Abstract
Antibodies and antibody derived fragments are excellent tools for the detection and purification of proteins. However, only few antibodies targeting Arabidopsis seed proteins are currently available. Here, we evaluate the process to make antibody libraries against crude protein extracts and more particularly to generate a VHH phage library against native Arabidopsis thaliana seed proteins. After immunising a dromedary with a crude Arabidopsis seed extract, we cloned the single-domain antigen-binding fragments from their heavy-chain only antibodies in a phage display vector and selected nanobodies (VHHs) against native Arabidopsis seed proteins. For 16 VHHs, the corresponding antigens were identified by affinity purification and MS/MS analysis. They were shown to bind the major Arabidopsis seed storage proteins albumin and globulin (14 to albumin and 2 to globulin). All 16 VHHs were suitable primary reagents for the detection of the Arabidopsis seed storage proteins by ELISA. Furthermore, several of the anti-albumin VHHs were used successfully for storage protein localisation via electron microscopy. The easy cloning, selection and production, together with the demonstrated functionality and applicability, strongly suggest that the VHH antibody format will play a more prominent role in future protein research, in particular for the study of native proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibodies have evolved as essential tools for protein detection and purification in biotechnological research and several ten thousands are nowadays available through commercial sources. This plethora of antibodies however is focused on detecting proteins of humans and animal model organisms. In addition, several post-genomic initiatives already exist of which the common goal is high-throughput development and production of antibodies against any human protein (e.g. the Human Protein Atlas, Affinomics, AffinityProteome) (Nilsson et al. 2005; Dübel et al. 2010; Stoevesandt and Taussig 2012). Plant scientists working on the model plant Arabidopsis thaliana don’t have such a vast array of antibody tools at their disposal: only a limited number of companies offer plant-specific antibodies to the research community.
If antibodies against a protein are not available, the researcher is left with three options. First, the protein can be expressed as a fusion to a protein tag (e.g. histidine, maltose binding protein, glutathione-S-transferase) against which several antibodies are available. However, the results from such an approach should be interpreted with care since a fusion protein remains an artificial representation of the native protein. Protein tags have been shown to interfere with protein stability, assembly into oligomers, localisation, function, accumulation and even with cell signalling pathways (Baens et al. 2006; Freydank et al. 2008; Yewdell et al. 2011). Moreover, several fluorescent protein tags have the tendency to form oligomers (Snapp 2009). The second option is to produce a limited batch of antibodies yourself. Due to time, money and facility issues, polyclonal antibodies are then preferred over monoclonal antibodies. The broad reactivity of polyclonal antibodies makes them good detection reagents across different protein techniques, but it increases the background signal or cross-reactivity that is often observed. A third option is to rely on pre-existing phage libraries that are available to the research community (Bernal and Willats 2004). One such library, an scFv phage library from which scFvs targeting plant proteins were successfully isolated, is described by Eeckhout et al. (2004). However, very few such libraries are available.
A crucial stage in producing your own antibodies via immunisation or in isolating antibodies via panning is the choice of antigen. Synthetic peptides have the advantage that the epitope recognised by the antibody is well defined. However, this approach does not always result in antibodies recognising the native protein (Jensen et al. 2009). Recombinant proteins are better suited, but their production is time-consuming, especially when they require complex folding steps and posttranslational modifications. Ideally a native antigen is used (Hu et al. 2008).
Dromedaries and other camelids produce, besides conventional antibodies, antibodies devoid of light chains (Muyldermans 2013). The N-terminal antigen-binding domain of these heavy-chain antibodies is known as a Nanobody® or VHH. Due to their small size and single-domain nature, VHHs are ideal entities for different biotechnological applications (Harmsen and De Haard 2007; Rothbauer et al. 2008; Vanlandschoot et al. 2011). They are easy to clone in different formats and show excellent tissue penetration (Hmila et al. 2008). In addition, VHHs are also known for their straightforward production in bacteria and yeast (Arbabi-Ghahroudi et al. 2005; Gorlani et al. 2012), high affinity (Lauwereys et al. 1998; Rothbauer et al. 2006) and high stability (Dumoulin et al. 2002).
The Arabidopsis seed proteome encompasses a few thousand different proteins, of which the seed storage proteins, albumin and globulin, are the most abundant (up to 84 % of the seed protein species) (Higashi et al. 2006; Baerenfaller et al. 2008). Due to their high abundance, they are amongst the first plant proteins studied and their synthesis pathway has been revealed to a large extent (Adachi et al. 2003; Hara-Nishimura et al. 1998; Herman and Larkins 1999; Müntz 1998). In the A. thaliana ecotype Columbia, albumin and globulin are encoded by a multi-gene family, i.e. five SESA genes and three CRA/CRU genes, respectively (van der Klei et al. 1993). For both proteins, each gene encodes a propolypeptide composed of two consecutive protein subunits. These subunits are referred to as the acidic (α) and basic (β) subunit, or the small (SS) and large (LS) subunit for globulins and albumins, respectively. The propolypeptide is cotranslationally transported into the endoplasmic reticulum, where intrachain disulfide bridges are formed (Krebbers et al. 1988; Pang et al. 1988). Hereafter, albumin and globulin are packed in vesicles, that are budding off from the post-Golgi network and directed towards prevacuoles or protein storage vacuoles (Herman and Larkins 1999). The final step is carried out by a vacuolar processing enzyme that cleaves the propolypeptide, resulting in a break-up between the two subunits. However, the subunits are still clustered together via the preformed disulfide bridges. In globulins, this cleavage also triggers the formation of α6–β6 hexamers (Adachi et al. 2003). Upon germination, the 15-kDa albumin monomers (SS–LS) and 300-kDa globulin hexamers (α6–β6) are degraded and serve as a carbon, nitrogen and sulfur source for the growing seedling (Fujiwara et al. 2002; Müntz 1998).
To evaluate the usefulness of an Arabidopsis VHH phage library for seed protein research, we immunised a dromedary with a crude Arabidopsis thaliana seed extract, selected a subset of 16 VHHs and identified their respective antigens in a reverse proteomic approach (Groot et al. 2009; Abbady et al. 2012). Next, we examined the capacity of the VHHs to recognise their antigens under native (ELISA, co-immunoprecipitation), western blot and fixed (EM) conditions. Finally, we also expanded their application towards detecting protein homologues in other plant species that are of interest in the field of allergy. Indeed, several albumin and globulin homologues (e.g. from mustard, sesame, peanut) are known to exhibit allergenic properties and some have reported antimicrobial and antifungal activities (Tandang et al. 2005; Jenkins et al. 2005).
Materials and methods
Protein extraction from seeds
Arabidopsis thaliana, white mustard (Sinapis alba), radish (Raphanus sativus), rapeseed (Brassica napus), sesame (Sesamum indicum) and rice (Oryza sativa subsp. japonica) seeds were cooled in liquid nitrogen and crushed for 2 min at 20 Hz with two steel, 4-mm balls. The powder was dissolved in 40 ml extraction buffer (50 mM Tris, 200 mM NaCl, 5 mM EDTA, 0.1 % Tween20, pH 8, cOmplete® protease inhibitor) per gram of crushed seeds and centrifuged for 30 min at 18,500 rpm to spin down the cell debris. Glycerol (20 %) was added to the supernatant and the protein concentrations were quantified by the method of Lowry (De Buck et al. 2012).
To remove the lipids from the Arabidopsis seed extract used for immunisation, a hexane extraction preceded the protein extraction (Goossens et al. 1999; De Buck et al. 2012).
Dromedary immunisation and VHH phage library construction
The dromedary immunisation, VHH phage library construction and the in vitro selection of seed protein-specific VHHs were performed by the VIB Nanobody Service Facility (http://www.vib.be/en/research/services/nanobody-service-facility/). A dromedary was immunised subcutaneously on a 7-day interval with 1.5 mg of Arabidopsis seed extract per injection. On day 39, anticoagulated blood was collected for immune response analysis and lymphocyte preparation. The immune response was verified by ELISA after successive affinity chromatography of the IgG subclasses on protein A and protein G columns (Hamers-Casterman et al. 1993). From the lymphocytes, RNA was isolated, cDNA was synthesised and used as template for PCR amplification of the VHH coding sequences as described by Saerens et al. (2008). Finally, the VHH phage library was constructed by cloning the VHH coding sequences in the pHEN4 phagemid vector via the PstI and NotI restriction sites and transforming the ligated material to TG1 E. coli cells as described in detail (Ghassabeh et al. 2010) (Fig. S1).
In vitro selection of anti-seed protein VHHs
The VHH library was super-infected with M13K07 helper phages and subjected to three rounds of in vitro selection on antigen-coated microtiter plates (20 μg Arabidopsis seed extract per well) as described by Saerens et al. (2008). The enrichment for antigen-specific phages after each round was determined by comparing the number of phages eluted from antigen-coated and blank wells (Parmley and Smith 1988). From each round, colonies were randomly selected and analysed by ELISA (2 μg Arabidopsis seed extract coated per well) for the presence of Arabidopsis-specific VHHs in their periplasmic extracts (Saerens et al. 2008).
VHH expression and purification via immobilised metal ion affinity chromatography (IMAC)
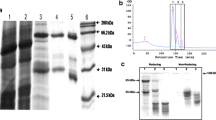
The VHH coding sequences were cloned from the pHEN4 into the pHEN6c vector using the PstI and BstEII restriction enzymes and transformed to electrocompetent WK6 E. coli cells. The pHEN6c expression vector is the same as pHEN4 except that the codons forming a NotI restriction site, hemagglutinin tag and gene pIII have been replaced by six His codons. The expressed VHHs end by amino acid sequences VTVSSHHHHHH. To produce VHHs on a 1–l scale, bacteria were grown at 37 °C in shake flasks containing Terrific broth medium supplemented with 0.1 % glucose, 0.01 % ampicillin and 2 mM MgCl2. At an OD600 of 0.6–0.9, VHH expression was induced by adding 1 mM IPTG. Eighteen hours after induction, the cells were pelleted and subjected to an osmotic shock by resuspending the cell pellet in 12 ml TES (0.2 M TRIS, 0.5 mM EDTA, 0.5 M sucrose, pH 8). After one hour on ice, 4.5 ml TES and 13.5 ml H2O were added and the cell extracts were incubated for another hour on ice. Subsequently, the periplasmic extract was prepared by 30 min centrifugation at 8,000 rpm. The extract was loaded on a 1 ml His-select nickel affinity resin (Sigma), equilibrated with phosphate-buffered saline (PBS). The column was washed extensively with PBS (approximately 120 ml), and the VHHs were eluted with PBS + 0.5 M imidazole and dialysed overnight against PBS (3,500 Da cutoff). The resulting VHH stocks were analysed on SDS-PAGE by diluting the samples in 5 × SDS sample buffer (1 M Tris–HCl, 40 % glycerol, 0.1 % SDS, 5 % DTT, 0.01 % bromophenol blue, pH 6.8), loading them on a 15 % polyacrylamide gel and staining the proteins with Coomassie Brilliant Blue. In addition, the same protocol was followed for the production of a GFP-binding VHH (GBP), which was used as a negative control in the different ELISAs, from WK6 bacteria containing pHEN6c-GBP plasmid (Rothbauer et al. 2008).
Reverse proteomic identification of the respective VHH antigens
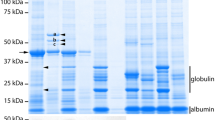
Ten VHHs were coupled to agarose beads according to manufacturer’s instructions (Pierce Co-immunoprecipitation Kit, Cat. No 26149). Briefly, by reductive amination 40 μg of purified VHH reacted 2 h with 50 μl of aldehyde-activated resin in the presence of 75 mM sodium cyanoborohydride on a 35 μm filter, resulting in covalently coupled VHHs. Unreacted aldehydes were quenched for 15 min with 1 M TRIS–HCl and 75 mM sodium cyanoborohydride. Subsequently, the beads were incubated overnight with 1.7 mg of Arabidopsis seed proteins. After washing the beads six times with the protein extraction buffer, 12.5 μl of the beads was diluted in 2× Novex® Tricine SDS sample buffer and 10× NuPAGE® reducing agent (Invitrogen), and denatured by heating. The samples were analysed on Novex® Tricine 10–20 % gradient gels (Invitrogen) and stained with Coomassie Brilliant Blue to visualise the affinity-purified antigens. Proteolysis and peptide isolation, acquisition of mass spectra by a 4800 MALDI TOF/TOF Proteomics Analyzer (AB SCIEX), and MS-based protein homology identification with a local search engine Mascot version 2.2 (Matrix Science) on the TAIR10 genomic database were performed as described in Van Leene et al. (2010).
Western blot
Different amounts of an Arabidopsis seed extract (34, 48 and 100 μg) were loaded on SDS-PAGE, in both reducing and non-reducing conditions, and blotted on PVDF membranes (Millipore). Next, the membranes were blocked with PBS + 0.1 % Tween (PBST) + 4 % skimmed milk. The seed proteins were detected with 0.5 and 1 μg/ml of VHHs, after which the bound VHHs were visualised with 1/1,000 mouse anti-His antibodies (Serotec, MCA1396) and 1/5,000 sheep anti-mouse antibodies fused with horse radish peroxidase (GE Healthcare, NXA931). The bound antibodies were detected by WesternBright ECL (Advansta, K-12045-D50).
Solid-phase ELISA
Different ELISA set-ups were performed to study the VHH binding characteristics. To analyse the binding of all VHHs to an Arabidopsis seed extract, 96-well plates (Maxisorp, Nunc) were coated overnight at 4 °C with 800 ng of Arabidopsis seed extract per well, diluted in coating buffer (0.1 M NaHCO3, pH 9.6). Subsequently, the wells were blocked with PBST + 2 % skimmed milk for 1 h and incubated with 90 ng of purified VHH per well for 1 h 30. Next, the bound VHHs were detected in a two-step reaction by incubating the wells for 1 h 30 with 1/1,000 mouse anti-His antibodies and 1/1,000 sheep anti-mouse antibodies fused to alkaline phosphatase (Sigma, A3563). After each step, the wells were washed three times with PBST. Finally, the reactions were analysed by adding 4-nitrophenyl phosphate (Sigma) as a substrate for the alkaline phosphatase. To determine the antigen concentration dependency, a serial dilution of the Arabidopsis seed protein extract from 3.9 ng to 16 μg per well was coated and detected with 90 ng of purified VHH. In another ELISA, to examine the cross-reaction with albumin and globulin homologues of other species, the wells were coated with 200 ng of the Arabidopsis, mustard, rapeseed, rice, radish and sesame seed protein extracts. Subsequently, 200 ng of purified VHH was added to the wells and detection was performed as described above. Finally, all VHHs were also analysed for their ability to bind Arabidopsis albumin in a sandwich-ELISA by coating with 1/500 diluted polyclonal anti-albumin antibodies (De Clercq et al. 1990). These were then incubated with 4 μg of Arabidopsis seed protein extract, after which 500 ng of all VHHs was assayed separately for their ability to bind the bound albumins. The VHHs were detected as described above. For all four ELISAs the reactions were quantified by measuring the absorption at 405 nm (VersaMax, Molecular Devices).
Transmission electron microscopy (EM) of Arabidopsis seeds
Arabidopsis seeds were imbibed overnight at 4 °C in dH2O after which embryos and endosperm were separated from the seed coat. The tissues were fixed by immersion in a fixative solution of 3 % paraformaldehyde and 0.3 % glutaraldehyde in 0.1 M NaCacodylate buffer (pH 7.2) under vacuum while rotating for 4 h at RT and for 14 h at 4 °C. Following three washes for 2 h in 0.1 M NaCacodylate buffer (pH 7.2) at 4 °C, the samples were dehydrated through a graded ethanol series and infiltrated stepwise over 3 days at 4 °C in LR-White, hard grade (London Resin), followed by embedding in capsules. Polymerisation was done by UV illumination for 24 h at 4 °C followed by 16 h at 60 °C. Ultrathin sections of gold interference colour were cut with an ultramicrotome (Leica EM UC6) and collected on formvar-coated copper slot grids. All steps of immunolabelling were performed in a humid chamber at RT. Grids were floated upside down on 25 μl of blocking solution (5 % BSA) for 30 min, followed by washing five times for 5 min with 1 % BSA in PBS. Incubation in a 1/10 VHH28 (0.1 mg/ml stock), 1/50 VHH34 (0.5 mg/ml stock), 1/10 VHH58 (0.1 mg/ml stock), and 1/10 VHH75 (0.1 mg/ml stock) dilution in 1 % BSA in PBS for 60 min was followed by washing five times for 5 min with 0.1 % BSA in PBS. The grids were then incubated with 1/100 mouse anti-His antibodies for 30 min, washed five times, incubated with 1/100 rabbit anti-mouse antibodies for 30 min (Jackson, 315-005-003), washed again five times and incubated finally with 10 nm colloidal gold-protein A conjugates (PAG10 nm, Department of Cell Biology, Utrecht University, Utrecht, The Netherlands). As negative controls, the same set-ups were performed without VHHs or without VHHs and mouse anti-His antibodies. Polyclonal rabbit anti-albumin antibodies (1/100) were used as a positive control for the anti-albumin VHHs and were detected in one step with the PAG10 nm conjugates. Grids were washed twice for 5 min with 0.1 % BSA in PBS, PBS, and double-distilled water. Sections were post-stained in a Leica EM AC20 for 40 min in uranyl acetate at 20 °C and for 10 min in lead citrate at 20 °C. Grids were viewed with a JEM 1010 transmission electron microscope (Jeol Ltd., Tokyo, Japan) operating at 80 kV.
Results
Generation of a VHH phage library against an Arabidopsis crude seed extract and selection, expression and purification of sixteen VHHs
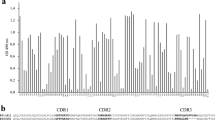
To construct a VHH library against native Arabidopsis seed proteins, a dromedary was immunised with a crude Arabidopsis seed extract. There was an immune response in all IgG subclasses to similar extents, as determined by ELISA (results not shown). By cloning the VHH coding sequences from the lymphocyte cDNA into the pHEN4 phagemid vector, a library of approximately 6 × 108 independent transformants was obtained. The library was panned against the Arabidopsis seed extract and the enrichment factors of phage particles eluted from wells coated with antigen versus those without antigen were 3-, 1,000- and 300-fold after the 1st, 2nd and 3rd round of panning, respectively. In total, 190 randomly selected colonies (47, 95 and 48 from the 1st, 2nd and 3rd round of panning, respectively) were analysed by ELISA and 84 scored positive for the presence of anti-seed protein VHHs in their periplasmic extracts. Subsequently, these 84 positive colonies were allocated to 26 groups, based on an RFLP analysis with the HinfI restriction enzyme. After sequencing one representative of each group, 19 different VHHs were identified. Based on their amino acid sequences, these 19 VHHs were again assigned to 13 groups (Table 1, Fig. 1).
a Protein homology tree of the seed protein binding VHHs. The VHH amino acid sequences were aligned by ClustalW. A neighbour-joining homology tree was constructed using MEGA 4.0 (Poisson correction, 500 bootstrap samples). The bootstrap support values are indicated for every node. Only the 16 VHHs that were successfully cloned, are represented. b Alignment of the VHH protein sequences. The CDR 1, 2 and 3 are indicated in bold. VHH11, VHH30, VHH34 and VHH49 carry an N-glycosylation site (Asn–X–Ser/Thr) in the CDR2 domain (marked in yellow)
To produce the VHHs in E. coli, 16 out of 19 VHH coding sequences were successfully cloned into the pHEN6c vector and transformed in E. coli WK6 cells. The pHEN6c vector ensures the periplasmic targeting of the VHHs and a C-terminal fusion to a His-tag for subsequent purification by IMAC. For each VHH, a 1 l culture was grown and VHH expression was induced with IPTG. After loading the periplasmic extract on an IMAC column, the VHHs all eluted as single bands in a one-step purification. The protein purity was assessed via Coomassie-stained polyacrylamide gel electrophoresis (Fig. S2). Yields varied from 0.5 to 4.1 mg per litre culture, except for VHH58 (9.3 mg/l) and VHH75 (7.9 mg/l) (Table 1).
Co-immunoprecipitation (Co-IP) and identification of the respective VHH antigens
As the respective antigens for each individual VHH were unknown, the binding partners for ten groups of VHHs were first identified in a reverse proteomics approach. By covalently coupling the VHHs to agarose beads, we were able to affinity capture the antigens from the Arabidopsis seed extract. The bound antigens were separated via polyacrylamide gel electrophoresis, stained with Coomassie and analysed via mass spectrometry. All ten elution profiles could be assigned to two groups of antigens. VHH58 and VHH75 showed a band pattern of six proteins at 20–50 kDa (Fig. 2). These bands were all identified via MS/MS as subunits (α or β) of the globulin seed storage proteins CRA1, CRU2 and CRU3. The VHH4, VHH11, VHH20, VHH25, VHH28, VHH30, VHH34 and VHH80 elution profiles all contained two clear bands with a molecular weight of 5–10 kDa, corresponding to the small and large subunits of the albumin seed storage proteins SESA2, SESA3 or SESA4. The two other Arabidopsis albumin protein family members, SESA1 and SESA5, were never found in any of the ten antigen identification set-ups (Table 1).
Albumin and globulin were identified as the VHH antigens after affinity capturing. The first elution profile, as shown for VHH28 and VHH30, shows two low molecular weight bands which were identified as the large (LS) and small (SS) albumin subunits of SESA2, SESA3 or SESA4. The second profile (e.g. VHH58) shows a typical pattern of six protein bands corresponding to subunits of the globulin protein complex (the α and β subunits of either CRA1, CRU2 or CRU3). The upper band contains both α and β subunits and is thought to represent the non-reduced α–β dimer. In every reaction some covalently linked VHH (arrow) also eluted from the beads. The identification of all distinct bands for all ten VHHs analysed are listed in Table S1
To verify albumin binding, a sandwich-ELISA was performed in which the wells were coated with polyclonal anti-albumin antibodies (De Clercq et al. 1990) and incubated with an Arabidopsis seed extract, after which all VHHs were assayed separately for their ability to recognise the bound albumins. The results were positive for all VHHs, except for 58 and 75, confirming the elution profiles identified after affinity purification and MS/MS (Table 1). In addition, VHHs belonging to the same group, as determined by aligning the amino acid sequences, indeed recognised the same antigen (Table 1, Fig. 1). Finally, this ELISA also indicated the 6th group of VHHs (VHH26), which was not included in the affinity capturing protocol explained above, as targeting the albumin seed storage proteins. In conclusion, of the 16 VHHs, 14 were directed against albumin and 2 against globulin.
Elucidation of the VHH binding characteristics
None of the anti-albumin and anti-globulin VHHs were able to detect their antigen under western blotting conditions (Fig. S3). To test their functionality, all 16 VHHs were analysed in a direct ELISA set-up. VHH24, VHH28, VHH75 and VHH58 clearly gave a lower signal compared to the other VHHs (Fig. 3a). The two anti-globulin VHHs (VHH58 and VHH75) only reached detectable levels when incubated longer (>20 min) with the ELISA substrate. Moreover, these VHHs were only functional in a narrow range of coated seed proteins (Fig. 3b). Interestingly, several VHHs could also be detected with protein A instead of anti-His antibodies (Table 1).
The VHH binding properties were evaluated in a direct ELISA. a Ninety ng of every VHH was allowed to react with 800 ng of coated A. thaliana seed extract. The absorbance was measured after three minutes. b A serial dilution of Arabidopsis seed extract was coated and detected with 90 ng of VHH as primary antibody. The OD405-values were measured after 37 min. The absorbance signals of the anti-globulin VHHs showed a peak around 1 μg of coated proteins, with VHH58 giving a higher signal than VHH75. VHH4 on the other hand, was still detecting albumin when 3.9 ng of Arabidopsis seed proteins was coated. The background threshold was determined as twice the OD405-blank, by using 90 ng GBP as primary antibody
The applicability of the VHHs was also tested in other closely related or economically important species: white mustard (Sinapis alba), radish (Raphanus sativus), rapeseed (Brassica napus) and sesame (Sesamum indicum). As a negative control, the monocot rice (Oryza sativa) was included, in which the seed storage proteins show no or low homology with the Arabidopsis albumins and globulins, respectively. For each species, seed extracts were prepared and coated in ELISA. One group of VHHs (group 11; VHH24/28) was identified to recognise the albumins in the other members of the Brassicaceae family (S. alba, R. sativus and B. napus), but not the albumins from S. indicum and O. sativa (Fig. 4). These results suggest that VHH24 and VHH28 bind distinct albumin epitopes, compared to the other VHHs. The Brassicaceae albumin sequences were also aligned by ClustalW (Fig. S4). No clear conclusions could be drawn from the alignment, except that both the small and large albumin subunits shared enough sequence homology with the other plant species to contain the epitope of the cross-reacting VHH24 and VHH28. Conversely, the globulins, despite being highly homologous within the Brassicaceae family [e.g. 84 % sequence identity between rapeseed CRU4 and Arabidopsis CRB (Sjodahl et al. 1991)], were not detected with the anti-globulin VHHs (VHH58/VHH75).
VHH24 and VHH28 recognised the albumin homologues in the Brassicaceae family in ELISA. VHH24 (not shown) and VHH28, belonging to the same group (Table 1), were identified as being cross-species reactive within the Brassicaceae family. They efficiently recognised the albumin homologues in S. alba, B. napus and R. sativus. All other VHHs (not shown) only recognised an Arabidopsis-specific albumin or globulin epitope. The background threshold was determined as twice the OD405-blank, by using GBP as primary antibody
The anti-albumin VHHs recognised their antigens in EM
The VHHs were further analysed for their ability to recognise the corresponding antigens after fixation. More in particular, the VHHs were used to visualise the vacuolar localisation of the albumins and globulins in mature Arabidopsis seeds by EM. Based on the binding characteristics of the VHHs in ELISA, four VHHs were selected as primary detection reagents: VHH34 (good albumin binder), VHH28 (intermediate, but cross-species albumin binder), VHH58 (globulin binder) and VHH75 (globulin binder). For VHH58 and VHH75, the very few visible immunogold particles were aspecifically dispersed throughout the sections, as in the negative control, whereas a vacuolar localisation of the globulins was anticipated. In contrast, VHH34 and VHH28 showed good, specific localisation of the albumins at the protein storage vacuoles (Fig. 5). Both VHHs showed a comparable amount of immunogold labelling.
EM immunolocalisation of albumin in Arabidopsis thaliana seeds. Using VHH34 and VHH28 as primary antibodies, specific signals were observed in the protein storage vacuoles (arrows). As negative control (neg. con.), no VHHs were added during the primary incubation. Rabbit polyclonal anti-albumin antibodies were used as a positive control (pos. con.). PSV protein storage vacuole, LB lipid body. Scale bar 200 nm
Discussion
By panning an immune VHH phage library against an Arabidopsis seed extract, 14 anti-albumin and 2 anti-globulin VHHs were isolated and their antigens were identified by affinity purification and MS/MS analysis. The globulin antigens were CRA1, CRU2 and CRU3. Because globulins form higher-order oligomers, the binding specificity of the anti-globulin VHHs, VHH58 and VHH75, towards either the α or β subunit (or a combination thereof) is unknown. The large SESA subunits were also vaguely visible in the Co-IP elution profiles of VHH58 and VHH75. The reason for this is unclear, but it is possible that the albumin proteins are sticky in nature and remained attached to the agarose beads during the Co-IP washing steps. Although the globulins were efficiently captured, eluted and identified by Co-IP, they were hardly detectable by VHH58 and VHH75 in ELISA. This could be attributed to an inefficient coating or a changed conformation upon coating of the globulins in ELISA, rather than to a low affinity of the VHHs. The observation that especially VHH75 showed reduced binding upon coating with higher amounts of seed proteins remains puzzling and can favour the first explanation. Indeed, if globulins are not coating well, addition of more seed proteins will result in competitive binding between the better coating seed proteins and the globulins.
The albumin antigens were identified as SESA2, SESA3 and SESA4. Although the SESA1 albumin subunits are homologous to the subunits of SESA2, SESA3 and SESA4 (e.g. 72 % sequence identity between the SESA1 and SESA4 propolypeptides), they were not detected during any of the antigen identification steps. SESA5 exhibits 52 % sequence identity with SESA4 and was also not identified as a VHH antigen. We hypothesise that either the SESA1 and SESA5 peptides were not efficiently ionised and detected by the mass spectrometer, or that the anti-albumin VHHs are indeed specific to the other three SESA subunits. In ELISA the anti-albumin VHH28 and VHH24 gave a rather low signal against the Arabidopsis seed proteins, compared to the other anti-albumin VHHs. Remarkably, they were the only two VHHs identified as recognising conserved albumin epitopes within the Brassicaceae family. Cross-species binding VHHs were indeed anticipated, based on the alignment of the Brassicaceae albumin proteins.
The antigen-binding behaviour of the 16 VHHs nicely corresponded to the VHH protein homology tree. VHH24 and VHH28 clustered together as the cross-species albumin binding VHHs, VHH58 and VHH75 represented the globulin binders and all other VHHs were Arabidopsis-specific albumin binding VHHs.
A similar approach to produce species-specific and cross-species binding VHHs was performed by Saerens et al. (2008). After an initial immunisation with Trypanosoma evansi, species-specific VHHs were isolated from the resulting phage library. In addition, by panning the same phage library to different Trypanosoma lysates, they identified VHHs recognising epitopes shared by several Trypanosoma species. Other recent studies also reported the generation of VHH phage libraries without access to purified antigens. To isolate VHHs able to discern Brucella amongst similar pathogens for diagnostic purposes, camels were immunised with a protein mixture, i.e. a heat-killed Brucella strain, and the corresponding chaperonin antigen was identified by immunoprecipitation and mass spectrometry (Abbady et al. 2011, 2012). Similarly, Groot et al. (2009) immunised a llama with membrane vesicles of a human carcinoma cell line to screen for VHHs targeting cancer cell surface markers and also identified the antigen, α3β1 integrin, by immunoprecipitation and mass spectrometry. In a third study, a VHH phage library was generated after immunising a dromedary with a crude Taenia solium antigen mix, from which T. solium-specific VHHs were isolated (Deckers et al. 2009).
Immunisation with a protein extract excludes the need for purified antigens, while guaranteeing their native conformation and posttranslational modifications that cannot be obtained by recombinant expression in bacteria (Saerens et al. 2008). The disadvantage of this approach, however, is the extra step required for each individual VHH to identify its cognate antigen. In addition, the immune response cannot be directed towards a particular target protein within the mixture; much depends on the abundance, immunogenicity and immunodominance of that protein. This explains why, by panning against a crude Arabidopsis seed extract, we only identified VHHs binding the major seed storage proteins. Adjusting the panning conditions or using purified antigens in a forward panning approach might lead to VHHs binding other Arabidopsis seed proteins.
Eeckhout et al. (2004) previously described another approach to screen for plant protein antibodies. They constructed a human scFv phage library and successfully isolated antibodies against 13 different proteins (seven of A. thaliana origin). Their scFvs were shown to be especially useful for ELISA and western blotting. In contrast, it is well established that VHHs have a high preference of conformational over linear epitopes as shown repeatedly in crystal structures (De Genst et al. 2006). Indeed, none of our 16 VHHs recognised their respective antigens in western blot. In addition, whereas Eeckhout and colleagues constructed a naive library from healthy humans, our phage library has been derived from a dromedary immunised with a crude Arabidopsis seed extract, ensuring the isolation of antibodies binding Arabidopsis seed proteins in their native conformation.
Neither VHH58, nor VHH75 showed a positive labelling of protein storage vacuoles in EM. This could be due to chemical fixation with aldehydes, resulting in the loss of antigenicity of the globulins (Claeys et al. 2004; Vanhecke et al. 2008). It will be interesting to see whether cryofixation (e.g. high-pressure freezing) would be more appropriate at maintaining this antigenicity and result in successful EM labelling. The cross-reactive anti-albumin VHH28 and Arabidopsis-specific anti-albumin VHH34, however, were successfully applied in EM with chemical fixation. VHHs have been used on fixed samples before, e.g. for imaging GFP-tagged proteins in mammalian or intact yeast cells (Leduc et al. 2013; Ries et al. 2012), or membrane proteins on HeLa cells (Groot et al. 2009), but not for EM purposes. In our hands, with VHHs as primary reagents, the number of gold particles per given area was still several orders of magnitude less as compared to the polyclonal anti-albumin antibodies used in the positive control. Possibly, the combination of several anti-albumin VHHs might mimick polyclonal antibodies and the sensitivity of individual VHHs in EM might be improved by fusing them to an Fc fragment. The avidity effect that comes with such bivalent VHH-Fc antibodies should make them more versatile tools for antigen detection (Moutel et al. 2009; Zhang et al. 2004; Zhu et al. 2010).
References
Abbady AQ, Al-Mariri A, Zarkawi M, Al-Assad A, Muyldermans S (2011) Evaluation of a nanobody phage display library constructed from a Brucella-immunised camel. Vet Immunol Immunopathol 142(1–2):49–56. doi:10.1016/j.vetimm.2011.04.004
Abbady AQ, Al-Daoude A, Al-Mariri A, Zarkawi M, Muyldermans S (2012) Chaperonin GroEL a Brucella immunodominant antigen identified using Nanobody and MALDI-TOF-MS technologies. Vet Immunol Immunopathol 146(3–4):254–263. doi:10.1016/j.vetimm.2012.01.015
Adachi M, Kanamori J, Masuda T, Yagasaki K, Kitamura K, Mikami B, Utsumi S (2003) Crystal structure of soybean 11S globulin: glycinin A3B4 homohexamer. Proc Natl Acad Sci USA 100(12):7395–7400. doi:10.1073/pnas.0832158100
Arbabi-Ghahroudi M, Tanha J, MacKenzie R (2005) Prokaryotic expression of antibodies. Cancer Metastasis Rev 24(4):501–519. doi:10.1007/s10555-005-6193-1
Baens M, Noels H, Broeckx V, Hagens S, Fevery S, Billiau AD, Vankelecom H, Marynen P (2006) The dark side of EGFP: defective polyubiquitination. PLoS One 1(1):e54. doi:10.1371/journal.pone.0000054
Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, Zimmermann P, Grossniklaus U, Gruissem W, Baginsky S (2008) Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320(5878):938–941. doi:10.1126/science.1157956
Bernal AJ, Willats WGT (2004) Plant science in the age of phage. Trends Plant Sci 9(10):465–468. doi:10.1016/j.tplants.2004.08.004
Claeys M, Vanhecke D, Couvreur M, Tytgat T, Coomans A, Borgonie G (2004) High-pressure freezing and freeze substitution of gravid Caenorhabditis elegans (Nematoda: Rhabditida) for transmission electron microscopy. Nematology 6(3):319–327. doi:10.1163/1568541042360564
De Buck S, Virdi V, De Meyer T, De Wilde K, Piron R, Nolf J, Van Lerberge E, De Paepe A, Depicker A (2012) Production of camel-like antibodies in plants. Methods Mol Biol 911:305–324
De Clercq A, Vandewiele M, De Rycke R, Van Damme J, Van Montagu M, Krebbers E, Vandekerckhove J (1990) Expression and processing of an Arabidopsis 2S albumin in transgenic tobacco. Plant Physiol 92(4):899–907. doi:10.1104/pp.92.4.899
De Genst E, Silence K, Decanniere K, Conrath K, Loris R, Kinne R, Muyldermans S, Wyns L (2006) Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci USA 103(12):4586–4591. doi:10.1073/pnas.0505379103
Deckers N, Saerens D, Kanobana K, Conrath K, Victor B, Wernery U, Vercruysse J, Muyldermans S, Dorny P (2009) Nanobodies, a promising tool for species-specific diagnosis of Taenia solium cysticercosis. Int J Parasit 39(5):625–633. doi:10.1016/j.ijpara.2008.10.012
Dübel S, Stoevesandt O, Taussig MJ, Hust M (2010) Generating recombinant antibodies to the complete human proteome. Trends Biotechnol 28(7):333–339. doi:10.1016/j.tibtech.2010.05.001
Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, Muyldermans S, Wyns L, Matagne A (2002) Single-domain antibody fragments with high conformational stability. Protein Sci 11(3):500–515. doi:10.1110/ps.34602
Eeckhout D, De Clercq A, Van De Slijke E, Van Leene J, Stals H, Casteels P, Persiau G, Vercammen D, Van Breusegem F, Zabeau M, Inzé D, Jespers L, Depicker A, De Jaeger G (2004) A technology platform for the fast production of monoclonal recombinant antibodies against plant proteins and peptides. J Immunol Methods 294(1–2):181–187. doi:10.1016/j.jim.2004.08.006
Freydank A-C, Brandt W, Dräger B (2008) Protein structure modeling indicates hexahistidine-tag interference with enzyme activity. Proteins 72(1):173–183. doi:10.1002/prot.21905
Fujiwara T, Nambara E, Yamagishi K, Goto DB, Naito S (2002) Storage proteins. In: Somerville CR, Meyerowitz EM (eds) The arabidopsis book, vol 1. American Society of Plant Physiologists, Rockville, pp 1–12. doi:10.1199/tab.0020
Ghassabeh GH, Saerens D, Muyldermans S (2010) Isolation of antigen-specific nanobodies. In: Kontermann R, Dübel S (eds) Antibody engineering, vol 2. Springer, Berlin, pp 251–266
Goossens A, Dillen W, De Clercq J, Van Montagu M, Angenon G (1999) The arcelin-5 gene of Phaseolus vulgaris directs high seed-specific expression in transgenic Phaseolus acutifolius and Arabidopsis plants. Plant Physiol 120(4):1095–1104
Gorlani A, de Haard H, Verrips T (2012) Expression of VHHs in Saccharomyces cerevisiae. Methods Mol Biol 911:277–286. doi:10.1007/978-1-61779-968-6_17
Groot AJ, El Khattabi M, Sachs N, van der Groep P, van der Wall E, van Diest PJ, Sonnenberg A, Verrips CT, Vooijs M (2009) Reverse proteomic antibody screening identifies anti adhesive VHH targeting VLA-3. Mol Immunol 46(10):2022–2028. doi:10.1016/j.molimm.2009.03.002
Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, Bendahman N, Hamers R (1993) Naturally occurring antibodies devoid of light chains. Nature 363(6428):446–448. doi:10.1038/363446a0
Hara-Nishimura I, Shimada T, Hatano K, Takeuchi Y, Nishimura M (1998) Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10(5):825–836
Harmsen MM, De Haard HJ (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 77(1):13–22. doi:10.1007/s00253-007-1142-2
Herman EM, Larkins BA (1999) Protein storage bodies and vacuoles. Plant Cell 11(4):601–613
Higashi Y, Hirai MY, Fujiwara T, Naito S, Noji M, Saito K (2006) Proteomic and transcriptomic analysis of Arabidopsis seeds: molecular evidence for successive processing of seed proteins and its implication in the stress response to sulfur nutrition. Plant J 48(4):557–571. doi:10.1111/j.1365-313X.2006.02900.x
Hmila I, Abdallah RBA-B, Saerens D, Benlasfar Z, Conrath K, Ayeb ME, Muyldermans S, Bouhaouala-Zahar B (2008) VHH, bivalent domains and chimeric Heavy chain-only antibodies with high neutralizing efficacy for scorpion toxin AahI’. Mol Immunol 45(14):3847–3856. doi:10.1016/j.molimm.2008.04.011
Hu W-G, Yin J, Jager S, Wong C, Fulton C, Rayner GA, Aw C, Fisher GR, Dai X, Nagata LP (2008) A novel approach to development of monoclonal antibodies using native antigen for immunization and recombinant antigen for screening. Hybridoma 27(4):307–311. doi:10.1089/hyb.2008.0011
Jenkins JA, Griffiths-Jones S, Shewry PR, Breiteneder H, Mills ENC (2005) Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: an in silico analysis. J Allergy Clin Immunol 115(1):163–170. doi:10.1016/j.jaci.2004.10.026
Jensen BC, Swigart PM, Simpson PC (2009) Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn-Schmiedeberg’s Arch Pharmacol 379(4):409–412. doi:10.1007/s00210-008-0368-6
Krebbers E, Herdies L, De Clercq A, Seurinck J, Leemans J, Van Damme J, Segura M, Gheysen G, Van Montagu M, Vandekerckhove J (1988) Determination of the processing sites of an Arabidopsis 2S albumin and characterization of the complete gene family. Plant Physiol 87(4):859–866. doi:10.1104/pp.87.4.859
Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Hölzer W, De Genst E, Wyns L, Muyldermans S (1998) Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J 17(13):3512–3520. doi:10.1093/emboj/17.13.3512
Leduc C, Si S, Gautier J, Soto-Ribeiro M, Wehrle-Haller B, Gautreau A, Giannone G, Cognet L, Lounis B (2013) A highly specific gold nanoprobe for live-cell single-molecule imaging. Nano Lett 13(4):1489–1494. doi:10.1021/nl304561g
Moutel S, El Marjou A, Vielemeyer O, Nizak C, Benaroch P, Dübel S, Perez F (2009) A multi-Fc-species system for recombinant antibody production. BMC Biotechnol 9:14. doi:10.1186/1472-6750-9-14
Müntz K (1998) Deposition of storage proteins. Plant Mol Biol 38(1–2):77–99. doi:10.1007/978-94-011-5298-3_4
Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem. doi:10.1146/annurev-biochem-063011-092449
Nilsson P, Paavilainen L, Larsson K, Ödling J, Sundberg M, Andersson A-C, Kampf C, Persson A, Al-Khalili Szigyarto C, Ottosson J, Björling E, Hober S, Wernérus H, Wester K, Pontén F, Uhlen M (2005) Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5(17):4327–4337. doi:10.1002/pmic.200500072
Pang PP, Pruitt RE, Meyerowitz EM (1988) Molecular cloning, genomic organization, expression and evolution of 12S seed storage protein genes of Arabidopsis thaliana. Plant Mol Biol 11(6):805–820. doi:10.1007/BF00019521
Parmley SF, Smith GP (1988) Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73(2):305–318. doi:10.1016/0378-1119(88)90495-7
Ries J, Kaplan C, Platonova E, Eghlidi H, Ewers H (2012) A simple, versatile method for GFP-based super-resolution microscopy via nanobodies. Nat Methods 9(6):582–584. doi:10.1038/nmeth.1991
Rothbauer U, Zolghadr K, Tillib S, Nowak D, Schermelleh L, Gahl A, Backmann N, Conrath K, Muyldermans S, Cardoso MC, Leonhardt H (2006) Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat Methods 3(11):887–889. doi:10.1038/nmeth953
Rothbauer U, Zolghadr K, Muyldermans S, Schepers A, Cardoso MC, Leonhardt H (2008) A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol Cell Proteomics 7(2):282–289. doi:10.1074/mcp.M700342-MCP200
Saerens D, Stijlemans B, Baral TN, Nguyen Thi GT, Wernery U, Magez S, De Baetselier P, Muyldermans S, Conrath K (2008) Parallel selection of multiple anti-infectome Nanobodies without access to purified antigens. J Immunol Methods 329(1–2):138–150. doi:10.1016/j.jim.2007.10.005
Sjodahl S, Rodin J, Rask L (1991) Characterization of the 12 s globulin complex of brassica-napus: evolutionary relationship to other 11–12 s storage globulins. Eur J Biochem 196(3):617–621
Snapp EL (2009) Fluorescent proteins: a cell biologist’s user guide. Trends Cell Biol 19(11):649–655. doi:10.1016/j.tcb.2009.08.002
Stoevesandt O, Taussig MJ (2012) European and international collaboration in affinity proteomics. New Biotechnol 29(5):511–514. doi:10.1016/j.nbt.2012.05.003
Tandang MRG, Atsuta N, Maruyama N, Adachi M, Utsumi S (2005) Evaluation of the solubility and emulsifying property of soybean proglycinin and rapeseed procruciferin in relation to structure modified by protein engineering. J Agric Food Chem 53(22):8736–8744. doi:10.1021/Jf050871y
van der Klei H, Van Damme J, Casteels P, Krebbers E (1993) A fifth 2S albumin isoform is present in Arabidopsis thaliana. Plant Physiol 101(4):1415–1416
Van Leene J, Hollunder J, Eeckhout D, Persiau G, Van De Slijke E, Stals H, Van Isterdael G, Verkest A, Neirynck S, Buffel Y, De Bodt S, Maere S, Laukens K, Pharazyn A, Ferreira PCG, Eloy N, Renne C, Meyer C, Faure J-D, Steinbrenner J, Beynon J, Larkin JC, Van de Peer Y, Hilson P, Kuiper M, De Veylder L, Van Onckelen H, Inzé D, Witters E, De Jaeger G (2010) Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol 6:397. doi:10.1038/msb.2010.53
Vanhecke D, Graber W, Studer D (2008) Close-to-native ultrastructural preservation by high pressure freezing. Methods Cell Biol 88:151–164. doi:10.1016/S0091-679X(08)00409-3
Vanlandschoot P, Stortelers C, Beirnaert E, Ibañez LI, Schepens B, Depla E, Saelens X (2011) Nanobodies®: new ammunition to battle viruses. Antiviral Res 92(3):389–407. doi:10.1016/j.antiviral.2011.09.002
Yewdell JW, Lacsina JR, Rechsteiner MC, Nicchitta CV (2011) Out with the old, in with the new? Comparing methods for measuring protein degradation. Cell Biol Int 35(5):457–462. doi:10.1042/CBI20110055
Zhang J, Tanha J, Hirama T, Khieu NH, To R, Tong-Sevinc H, Stone E, Brisson J-R, MacKenzie CR (2004) Pentamerization of single-domain antibodies from phage libraries: a novel strategy for the rapid generation of high-avidity antibody reagents. J Mol Biol 335(1):49–56. doi:10.1016/j.jmb.2003.09.034
Zhu X, Wang L, Liu R, Flutter B, Li S, Ding J, Tao H, Liu C, Sun M, Gao B (2010) COMBODY: one-domain antibody multimer with improved avidity. Immunol Cell Biol 88(6):667–675. doi:10.1038/icb.2010.21
Acknowledgments
The authors thank Geert De Jaeger and his research group for advice and help, Gholamreza Hassanzadeh Ghassabeh for helpful discussions and for providing the pHEN6c-GBP vector, Jonah Nolf and Gijs De Cort for technical assistance and Annick Bleys for help in preparing the manuscript. T.D.M. is indebted to the Agency for Innovation by Science and Technology (IWT) for a predoctoral fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Meyer, T., Eeckhout, D., De Rycke, R. et al. Generation of VHH antibodies against the Arabidopsis thaliana seed storage proteins. Plant Mol Biol 84, 83–93 (2014). https://doi.org/10.1007/s11103-013-0118-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-013-0118-0