific hordein promoters in transgenic barley. Plant Cell Rep 21:1108–1120. https://doi.org/10.1007/s00299-003-0630-9
Choi HW, Yu XH, Lemaux PG, Cho MJ (2009) Stability and inheritance of endosperm-specific expression of two transgenes in progeny from crossing independently transformed barley plants. Plant Cell Rep 28:1265–1272. https://doi.org/10.1007/s00299-009-0726-y
Choudhury P, Dutta KN, Singh A, Malakar D, Pillai M, Talukdar NC, Samanta SK, Devi R (2020) Assessment of nutritional value and quantitative analysis of bioactive phytochemicals through targeted LC-MS/MS method in selected scented and pigmented rice varietals. J Food Sci 85:1781–1792. https://doi.org/10.1111/1750-3841.15164
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2017) Identification of alternatively spliced transcripts of rice phytochelatin synthase 2 gene OsPCS2 involved in mitigation of cadmium and arsenic stresses. Plant Mol Biol 94:167–183. https://doi.org/10.1007/s11103-017-0600-1
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2018) Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol Biol 98:101–120. https://doi.org/10.1007/s11103-018-0766-1
Datta SK (2004) Rice biotechnology: a need for developing countries. AgBioForum 7:31–35. https://agbioforum.org/rice-biotechnology-a-need-for-developing-countries/
Ekta, Biswas D, Mukherjee G, Maiti MK (2023) Rice Big Grain1 enhances biomass and plant growth-promoting traits in rhizospheric yeast Candida tropicalis. Appl Microbiol Biotechnol 107:6553–6571. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00253-023-12740-9
Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, Li X, Zhang Q (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112:1164–1171. https://doi.org/10.1007/s00122-006-0218-1
Farrell TC, Fox KM, Williams RL, Fukai S, Lewin LG, Farrell TC, Fox KM, Williams RL, Fukai S, Lewin LG (2006) Minimising cold damage during reproductive development among temperate rice genotypes. II. Genotypic variation and flowering traits related to cold tolerance screening. Aust J Agric Res 57:89–100. https://doi.org/10.1071/AR05186
Faruq G, Prodhan ZH, Nezhadahmadi A (2015) Effects of ageing on selected cooking quality parameters of rice. Int J Food Prop 18:922–933. https://doi.org/10.1080/10942912.2014.913062
Guo L, Ma M, Wu L, Zhou M, Li M, Wu B, Li L, Liu X, Jing R, Chen W, Zhao H (2022) Modified expression of TaCYP78A5 enhances grain weight with yield potential by accumulating auxin in wheat (Triticum aestivum L). Plant Biotechnol J 20:168–182. https://doi.org/10.1111/pbi.13704
Hardon J, Williams C, Watson I (1969) Leaf area and yield in the oil palm in Malaya. Exp Agric 5:25–32. https://doi.org/10.1017/S0014479700009935
Haripriya Anand M, Byju G (2008) Chlorophyll meter and leaf colour chart to estimate chlorophyll content, leaf colour, and yield of cassava. Photosynthetica 46:511–516. https://doi.org/10.1007/s11099-008-0087-8
Hu Q, Wang W, Lu Q, Huang J, Peng S, Cui K (2021) Abnormal anther development leads to lower spikelet fertility in rice (Oryza sativa L.) under high temperature during the panicle initiation stage. BMC Plant Biol 21:428. https://doi.org/10.1186/s12870-021-03209-w
Hussain A, Shahzad A, Tabassum S, Hafeez H, Khattak JZK (2018) Salt stress tolerance of transgenic rice (Oryza sativa L.) expressing AtDREB1A gene under inducible or constitutive promoters. Biologia 73:31–41. https://doi.org/10.2478/s11756-018-0010-0
Hussain B, Raza Q, Atif RM, Ahmad MQ (2022) New breeding techniques (NBTs) and biotechnology for boosting rice grain yield to feed 5 billion in 2050. In: Sarwar N, Ahmad S, Hasanuzzaman M (eds) Modern techniques of rice crop production. Springer, Singapore, pp 681–700. https://doi.org/10.1007/978-981-16-4955-4_33
Jena KK, Kim SR (2020) Genomics, biotechnology and plant breeding for the improvement of rice production. In: Gosal SS, Wani SH (eds) Accelerated plant breeding, cereal crops. Springer, Cham, Switzerland, pp 217–232. https://doi.org/10.1007/978-3-030-41866-3_9
Jiang H, Zhang A, Liu X, Chen J (2022) Grain size associated genes and the molecular regulatory mechanism in rice. Int J Mol Sci 23:3169. https://www.mdpi.com/1422-0067/23/6/3169
Khush GS, Cruz ND (2000) Rice grain quality evaluation procedures. In: Singh RK, Singh US, Khush GS (eds) Aromatic rices. Oxford & IBH publishing co. pvt. ltd., New Delhi, pp 15–28
Li W, Yang S, Xu P, Zhang D, Tong Y, Chen L, Jia B, Li A, Lian C, Ru D, Zhang B, Liu M, Chen C, Fu W et al (2022) SARS-CoV-2 RNA elements share human sequence identity and upregulate hyaluronan via NamiRNA-enhancer network. EBioMedicine 76:103861. https://doi.org/10.1016/j.ebiom.2022.103861
Liu D (2009) Design of gene constructs for transgenic maize. In: Scott MP (ed) Transgenic maize, methods in molecular biology. Humana, Totowa, NJ, pp 3–20. https://doi.org/10.1007/978-1-59745-494-0_1
Liu L, Tong H, Xiao Y, Che R, Xu F, Hu B, Liang C, Chu J, Li J, Chu C (2015) Activation of big Grain1 significantly improves grain size by regulating auxin transport in rice. Proc Natl Acad Sci 112:11102–11107. https://doi.org/10.1073/pnas.1512748112
Lo S, Cheng M, Hsing YC, Chen Y, Lee K, Hong Y, Hsiao Y, Hsiao A, Chen P, Wong L, Chen N, Reuzeau C, Ho TD, Yu S (2020) Rice Big Grain 1 promotes cell division to enhance organ development, stress tolerance and grain yield. Plant Biotechnol J 18:1969–1983. https://doi.org/10.1111/PBI.13357
Milner MJ, Bowden S, Craze M, Wallington EJ (2021) Ectopic expression of TaBG1 increases seed size and alters nutritional characteristics of the grain in wheat but does not lead to increased yields. BMC Plant Biol 21:524. https://doi.org/10.1186/s12870-021-03294-x
Mohapatra D, Bal S (2006) Cooking quality and instrumental textural attributes of cooked rice for different milling fractions. J Food Eng 73:253–259. https://doi.org/10.1016/j.jfoodeng.2005.01.028
Muhammad B, Adnan M, Munsif F, Fahad S, Saeed M, Wahid F, Arif M, Amanullah D Jr, Saud S, Noor M, Zamin M, Subhan F, Saeed B, Raza MA, Mian IA (2019) Substituting urea by organic wastes for improving maize yield in alkaline soil. J Plant Nutr 42:2423–2434. https://doi.org/10.1080/01904167.2019.1659344
Nguyen V, Fleury D, Timmins A, Laga H, Hayden M, Mather D, Okada T (2015) Addition of rye chromosome 4R to wheat increases anther length and pollen grain number. Theor Appl Genet 128:953–964. https://doi.org/10.1007/s00122-015-2482-4
Nishiyama I (1982) Male sterility caused by cooling treatment at the young microspore stage in rice plants: XXIII. Anther length, pollen number and the difference in susceptibility to coolness among spikelets on the panicle. Jpn J Crop Sci 51:462–469. https://doi.org/10.1626/jcs.51.462
Pandey SP, Singh AP, Srivastava S, Chandrashekar K, Sane AP (2019) A strong early acting wound-inducible promoter, RbPCD1pro, activates cryIAc expression within minutes of wounding to impart efficient protection against insects. Plant Biotechnol J 17:1458–1470. https://doi.org/10.1111/pbi.13071
Pennacchio LA, Bickmore W, Dean A, Nobrega MA, Bejerano G (2013) Enhancers: five essential questions. Nat Rev Genet 14:288–295. https://doi.org/10.1038/nrg3458
Qu LQ, Xing YP, Liu WX, Xu XP, Song YR (2008) Expression pattern and activity of six glutelin gene promoters in transgenic rice. J Exp Bot 59:2417–2424. https://doi.org/10.1093/jxb/ern110
Rebetzke GJ, Richards RA (2000) Gibberellic acid-sensitive dwarfing genes reduce plant height to increase kernel number and grain yield of wheat. Aust J Agric Res 51:235–246. https://doi.org/10.1071/ar99043
Rebetzke GJ, Ellis MH, Bonnett DG, Condon AG, Falk D, Richards RA (2011) The Rht13 dwarfing gene reduces peduncle length and plant height to increase grain number and yield of wheat. Field Crops Res 124:323–331. https://doi.org/10.1016/j.fcr.2011.06.022
Semagn K, Bjørnstad Å, Ndjiondjop M (2006) An overview of molecular marker methods for plants. Afr J Biotechnol 5:2540–2568. https://www.ajol.info/index.php/ajb/article/view/56080
Simkin AJ, McAusland L, Headland LR, Lawson T, Raines CA (2015) Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J Exp Bot 66:4075–4090. https://doi.org/10.1093/jxb/erv204
Singh N, Kaur L, Singh Sodhi N, Singh Sekhon K (2005) Physicochemical, cooking and textural properties of milled rice from different Indian rice cultivars. Food Chem 89:253–259. https://doi.org/10.1016/j.foodchem.2004.02.032
Singh G, Singh S, Singh RK (2012) Effect of fertility management on yield and economics of traditional scented rice varieties in low lands. Ann Plant soil res 14:1–4
Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39:623–630. https://doi.org/10.1038/ng2014
Sperotto RA, Ricachenevsky FK, Waldow VA, Müller ALH, Dressler VL, Fett JP (2013) Rice grain Fe, Mn and Zn accumulation: how important are flag leaves and seed number? Plant Soil Environ 59:262–266
Spielmeyer W, Ellis MH, Chandler PM (2002) Semidwarf (sd-1), green revolution rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci 99:9043–9048. https://doi.org/10.1073/pnas.132266399
Tone Y, Kidani Y, Ogawa C, Yamamoto K, Tsuda M, Peter C, Waldmann H, Tone M (2014) Gene expression in the gitr locus is regulated by NF-κB and Foxp3 through an enhancer. J Immunol 192:3915–3924. https://doi.org/10.4049/jimmunol.1302174
Vaikuntapu PR, Dutta S, Samudrala RB, Rao VRVN, Kalam S, Podile AR (2014) Preferential promotion of Lycopersicon esculentum (Tomato) growth by plant growth promoting bacteria associated with tomato. Indian J Microbiol 54:403-412. https://doi.org/10.1007/s12088-014-0470-z
Verma DK, Srivastav PP (2017) Proximate composition, mineral content and fatty acids analyses of aromatic and non-aromatic Indian rice. Rice Sci 24:21–31. https://doi.org/10.1016/j.rsci.2016.05.005
Wang P, Zhou G, Yu H, Yu S (2011) Fine mapping a major QTL for flag leaf size and yield-related traits in rice. Theor Appl Genet 123:1319–1330. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s00122-011-1669-6#citeas
Wassmann R, Nelson G, Peng S, Sumfleth K, Jagadish K, Hosen Y, Rosegrant M (2010) Rice and global climate change. In: Pandey S, Byerlee D, Dawe D, Dobermann A, Mohanty S, Rozelle S, Hardy B (eds) Rice in the global economy: strategic research and policy issues for food security. Los Banos, International Rice Research Institute, pp 411–432. https://cgspace.cgiar.org/handle/10568/33417
Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15:2532–2550. https://doi.org/10.1105/tpc.014928
Winer J, Jung CKS, Shackel I, Williams PM (1999) Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270:41–49. https://doi.org/10.1006/ABIO.1999.408
Wood CW, Reeves DW, Himelrick DG (1993) Relationships between chlorophyll meter readings and leaf chlorophyll concentration, N status, and crop yield: a review. Proc Agron Soc NZ 23:1–9
Xing A, Gao Y, Ye L, Zhang W, Cai L, Ching A, Llaca V, Johnson B, Liu L, Yang X, Kang D, Yan J, Li J (2015) A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J Exp Bot 66:3791–3802. https://doi.org/10.1093/jxb/erv182
Yadav S, Kumar V (2019) Feeding the world while caring for the planet. Direct Seeded Rice Consor 1:3–4. https://ricetoday.irri.org/feeding-the-world-while-caring-for-the-planet
Ye C, Guo J, Zhou X-q, Da-g C, Liu J, Peng X, Jaremko M, Jaremko Ł, Guo T, Liu C-g, Chen K (2023) The dsup coordinates grain development and abiotic stress in rice. Plant Physiol Biochem 205:108184. https://doi.org/10.1016/j.plaphy.2023.108184
Yoshida H, Tanigawa T, Kuriyama I, Yoshida N, Tomiyama Y, Mizushina Y (2011) Variation in fatty acid distribution of different acyl lipids in rice (Oryza sativa L.) brans. Nutrients 3:505–514. https://doi.org/10.3390/nu3040505
Zhang WH, WuJ, Weng L, Zhang H, Zhang J, Wu A (2020) An improved phenol-sulfuric acid method for the determination of carbohydrates in the presence of persulfate. Carbohydr Polym 227:115332. https://doi.org/10.1016/j.carbpol.2019.115332
Zou J, Chen Z, Zhang S, Zhang W, Jiang G, Zhao X, Zhai W, Pan X, Zhu L (2005) Characterizations and fine mapping of a mutant gene for high tillering and dwarf in rice (Oryza sativa L). Planta 222:604–612. https://doi.org/10.1007/s00425-005-0007-0
Acknowledgements
Ekta acknowledges the University Grant Commission (UGC), Govt. of India, for providing fellowship. The authors are grateful to Prof. Somnath Bhattacharya, Bidhan Chandra Krishi Viswavidyalaya (BCKV), West Bengal, for providing rice germplasms for the study. Food Security Project (F. No. 4–25/2013-TS-1) fund of IIT Kharagpur for developing research infrastructure is also acknowledged.
Funding
This study was not sponsored by any funding agency.
Author information
Authors and Affiliations
Contributions
Ekta conceptualized and designed the experiments, conducted the analysis, prepared the original draft, and reviewed and edited the manuscript. Mrinal K. Maiti conceptualized and supervised the research, involved in acquisition of resource and fund, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekta, Maiti, M.K. Rice Big Grain1 improves grain yield in ectopically expressing rice and heterologously expressing tobacco plants. Plant Mol Biol 114, 73 (2024). https://doi.org/10.1007/s11103-024-01472-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11103-024-01472-7