Abstract
Objective
With the resection of pituitary lesions, the anterior pituitary gland often obstructs transsphenoidal access to the lesion. In such cases, a gland incision and/or partial gland resection may be required to obtain adequate exposure. We investigate this technique and determine the associated risk of post-operative hypopituitarism.
Methods
All patients who underwent surgical resection of a pituitary adenoma or Rathke cleft cyst (RCC) between July 2007 and January 2013 were analyzed for pre- and post-operative hormone function. The cohort of patients with gland incision/resection were compared to a case-matched control cohort of pituitary surgery patients. Total hypophysectomy patients were excluded from outcome analysis.
Results
Of 372 operations over this period, an anterior pituitary gland incision or partial gland resection was performed in 79 cases (21.2 %). These include 53 gland incisions, 12 partial hemi-hypophysectomies and 14 resections of thinned/attenuated anterior gland. Diagnoses included 64 adenomas and 15 RCCs. New permanent hypopituitarism occurred in three patients (3.8 %), including permanent DI (3) and growth hormone deficiency (1). There was no significant difference in the rate of worsening gland dysfunction nor gain of function. Compared to a control cohort, there was a significantly lower incidence of transient DI (1.25 vs. 11.1 %, p = 0.009) but no significant difference in permanent DI (3.8 vs. 4.0 %) in the gland incision group.
Conclusion
Selective gland incisions and gland resections were performed in over 20 % of our cases. This technique appears to minimize traction on compressed normal pituitary gland during removal of large lesions and facilitates better visualization and removal of cysts, microadenomas and macroadenomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pituitary gland dysfunction is a recognized post-operative complication following transsphenoidal surgery for pituitary adenomas and Rathke’s cleft cysts (RCC). Higher rates of long-term pituitary gland failure are typically seen with larger tumor sizes (>2 cm), a history of apoplexy and in reoperations for Cushing’s disease particularly when partial or total hypophysectomy is performed [1–6]. For pituitary adenoma resection, it is the current practice to perform selective adenomectomy utilizing the tumor pseudocapsule in order to maximize tumor removal while minimizing the risk of injury to the normal gland [7, 8]. The growth patterns of many pituitary adenomas and Rathke’s cleft cysts (RCCs), however, often result in anterior displacement of the anterior pituitary gland, obstructing direct surgical access from an endonasal transsphenoidal approach. In such cases, we have found it useful to incise or partially resect a portion of the attenuated anterior pituitary gland in order to gain adequate access to the lesion while also minimizing traction on the remainder of the normal gland.
Additionally, gland incisions or partial resections are often necessary to remove small functional pituitary adenomas. Ultimately, this technique can help ensure complete removal of a tumor that is otherwise difficult to visualize. While rates of new post-operative anterior or posterior pituitary failure range from as low as 0.4 % in standard procedures to as high as 33 % in cases requiring a hemihypophysectomy [5], it is our belief that the pituitary gland tolerates incisions or partial resections relatively well. Herein we investigate the frequency of this technique performed in our practice and evaluate the associated risk of post-operative hypopituitarism.
Methods
All patients who underwent endoscopic-assisted or fully endoscopic transsphenoidal resection of a pituitary adenoma or RCC at our institution between July 2007 and January 2013 were reviewed. Patients who had required a pituitary gland incision or partial gland resection were identified. In patients who underwent hemihypophysectomy, the surgical technique preserved the insertion of the infundibulum to the remaining gland and typically did not extend directly to the midline.
A case-matched cohort was randomly identified from the remaining patients to serve as a control group. Each patient’s routine pre- and post-operative hormonal testing (minimum 3 month post-surgical follow up) was then retrospectively evaluated in order to determine the overall impact on pituitary gland function. Patient’s who underwent a total hypophysectomy were excluded from post-operative outcomes analysis. All operations were done by the senior author (DFK) at Saint John’s Health Center. Medical records were reviewed to evaluate pre- and post-operative hormonal status, pathology reports, magnetic resonance imaging (MRI) characteristics, operative notes, and clinic follow-up notes from the patient’s neurosurgeon and endocrinologist. All patients were required to have a minimum of three months post-operative follow up.
Pre-, peri- and post-operative hormonal assessment
The following pre- and post-operative pituitary function tests (at least 3 months after surgery) were utilized for assessing each patient’s hormonal axes: morning plasma ACTH and serum cortisol, thyroid stimulating hormone (TSH), Total T4 or free T4, leutinizing hormone (LH), follicle stimulating hormone (FSH), prolactin (PRL), growth hormone (GH) and insulin-like growth factor 1 (IGF-1). Results were therefore interpreted as normal or abnormal based on each laboratory’s reference range. Secondary hypothyroidism was diagnosed if the patient had the combination of either a low Free T4/low TSH or a low Free T4/normal TSH. Hypogonadism in women was diagnosed if amenorrhea and/or infertility were present and if gonadotropins were low or low normal in the setting of low estradiol levels. Secondary hypogonadism in men was diagnosed if the patient had low serum testosterone (or low estradiol) in the context of either normal or low gonadotropin levels. GH deficiency was based upon low age- and sex adjusted IGF-1 and in some instances by GHRH-arginine or the insulin tolerance test. Any patient who had three other anterior hormonal axis deficiencies (corticotroph, thyrotroph and gonadotroph) was categorized as being GH deficient, whether in the pre-operative or post-operative state. Posterior pituitary function was assessed based upon urine specific gravity, serum sodium and urine output. Patients were diagnosed with post-operative diabetes insipidus (DI) if urine specific gravity was ≤1.005 and urine volume was greater than 200 cc per hour for at least three consecutive hours with normo- or hypernatremia.
Statistical analysis
Statistical analysis was performed with the SPSS software (v13.0 SPSS Inc., Chicago, IL). Parametric variables analyzed with Pearson Chi squared method assuming 2-tailed data sets and/or Fisher’s Exact Test for small sample sizes. Continuous variables analyzed with ANalysis Of VAriance (ANOVA) methods. Statistical significance was defined for p values p < 0.05.
IRB
Informed consent was waived for this study as this was a retrospective chart review. This was reviewed and approved by our institution’s review board (IRB).
Results
Of 372 total operations over this period, an anterior pituitary gland incision or partial gland resection was performed in 79 cases (21.2 %). In 53 operations, only a vertical or horizontal gland incision was made (Fig. 1) while the remaining 26 cases involved some degree of gland resection including 12 partial hemi-hypophysectomies and 14 resections of thinned/attenuated anterior gland draped over a large macroadenoma (Fig. 2). Diagnoses included 64 pituitary adenomas (17 endocrine-inactive, 28 Cushing’s disease, 13 prolactinomas, 6 acromegaly) and 15 RCCs. One patient with total hypophysectomy was excluded from endocrine outcomes evaluation. The control cohort was well matched to the gland incision cohort (Table 1), with a bias towards more Cushing’s disease patients in the gland incision group, due to the higher number of microadenomas in this diagnosis group.
Intraoperative photographs with 0- and 30-degree 4 mm endoscopes demonstrating drainage of a supraglandular Rathke’s cleft cyst requiring an incision into the normal anterior pituitary gland (PG); a anterior pituitary gland (PG) surface following dural opening; b vertical pituitary gland (PG) incision being performed using a straight feather blade; c, d dissection performed through the pituitary gland (PG) incision revealing characteristic Rathke’s cleft cyst contents (Cy); e removal of cyst contents (Cy); f final view of cyst cavity (CyC) with diaphragma sellae left intact
Intraoperative photographs with 0- and 30-degree 4 mm endoscopes demonstrating; a small attenuated portion of the anterior pituitary gland (PG) draped over a macroadenoma; b partial resection of the attenuated anterior pituitary gland (PG) being performed revealing underlying soft tumor (T); c–e pseudocapsular dissection followed by complete tumor (T) removal with the remainder of the pituitary gland (PG) left intact; f final view of resection cavity
Within the gland incision/resection cohort, new permanent hypopituitarism occurred in three patients (3.8 %): two with macroadenomas (3.2 and 3.5 cm) and one with RCC (1.2 cm). Of these patients, one macroadenoma patient presented with apoplexy. All three of these patients developed permanent DI and one macroadenoma patient developed growth hormone deficiency. Four additional patients experienced transient post-operative hyponatremia (Table 2).
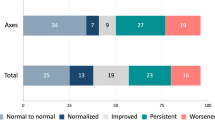
In patients with normal pre-operative endocrine function (n = 99), there was no significant difference in post-operative dysfunction between the gland incision/resection and control cohorts (9.8 vs. 5.2 %, p = 0.31). In patients with pre-operative pituitary dysfunction (n = 79), there was no further gland dysfunction in the gland incision/resection cohort compared to an 8.6 % rate of gland dysfunction in the control cohort (p = 0.2). There was no significant difference in gain of function in this subset of patients comparing the gland incision/resection and control cohorts (38.1 vs. 32.8 %, p = 0.43)—(Fig. 3). The specific pituitary axes incidences of baseline and post-operative loss or gain of function are detailed in Table 3.
Diabetes Insipidus was defined to be permanent if it persisted at the time of endocrine evaluation (minimum interval of 3 months). With these parameters, there was a significantly lower incidence of transient DI (1.25 vs. 11.1 %, p = 0.009) comparing the gland incision/resection to the control cohorts. However, there was no significant difference in the incidence of permanent DI in the gland incision/resection cohort compared to the control cohort (3.8 vs. 4.0 %)—(Fig. 4).
Within the gland incision/resection cohort, endocrine outcomes were evaluated based on extent of gland violation (incision, wedge resection, hemihypophysectomy—see Table 4). The single total hypophysectomy patient was excluded from this analysis. The incidence of post-operative anterior pituitary gland dysfunction had no significant difference between incision and resection groups (3.8 vs. 7.1 %, p = 0.64). These same patients also developed permanent diabetes insipidus (3.8 vs. 7.1 %, p = 0.64). The rate of pituitary function recovery was 15.1 % in the gland incision group compared to 4.0 % in the gland resection group (p = 0.14)—(Fig. 5).
Discussion
Gland incision or resection techniques have been utilized for the resection of macro- and micro-adenomas since the advent of pituitary microsurgery. Although these have been described in the literature, particularly when applying the pseudocapsular dissection techniques, there has not been a systematic evaluation of the impact to the pituitary gland and its function [8]. This study demonstrates equivocal pituitary dysfunction outcomes comparing gland-incision/resection patients with a control cohort (9.8 vs. 5.2 %). There were significantly fewer patients with transient diabetes insipidus (1.25 vs. 11.1 %), but no significant difference in the incidence of long-term DI (3.8 vs. 4.0 %). Within the gland-incision/resection cohort, there was no significant different in the rate of pituitary dysfunction with regards to technique (incision only, wedge resection, hemihypophysectomy).
Over the past 25 years, pituitary dysfunction following pituitary adenoma resection has been reported between 2 and 22 % [9–11]. Fatemi et al. [2] demonstrated the association between tumor size and post-operative dysfunction as well as patient age and surgical experience. Nemergut et al. [12] correlated increased pituitary dysfunction with the incidence of intra-operative CSF leakage, suggesting more vigorous tumor resection and manipulation. The vulnerability of the pituitary gland vasculature when compressed by large tumors has been frequently reported as well [13]. Hence, efforts are made to avoid unnecessary manipulation of the pituitary gland, and particularly the infundibulum, during pituitary tumor resection.
The introduction of neuroendoscopy to pituitary surgery has improved visualization of the pituitary and parasellar structures. Hence, one would assume that pituitary gland identification and preservation would be more likely and could result in improved pituitary outcomes. Endoscopic series have reported similar overall outcomes to ours (which includes both fully endoscopic and endoscope-assisted approaches) with pituitary dysfunction rates ranging from 3 to 13 % [11, 14, 15]. the incidence of new post-operative long-term DI is also similar with rates ranging from 1 to 4 % [16, 17]. It is unclear in these studies as to the rate of deliberate gland incision or resection for adequate comparison.
Incising the gland or resecting a small section of thin, attenuated gland can help decrease the traction onto the remaining normal pituitary gland. This can help prevent new pituitary dysfunction, perhaps at the cost of potential return of function. In this series, there was no measurable difference of new loss of function (regardless of pre-operative dysfunction) between either cohort. Nor was there difference in gain of function amongst the cohorts with existing pituitary dsyfunction.
Of the twelve patients in this series who underwent partial hemi-hypophysectomy for microadenoma, none developed new pituitary dysfunction in any axis. Though generally thought to be relatively safe for pituitary function, hemi-hypophysectomy has been shown to be associated with pituitary dsyfunction in a few studies, with rates up to 50 % [18–20]. The significant variability may be due to the extent of pituitary gland resection for partial or hemi-hypophysectomies (as detailed in the methods section). Additionally, as most of these patients had ACTH secreting tumors, the native pituitary corticotroph axis was difficult to assess and this would be difficult to extrapolate to all patients.
The approach to intra-glandular microadenomas or Rathke’s cleft cysts (RCCs) may also require the incision of the normal pituitary gland [7, 8, 21, 22]. The approach to retroglandular or supraglandular RCCs has also been performed using a low, vertical incision through the midline pituitary gland. This is in contrast to the completely supraglandular, supradiaphragmatic approach which has a higher chance of post-operative CSF leak [23, 24]. Given that the vascularity of the midline pituitary gland is also vertical in orientation, such a low, vertical incision has thought to be relatively safe for pituitary gland function [13].
Post-operative diabetes insipidus has been associated with lesions intimately involving the posterior lobe, such as RCC and craniopharyngiomas [12, 25, 26]. Larger macroadenomas causing infundibular compromise has also been associated with post-operative DI [2]. The overall rate of long-term (permanent) DI has been reported between 0.4 and 15 %, though most studies are less then 3 % [2, 10, 11]. In this series, there was no significant difference in the rate of long-term DI between the gland-incision/resection and control cohorts (3.8 vs. 4.0 %). Interestingly, there was only one patient (1.25 %) in the gland-incision/resection cohort that developed transient DI compared to 11 (11.1 %) in the control cohort. These data suggest that the theoretically increased pituitary gland manipulation that occurs without gland incision/resection causes a measurable amount of infundibular traction, resulting in transient DI.
Limitations of this study are apparent in its retrospective nature. Though only 21.2 % of patients underwent deliberate gland incision/resection, perhaps more would have been performed if prompted by a prospective study. Second, the assessment of pituitary dysfunction was not standardized, given the varying management parameters by the treating endocrinologists. Third, it is very challenging to measure the volume and function of the attenuated pituitary gland resected, preventing a volume-based analysis of pituitary dysfunction. Fourth, the control cohort was randomly selected from the larger group of non gland-incised patients. Hence, these were not directly case-matched, diminishing direct comparison. This is evident in the increased rate of Cushing’s disease in the gland incision/resection cohort. To better assess the impact of gland incision/resection on pituitary function, a prospective, cohort-controlled study is necessary.
Conclusion
Incisions or partial resections of the pituitary gland appear to be generally well-tolerated and, in the great majority of patients, are not associated with new post-operative hypopituitarism. This technique which was performed in 21.2 % of our cases, can therefore be utilized when necessary to gain access to pituitary adenomas or RCCs. It appears to minimize traction on the normal pituitary gland during removal of large tumors or cysts and facilitates better visualization of microadenomas or small cystic lesions embedded within or behind the anterior gland. Although a small subset of patients did develop new pituitary dysfunction, this deterioration may be more related to larger tumor or cyst size, infundibular lesion extension and/or multiple pre-operative hormonal axis deficiencies, and less so to the performance of a gland incision or partial resection.
References
Chee GH, Mathias DB, James RA, Kendall-Taylor P (2001) Transsphenoidal pituitary surgery in Cushing’s disease: Can we predict outcome? Clin Endocrinol (Oxf) 54:617–626
Fatemi N, Dusick JR, Mattozo C, McArthur DL, Cohan P, Boscardin J, Wang C, Swerdloff RS, Kelly DF (2008) Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery 63(4):709–718. doi:10.1227/01.NEU.0000325725.77132.90 discussion 718–709
McCance DR, Gordon DS, Fannin TF, Hadden DR, Kennedy L, Sheridan B, Atkinson AB (1993) Assessment of endocrine function after transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf) 38:79–86
Randeva HS, Schoebel J, Byrne J, Esiri M, Adams CB, Wass JA (1999) Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol (Oxf) 51:181–188
Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF (2002) Long-term follow-up results of transsphenoidal surgery for Cushing’s disease in a single centre using strict criteria for remission. Clin Endocrinol (Oxf) 56:541–551
Semple PL, Webb MK, de Villiers JC, Laws ERJ (2005) Pituitary apoplexy. Neurosurgery 56:65–72
Jagannathan J, Smith R, DeVroom HL, Vortmeyer AO, Stratakis CA, Nieman LK, Oldfield EH (2009) Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J Neurosurg 111(3):531–539. doi:10.3171/2008.8.JNS08339
Oldfield EH, Vortmeyer AO (2006) Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg 104(1):7–19. doi:10.3171/jns.2006.104.1.7
Arafah BUM (1986) Reversible hypopituitarism in patients with large nonfunctioning pituitary adenomas*. J Clin Endocrinol Metab 62(6):1173–1179
Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB (1998) Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab 83(10):3411–3418
Cappabianca P, Cavallo LM, Colao A, de Divitiis E (2002) Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 97(2):293–298
Nemergut EC, Zuo Z, Jane JA Jr, Laws ER Jr (2005) Predictors of diabetes insipidus after transsphenoidal surgery: a review of 881 patients. J Neurosurg 103(3):448–454
Gorczyca W, Hardy J (1987) Arterial supply of the human anterior pituitary gland. Neurosurgery 20(3):369–378
Dehdashti AR, Ganna A, Karabatsou K, Gentili F (2008) Pure endoscopic endonasal approach for pituitary adenomas: early surgical results in 200 patients and comparison with previous microsurgical series. Neurosurgery 62(5):1006–1017
Gondim JA, Almeida JPC, Albuquerque LAF, Schops M, Gomes E, Ferraz T, Sobreira W, Kretzmann MT (2011) Endoscopic endonasal approach for pituitary adenoma: surgical complications in 301 patients. Pituitary 14(2):174–183
O’Malley BW Jr, Grady MS, Gabel BC, Cohen MA, Heuer GG, Pisapia J, Bohman L-E, Leibowitz JM (2008) Comparison of endoscopic and microscopic removal of pituitary adenomas: single-surgeon experience and the learning curve. Neurosurg Focus 25(6):E10
Tabaee A, Anand VK, Barrón Y, Hiltzik DH, Brown SM, Kacker A, Mazumdar M, Schwartz TH (2009) Endoscopic pituitary surgery: a systematic review and meta-analysis: Clinical article. J Neurosurg 111(3):545–554
Friedman RB, Oldfield EH, Nieman LK, Chrousos GP, Doppman JL, Cutler GB Jr, Loriaux DL (1989) Repeat transsphenoidal surgery for Cushing’s disease. J Neurosurg 71(4):520–527
Biller B, Grossman AB, Stewart P, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus A, Hofland L (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462
Benveniste RJ, King WA, Walsh J, Lee JS, Delman BN, Post KD (2005) Repeated transsphenoidal surgery to treat recurrent or residual pituitary adenoma. J Neurosurg 102(6):1004–1012
Zada G, Kelly DF, Cohan P, Wang C, Swerdloff R (2003) Endonasal transsphenoidal approach to treat pituitary adenomas and other sellar lesions: an assessment of efficacy, safety, and patient impressions of the surgery. J Neurosurg 98(2):350–358
Cohan P, Foulad A, Esposito F, Martin N, Kelly D (2004) Symptomatic Rathke’s cleft cysts: a report of 24 cases. J Endocrinol Invest 27(10):943–948
Barrow DL, Spector RH, Takei Y, Tindall GT (1985) Symptomatic Rathke’s cleft cysts located entirely in the suprasellar region: review of diagnosis, management, and pathogenesis. Neurosurgery 16(6):766–772
Kato T, Sawamura Y, Abe H, Nagashima M (1998) Transsphenoidal-transtuberculum sellae approach for supradiaphragmatic tumours: Technical note. Acta Neurochir (Wien) 140(7):715–719
Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH (2005) Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg 102(2):189–193
Ghirardello S, Hopper N, Albanese A, Maghnie M (2006) Diabetes insipidus in craniopharyngioma: postoperative management of water and electrolyte disorders. J Pediatr Endocrinol Metab 19:413
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkhoudarian, G., Cutler, A.R., Yost, S. et al. Impact of selective pituitary gland incision or resection on hormonal function after adenoma or cyst resection. Pituitary 18, 868–875 (2015). https://doi.org/10.1007/s11102-015-0664-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0664-3