Abstract
Background/Purpose
Recent studies have reported that sepsis survivors show impaired central nervous system functions. The osmoregulation in this post-sepsis condition has not been well investigated. In the present study, we evaluated the secretion of neurohypophyseal hormones, arginine vasopressin (AVP) and oxytocin (OT), and water intake induced by osmotic challenge in survivor rats.
Methods
Wistar rats were submitted to sepsis by cecal ligation and puncture (CLP). Five days after CLP surgery, the survivor and naive animals were stimulated with an osmotic challenge consisting of hypertonic saline administration. Thirty minutes later, blood and brain were collected for determination of osmolality, nitrite, interleukin (IL)-1β, IL-6, AVP and OT levels and c-fos expression analysis of hypothalamic supraoptic nuclei (SON), respectively. In another set of sepsis survivor animals, water intake was measured for 240 min after the osmotic stimulus.
Results
High levels of nitrite and IL-1β, but not IL-6, were found in the plasma of sepsis survivors and this long-term systemic inflammation was not altered by the osmotic challenge. Moreover, the AVP and OT secretion (but not the osmolality) and c-fos expression in SON were significantly attenuated in CLP survivor animals. Additionally, there was no alteration in the water intake response induced by osmotic challenge in the sepsis survivor group.
Conclusion
The results suggest that the inflammatory components mediated a persistent impairment in the component of the osmoregulatory reflex affecting the secretion of neurohypophyseal hormones in sepsis survivor animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is a systemic inflammatory syndrome characterized by exacerbated activation of the innate immune system followed by severe dysfunctions of vital organs [1]. Impairment of neurohypophyseal hormones secretion has been considered one of the main pathophysiological events in sepsis [2, 3]. These hormones are synthesized by magnocellular neurons in hypothalamic supraoptic (SON) and paraventricular nuclei (PVN) and, in physiological conditions, are secreted in response to stimuli such as hyperosmolality, hypovolemia, stress and angiotensin II, playing a critical role in maintaining fluid and electrolytes homeostasis [4]. Recently, studies in sepsis models have shown alterations in the osmoregulatory neurons associated with arginine vasopressin (AVP) secretion and thirst [5]. Furthermore, during the early phase of sepsis, blood AVP and oxytocin (OT) levels are initially increased in response to hypotension/hypovolemia; but with the progression of the disease (late phase), these hormones are found in basal levels, despite the presence of a lasting hypotension [3, 6–8]. It leads us to the hypothesis of a persistent impairment in central control of homeostasis.
Moreover, a particular attention has been given to patients who survived from sepsis, because they show pathophysiological dysfunctions in several organ systems. For example, long-term immunological alteration has been associated with an elevated susceptibility of acquiring secondary infections [9, 10]. In addition, besides the deleterious consequences in the periphery, dysfunctions in the central nervous system (CNS) are also observed in sepsis survivors, characterized by psychological disorders and long-term cognitive deficits [11].
Although evidence have also pointed to a persistent impairment in the osmoregulation in sepsis survivor patients after osmotic challenge [12, 13], this important secondary dysfunction has not received the merit attention and, therefore, has not been investigated. In this context, in the present study, we investigated the AVP and OT secretion and the water intake induced by an osmotic challenge in a sepsis survivor model.
Materials and methods
Animals
All experiments were performed using male Wistar rats with an average body weight of 300 ± 25 g. The animals were obtained from the Central Animal Facility at the University of São Paulo, campus of Ribeirão Preto, and were housed in the same room under standard 12-h-light/12-h-dark cycle, room temperature (25 ± 2 °C) and ad libitum access to food (Nuvilab CR-1, NUVITAL) and water.
Sepsis model
Polymicrobial peritonitis was induced by the surgical procedure of cecal ligation and puncture (CLP). Briefly, the rats were anesthetized by an intraperitoneal (i.p.) injection of tribromoethanol (TBE) (250 mg/kg) and then a midline laparotomy was carried out under sterile conditions. The cecum was exposed, ligated below the ileocecal valve, and then gently punctured once with a sterile 14-gauge needle. Subsequently, the cecum was returned to the abdominal cavity and the incision was sutured in layers. These rats were given a subcutaneous injection of isotonic saline (0.9%; 5 mL/250 g) solution to compensate for fluid loss during surgery.
Experimental design
Five days after CLP surgery, the survivor animals were submitted to an osmotic challenge consisting of hypertonic saline administration (i.p.; 2 mol/L NaCl), in a volume corresponding to 1% of body weight. Isotonic saline was administrated in the control group (i.p.; 0.01 mol/L NaCl). After thirty minutes, the animals were anesthetized with TBE and blood was collected for plasma evaluation of osmolality, nitrite, IL-1β, IL-6 and, AVP and OT levels. Then, the same animals were perfused with 250 mL of PBS followed by 250 mL of fixative solution (4% paraformaldehyde in 0.1 mol/L phosphate buffer). The brains were removed, post-fixed in the fixative solution for 4 h, placed in PBS containing 30% sucrose and stored at 4 °C. In another group, hypertonic saline was administrated in sepsis survivor animals and the water drinking was measured for 240 min.
Plasma cytokines and nitrite quantification
The IL-1β and IL-6 plasma levels were determined using ELISA kits for each cytokine (R&D Systems, Minneapolis, Minn., USA) according to the manufacturer’s instructions. The detection limits for IL-1β and IL-6 were 5 and 10 pg/mL, respectively. The determination of plasma nitrite was performed by the technique of chemiluminescence NO/ozone as described previously [14].
Osmolality and plasma AVP and OT levels determination
AVP and OT were extracted from 500 μL of plasma using acetone and petroleum ether. Dried sample extracts were then stored at −20 °C until quantification by ELISA (Enzo Life Sciences Inc. Farmingdale NY, USA). The AVP and OT ELISA sensitivities were 3.39 and 15.0 pg/mL, respectively. Plasma osmolality was determined by freezing point depression using an osmometer (Precision System, Inc., Natick, MA, USA).
Immunohistochemistry
Brain coronal sections were cut at 40 μm thickness in cryostat (Microm HM 505 E) and processed for immunocytochemistry. Free floating sections were washed three times in PBS (0.01 mol\L; pH 7.4) and submitted to antigen retrieval protocol: incubation in Tris/EDTA (10/1 mmol/L; pH 9.0) solution for 5 min and then heated for 30 min in water bath at 70 °C in 10 mmol/L sodium citrate buffer (pH 6.0). After three new rinses, sections were incubated in PBS containing 1% hydrogen peroxidase for 10 min with PBS to block endogenous peroxidases. Nonspecific binding sites were then blocked for 60 min in PBS containing 5% normal goat serum (NGS) and 0.3% Triton X-100. Subsequently, sections were incubated for 24 h at room with: c-fos-antibody generated in rabbit (sc-52, Santa Cruz) diluted 1:100 in PBS containing 1% NGS, 1% bovine serum albumin and 0.3% Triton-X 100. After rising, the sections were incubated for 1.5 h at room temperature with biotinylated anti-rabbit IgG (1:200; Vector), washed again in PBS, placed for 30 min in avidin–biotin peroxidase complex (ABC, Vectastain). C-fos immunoreactivity was visualized by incubation with 0.02% 3,3′-diaminobenzidine tetrachloride and 0.2% hydrogen peroxide. The sections were mounted on gelatin-coated slides, dehydrated and cover slipped with Entellan (Merck). As a control for staining specificity, first antibody was omitted in some sections, resulting in the complete elimination for immunostaining. Since this antibody also recognizes Fos-related proteins, the staining we have obtained was described as Fos-like immunoreactivity.
Quantification of Fos-like immunoreactivity (FLI)
The brain nuclei exhibiting FLI were identified and delimited according to the rat brain atlas of Paxinos and Watson [15]. The number of FLI nuclear profiles in the sections was counted at one level of the SON (−1.4 mm from the bregma). Nuclei with FLI were quantified using a system that includes a Zeiss microscope equipped with a digital camera. Images were digitalized and analyzed using Image J (version 1.49v—National Institute of Health, USA) software.
Metabolic cages
In order to accurately assess the water intake, in the third day after CLP surgery, naive or sepsis survivor animals were housed in metabolic cages for adaptation, with food and water ad libitum. Two days later, they were submitted to an injection of hypertonic saline (i.p.), and water intake was assessed (30, 60, 120, 180 and 240 min).
Statistical analysis
All results are expressed as mean ± SEM. For statistical analysis, we used two-way ANOVA followed by post hoc Tukey test. P values of < 0.05 were considered statistically significant in all cases. The software used was GraphPad Prism version 5.0 (GraphPad Software, Inc. 2007).
Result
Neurohypophyseal hormones secretion is attenuated in sepsis survivor rats after osmotic challenge
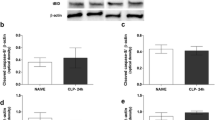
We assessed the plasma neurohypophyseal hormones, cytokines and nitrite levels and osmolality after 30 min of administration of hypertonic saline in sepsis survivor rats (survival rate ~ 70%—Fig. 1a). We detected a persistent increased plasma level of IL-1β and nitrite, but not IL-6, five days post-CLP surgery (Fig. 1b, c, d, respectively). The hypertonic saline injection did not alter the plasma IL-1β and nitrite levels in naive or septic animals, when compared with the respective isotonic saline-treated control groups. In addition, as expected, the osmotic challenge increased the plasma osmolality (Fig. 2a) and AVP and OT secretion, in naive and sepsis survivor rats (Fig. 2b, c). However, both hormones (but not the osmolality) were significantly attenuated in survivor group as compared with the control (Fig. 2b, c). Additionally, we evaluated whether the AVP and OT secretion and thirst are similarly affected, since under normal conditions, both can be stimulated via osmoreceptors during systemic hypertonicity. Here, we observed that the osmotic challenge increased water intake in both naive and survivor rats, but without differences between the groups (Fig. 2d).
Persistent inflammatory mediators in sepsis survivors. a Survival curve from naive rats or submitted to CLP surgery (n = 14–20 animals/group). After 5 days, the survivor animals were challenged with isotonic (control) or hypertonic saline administration. b Plasma IL-1β, c Nitrite, d IL-6. The data represent the means ± SEM of 5–8 animals per group. *P < 0.01 (c) and 0.001 (b) versus Naive-control; **P < 0.05 (c) and 0.01 (b) versus Naive-control; ***P < 0.01 (b) and 0.001 (c) versus Naive-osmotic challenge; # P < 0.05 (b, c) versus Naive-osmotic challenge
Neurohypophyseal hormones secretion is attenuated in sepsis survivor rats after osmotic challenge. a Osmolality, b AVP and c OT levels were evaluated 30 min after hypertonic saline administration. d Cumulative water intake was measured during 240 min after osmotic challenge in sepsis survivor animals. Naive rats were used as the control group. The data represent the means ± SEM of 4–8 animals per group. *P < 0.001 (a, b, c) versus naive-control; #P < 0.001 (b, c) versus naive-osmotic challenge; ·P < 0.01 versus CLP-control
Neuronal activation is attenuated in sepsis survivor rats after osmotic challenge
Following, we assessed a possible hypothalamic dysfunction in sepsis survivor through c-fos expression analysis, an immediate-early gene used as a marker of activated vasopressinergic and oxytocinergic neurons by osmotic stimulation [16, 17]. As expected, we observed that in naive rats, saline hypertonic injection increased significantly the number of Fos-labeled neurons in SON as compared with isotonic saline administration (Fig. 3a, b, e). However, in the sepsis survivors, the osmotic challenge did not enhance the c- fos expression in SON showing a degree of activation similar to the saline-treated group (Fig. 3a, d, e).
Neuronal activation is attenuated in sepsis survivor rats after osmotic challenge. Photomicrographs illustrating nuclear labelling of FLI neurons in the SON. The number of FLI labelled neurons were performed 30 min after the a, b naive and c, d sepsis survivor groups received, a, c isotonic saline or b, d osmotic challenge. The data represent the means ± SEM of 5–8 animals per group. ***P < 0.001 (e) versus naive-control; # P < 0.001 (e) versus naive-osmotic challenge. Objective magnification: ×40 (oil immersion). Scale bar = 50 μm
Discussion
In the current study, we showed a long-term neuroendocrine dysfunction in an animal model of sepsis characterized by an impairment of AVP and OT secretion following an acute osmotic stimulus associated with abnormal activation of hypothalamic neurons.
Moreover, we observed persistent increased plasma levels of IL-1β and nitrite, but not IL-6, in these septic survivals animals. Here, we used the systemic cytokine levels to define the animals as sepsis survivors. For example, high IL-6 levels have a positive association with elevated sepsis mortality and, therefore, its presence has been considered a good predictor of death [18, 19]. However, in our study, IL-6 was not detected in the plasma of surviving animals in contrast with the persistent high IL-1β levels. The same pattern of blood cytokines in sepsis survivor rats was already reported in another study using the same sepsis model suggesting that, in our conditions, the levels of cytokines are reproducible but not an isolated data [20]. Although high IL-1β levels are not associated with high mortality of septic animals, this cytokine could contribute to the dysfunction of neurohypophyseal hormones secretion as we already observed in septic rats [14, 21]. IL-1β can reach the CNS through the circumventricular organs, structures lacking a blood–brain barrier, modifying the function of magnocellular neurons present in the SON [2]. Our group already reported an increase of IL-1β and NO production in hypothalamus associated with the impairment of AVP secretion one day after sepsis induction and that IL-1β can also induce central iNOS expression amplifying mechanisms that results in the activation of apoptotic pathways [7, 14]. In this study, we observed increased nitrite plasma levels in all survivor animals groups, without difference between groups. Nitrate is a metabolite, that indirectly indicates the formation of NO [22]. Peripherally, high levels of NO results in decreased blood pressure and a deficient response to vasoconstrictors, contributing to the cardiovascular failure during septic shock [7, 23].
We observed that this impaired hormonal secretion was accompanied by an attenuated neuronal activation in SON. It may reflect an overall decrease in the activation of both vasopressinergic and oxytocinergic magnocellular neurons harbored in SON even in the presence of osmotic stimulation.
In the present study it was not evidenced an alteration in thirst in sepsis survivor rats, suggesting that only the endocrine component of osmoregulatory reflex is impaired. Differential disruptions in the components of osmoregulatory response have been evidenced during sepsis in an experimental model [5]. Therefore, the components of the osmoregulatory reflex, that include also organum vasculosum laminae terminalis and median preoptic nucleus, could have different recovery times. The neuronal dysfunction has been observed in human and experimental septic shock, where the AVP post-transcriptional synthesis is decreased mainly in the SON [24–26]. Recently we demonstrated in this nucleus the presence of cellular death markers specifically in vasopressinergic neurons [27].
Post-sepsis clinical evidences pointed to the impairment of AVP secretion following an acute osmotic challenge [12, 13]. Herein, we provide the first experimental data demonstrating a persistent impairment of neurohypophyseal hormones in CLP survivor animals mimicking secondary neuroendocrine dysfunctions observed in patients. Alterations in this neuroendocrine function may decrease the life quality of survivor patients and, therefore, this long-term model could be useful to comprehend pathological events involved in this hypothalamic hormones dysfunction.
In conclusion, our results showed that persistent inflammatory mediators may contribute for the alterations in hypothalamic neuronal activation, providing a hypothetic mechanistic explanation for the impairment in the endocrine component of the osmoregulatory reflex in sepsis survivors. Further studies are necessary to determine the cause of this disturbance in the hypothalamic function. It is possible that neurodegeneration or synaptic dysfunction may be involved in long-term brain events after sepsis and that neurohypophyseal hormones secretion deficiency is just part of vast brain function impairment.
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 315(8):801–810. doi:10.1001/jama.2016.0287
Wahab F, Atika B, Oliveira-Pelegrin GR, Rocha MJ (2013) Recent advances in the understanding of sepsis-induced alterations in the neuroendocrine system. Endocr Metab Immune Disord Drug Targets 13(4):335–347
Oliveira-Pelegrin GR, Saia RS, Cárnio EC, Rocha MJ (2013) Oxytocin affects nitric oxide and cytokine production by sepsis-sensitized macrophages. Neuroimmunomodulation 20(2):65–71. doi:10.1159/000345044
Cunningham ET, Sawchenko PE (1991) Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci 14(9):406–411
Stare J, Siami S, Trudel E, Prager-Khoutorsky M, Sharshar T, Bourque CW (2015) Effects of peritoneal sepsis on rat central osmoregulatory neurons mediating thirst and vasopressin release. J Neurosci 35(35):12188–12197. doi:10.1523/JNEUROSCI.5420-13.2015
Landry DW, Levin HR, Gallant EM, Ashton RC, Seo S, D’Alessandro D, Oz MC, Oliver JA (1997) Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation 95(5):1122–1125
Corrêa PB, Pancoto JA, de Oliveira-Pelegrin GR, Cárnio EC, Rocha MJ (2007) Participation of iNOS-derived NO in hypothalamic activation and vasopressin release during polymicrobial sepsis. J Neuroimmunol 183(1–2):17–25. doi:10.1016/j.jneuroim.2006.10.021
Oliveira-Pelegrin GR, Ravanelli MI, Branco LG, Rocha MJ (2009) Thermoregulation and vasopressin secretion during polymicrobial sepsis. Neuroimmunomodulation 16(1):45–53. doi:10.1159/000179666
Benjamim CF, Hogaboam CM, Kunkel SL (2004) The chronic consequences of severe sepsis. J Leukoc Biol 75(3):408–412. doi:10.1189/jlb.0503214
Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS (2014) Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med 29(2):87–95. doi:10.1177/0885066612467162
Mazeraud A, Pascal Q, Verdonk F, Heming N, Chrétien F, Sharshar T (2016) Neuroanatomy and physiology of brain dysfunction in sepsis. Clin Chest Med 37(2):333–345. doi:10.1016/j.ccm.2016.01.013
Siami S, Bailly-Salin J, Polito A, Porcher R, Blanchard A, Haymann JP, Sharshar T (2010) Osmoregulation of vasopressin secretion is altered in the postacute phase of septic shock. Crit Care Med 38(10):1962–1969. doi:10.1097/CCM.0b013e3181eb9acf
Siami S, Polito A, Porcher R, Hissem T, Blanchard A, Boucly C, Carlier R, Annane D, Sharshar T (2013) Thirst perception and osmoregulation of vasopressin secretion are altered during recovery from septic shock. PLoS One 8(11):e80190. doi:10.1371/journal.pone.0080190
Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, Rocha MJ (2015) Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrinology 49(1):215–221. doi:10.1007/s12020-014-0452-2
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Ruginsk SG, Uchoa ET, Elias LL, Antunes-Rodrigues J (2013) Anandamide modulates the neuroendocrine responses induced by extracellular volume expansion. Clin Exp Pharmacol Physiol 40(10):698–705. doi:10.1111/1440-1681.12155
Ruginsk SG, Oliveira FR, Margatho LO, Vivas L, Elias LL, Antunes-Rodrigues J (2007) Glucocorticoid modulation of neuronal activity and hormone secretion induced by blood volume expansion. Exp Neurol 206(2):192–200. doi:10.1016/j.expneurol.2007.04.012
Pallás Beneyto LA, Rodríguez Luis O, Saiz Sánchez C, Cotell Simón O, Bautista Rentero D, Miguel Bayarri V (2016) Prognostic value of interleukin 6 for death of patients with sepsis. Med Clin (Barc). doi:10.1016/j.medcli.2016.06.001
Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM (2005) Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol 289(4):R1048–R1053. doi:10.1152/ajpregu.00312.2005
Griffin MJ, Letson HL, Dobson GP (2016) Small-volume adenosine, lidocaine and Mg2+ (ALM) 4 h infusion leads to 88% survival after 6 days of experimental sepsis in the rat without antibiotics. Clin Vaccine Immunol. doi:10.1128/CVI.00390-16
Wahab F, Santos-Junior NN, de Almeida Rodrigues RP, Costa LH, Catalão CH, Rocha MJ (2016) Interleukin-1 receptor antagonist decreases hypothalamic oxidative stress during experimental sepsis. Mol Neurobiol 53(6):3992–3998. doi:10.1007/s12035-015-9338-4
Krafte-Jacobs B, Brilli R, Szabó C, Denenberg A, Moore L, Salzman AL (1997) Circulating methemoglobin and nitrite/nitrate concentrations as indicators of nitric oxide overproduction in critically ill children with septic shock. Crit Care Med 25(9):1588–1593
Bruhn FH, Corrêa PB, Oliveira-Pelegrin GR, Rocha MJ (2009) Blocking systemic nitric oxide production alters neuronal activation in brain structures involved in cardiovascular regulation during polymicrobial sepsis. Neurosci Lett 453(3):141–146. doi:10.1016/j.neulet.2009.02.030
Sonneville R, Guidoux C, Barrett L, Viltart O, Mattot V, Polito A, Siami S, de la Grandmaison GL, Blanchard A, Singer M, Annane D, Gray F, Brouland JP, Sharshar T (2010) Vasopressin synthesis by the magnocellular neurons is different in the supraoptic nucleus and in the paraventricular nucleus in human and experimental septic shock. Brain Pathol 20(3):613–622. doi:10.1111/j.1750-3639.2009.00355.x
Oliveira-Pelegrin GR, de Azevedo SV, Yao ST, Murphy D, Rocha MJ (2010) Central NOS inhibition differentially affects vasopressin gene expression in hypothalamic nuclei in septic rats. J Neuroimmunol 227(1–2):80–86. doi:10.1016/j.jneuroim.2010.06.019
Oliveira-Pelegrin GR, Aguila FA, Basso PJ, Rocha MJ (2010) Role of central NO-cGMP pathway in vasopressin and oxytocin gene expression during sepsis. Peptides 31(10):1847–1852. doi:10.1016/j.peptides.2010.06.031
da Costa LH, Júnior NN, Catalão CH, Sharshar T, Chrétien F, da Rocha MJ (2016) Vasopressin impairment during sepsis is associated with hypothalamic intrinsic apoptotic pathway and microglial activation. Mol Neurobiol. doi:10.1007/s12035-016-0094-x
Acknowledgements
The authors thank Nadir Martins Fernandes and Marcelo Batalhão for technical assistance in the measurement of nitrite.
Funding
This work was supported by the State Research Support Foundation São Paulo (grant number: 2015-12152-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were done following the Ethics Committee of Animal Experimentation at the University of São Paulo (CEUA)-Campus Ribeirão Preto.
Rights and permissions
About this article
Cite this article
Santos-Junior, N.N., Costa, L.H.A., Catalão, C.H.R. et al. Impairment of osmotic challenge-induced neurohypophyseal hormones secretion in sepsis survivor rats. Pituitary 20, 515–521 (2017). https://doi.org/10.1007/s11102-017-0812-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-017-0812-z