Abstract
Background
The association of GHR-exon 3 and -202 A/C IGFBP3 polymorphisms with clinical presentation, biochemical measurements and response to therapies in acromegaly have been suggested.
Objective
To evaluate the presence of these polymorphisms in acromegaly and their influence on clinical and laboratorial characteristics of patients at diagnosis and after treatment in a large cohort of acromegalic patients.
Patients and methods
This is a cross-sectional study developed in a single tertiary reference center. Clinical data were obtained from the medical records of 186 acromegalic patients (116 women, age range 21–88 years). GH and IGF1 levels and GHR-exon 3 and -202 A/C IGFBP3 polymorphisms were evaluated in the same hospital.
Results
At diagnosis, serum GH concentrations were lower in patients with GHR-d3 genotype than those with GHR-fl, whereas an association of lower IGFBP3 levels with d3 allele was observed only after neurosurgical or medical treatments. However, these associations were not confirmed in posterior statistical analysis.
Conclusion
Our results suggest that GHR-exon 3 and -202 A/C IGFBP3 polymorphisms did not show any consistent association on clinical and laboratorial features of acromegalic patients even after treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acromegaly is a rare disorder caused by growth hormone (GH) hypersecretion which, through binding to the growth hormone receptor (GHR), leads to increased production of insulin-like growth factor 1 (IGF-1). In most, but not in all patients, the clinical phenotype correlates with the degree of GH/IGF-1 hypersecretion and the relationship between GH and IGF1 levels is linear in active disease [1, 2]. Ideally, serum GH and IGF-1 values should be concordant at diagnosis and in the assessment of disease activity during treatment. Nevertheless, discrepancy between GH and IGF-1 levels (normal GH and elevated IGF1 or vice versa) has been reported [3–8].
Common genetic variants within GH-IGF-1 axis may cause differences in the GH/GHR signaling pathways and were described as an important contributor to height variation in the general population and to rhGH therapy response in growth retarding disorders [9]. GHR-exon 3 polymorphism in the GHR gene is one of these variations. This polymorphism gives rise to two isoforms of GHR transcripts differing by the retention (GHR-fl) or exclusion (GHR-d3) of exon 3 [10, 11]. The effect of GHR-exon 3 polymorphism is still unclear, as it does not affect the affinity, binding capacity, or internalization of d3 and fl GHR isoforms. However, it could facilitate ligand-induced activation of GHR [10, 12–15].
In clinical practice, the presence of the GHR-d3 genotype has been associated with enhanced sensitivity to exogenous recombinant human GH administration in children with Turner syndrome, in those with short stature small-for-gestational-age, and in those with severe GH deficiency [2, 5]. In acromegaly, patients harboring the GHR-d3 allele have lower levels of circulating GH compared with the GHR-fl homozygous carries [16]. Moreover, the presence of GHR-d3 isoform was associated with more severe clinical picture and a lower chance of biochemical control [17].
The polymorphism -202 A/C (rs2854744) in the promoter region of the Insulin-like growth factor binding protein-3 (IGFBP3) gene, which encodes a protein that regulates IGF-1 action, has also been involved in GH therapy pharmacogenetics of GH deficiency and in children born small for gestational age [18, 19]. The -202 A allele was associated with a significantly decreased circulating level of IGFBP-3 in both in vitro and in vivo studies [20, 21]. However, only one study evaluated the -202 A/C IGFBP3 in acromegaly showing no significant difference in clinical characteristics according this genotype.
This study aimed to evaluate the possible influences of GHR-d3 and -202 A/C IGFBP3 polymorphisms on the clinical and metabolic presentation of a large population of Brazilian acromegalic patients, at diagnosis and after treatment, and to investigate the impact of these polymorphisms on the prevalence of long-term complications of acromegaly.
Materials and methods
Patient characteristics
A cohort of 186 acromegalics patients (116 females) followed at the Neuroendocrinology Unit of the Clinical Hospital of the University of São Paulo Medical School were evaluated. Mean age was 43.6.8 (±13.63) years, ranging from 21–88 years. The interval from the presumed onset of symptoms until diagnosis was 8 ± 6 years; mean body mass index (BMI) was 31.77 (±18.8) kg/m2. The clinical suspicion of acromegaly was confirmed by serum IGF-I level above the upper limit of normal range and by lack of GH suppression <1.0 ng/mL during an oral glucose load. At diagnosis, GH and IGF-1 levels were 3.46 ± 16.31 and 937.77 ± 438.30, respectively; and the baseline % ULNR-IGF-1 was 334 ± 160. All patients had a magnetic resonance imaging (MRI) of the sella region, and 97 % (n = 181) harbored macroadenomas (10 mm in diameter). Four macroadenomas showed radiological evidence of tumor necrosis or hemorrhage, suggesting apoplexy. Data was expressed as mean ± SD.
At the time of diagnosis, two or more comorbidities were presented in acromegalic patients: hypertension (74 %), type 2 diabetes (38 %), glucose intolerance (35 %), arthropathy (73 %); cardiovascular disease (38 %), carpal tunnel syndrome (5 %); colon polyps (36 %); goiter (61 %) and papillary thyroid cancer (3.7 %). Two patients had colorectal cancer, 50 hyperplastic polyps, 3 adenomatous/hyperplastic polyps, 20 with adenomatous polyps and 101 had normal colonoscopy (Table 1).
The primary treatment was transsphenoidal surgery (TSS) in 120 patients, somatostatin analogs in 52, dopamine agonists in 13 patients (2 on bromocriptine and 11 on cabergoline) and radiotherapy in one patient. In those patients submitted to TSS, the GH-secreting nature of their tumors was confirmed by positive immuno-histochemical staining for GH. Clinical, biochemical and imaging assessment were performed 3–6 months postoperatively. For those that did not obtain control of the disease, adjuvant treatment with surgery, radiotherapy and/or medication was introduced (Table 2).
Local ethics committee approved this study and all patients provided written informed consent.
Hormone assays
Serum GH concentration was measured before 1994 with immunoradiometric assay (IRMA) in eleven patients; subsequently (1994 onwards), GH samples of the others patients were measured by immunofluorometric assay (IFMA) (AutoDELFIA, Wallac, Turku, Finland) with monoclonal antibodies. IGF-1 was measured by RIA after ethanol extraction (Diagnostic Systems Laboratories, Webster, TX) or by chemiluminescence assays (CLIA) (IMMULITE; Diagnostic Products Corp., Los Angeles,CA). IGFBP3 was measured by IRMA (Diagnostic Systems Laboratories) or CLIA (IMMULITE). IGF-1 and IGFBP3 absolute levels were standardized for age and sex, according to reference values provided by the respective assay kits. To achieve a standardized comparability value among the two different assay used along these years, IGF-I levels were also expressed as the percentage above the upper limit of the normal range (% ULNR-IGF-1).
Definition of disease control/disease remission
Normal serum IGF-I levels adjusted for age and random GH levels below 1.0 ng/mL defined biochemical control during medical treatment. In patients submitted to surgery, disease remission was defined by normal IGF-1 with GH nadir below 1.0 ng/mL during oral 75-g glucose load (OGTT), performed approximately 3–4 months after the procedure. Controlled patients after surgery and/or radiotherapy or during drug treatment were collectively defined as being “in remission” of the disease. The number of previous treatments until the visit study was recorded. This number was used as a potential predictive factor of aggressive phenotype, characterized by persistence of disease after surgical, medical therapy and radiotherapy, reflecting a poor response to usual treatment.
GHR-exon 3 and -202 A/C IGFBP3 genotyping
Genomic DNA was obtained from peripheral blood leukocytes using standard methods from all patients. DNA was amplified by multiplex PCR to determine the GHR-exon 3 polymorphism using previously described primers and PCR condition (16). The -202 A/C IGFBP3 (rs2854744) polymorphism was determined by a specific real-time PCR-based genotyping assay (ID 186389191-1, TaqMan SNP genotyping assay; Applied Biosystems) according to the manufacturer’s instructions in a StepOnePlus™ Real Time PCR system (Applied Biosystems, Foster City, CA). Fifteen percent of all samples were randomly genotyped by direct sequencing for checking of assay performance and quality control. The agreement of the genotypes determined in both analyses was 100 %.
Statistical analysis
Qualitative variables are expressed as frequencies and percentages, whereas quantitative data are presented as mean ± SD. The distribution of genotypes was determined and then compared with clinical and hormonal characteristics at diagnosis and after treatment. ANOVA followed by a Tukey test was used for comparisons according to the additive model, whereas the t test was used for comparisons according to the dominant model. Numerical variables that did not demonstrate parametric distribution were analyzed by Kruskal–Wallis one-way ANOVA on ranks or Mann–Whitney rank sum test. Nominal variables were compared by a X2 or Fisher exact test, as appropriate. Serum GH levels were log10 transformed in order to normalize their distributions. To assess whether genetic variants had an independent effect, we performed single-followed by multiple-regression analyses adjusting for the established influential factors. SigmaStat for Windows (version 3.5; SPSS, Inc., San Rafael, CA) performed all statistical analyses. P value <0.05 was considered statistically significant.
Results
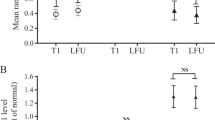
Eighty-three (45 %) patients were to be homozygous for GHR-fl, 81 (44 %) heterozygous and 22 (12 %) homozygous carriers of the GHR-d3. At diagnosis, serum GH concentrations were lower in patients with GHR-d3 allele (P = 0.026 and P = 0.007, according addictive and dominant model, respectively; Table 3). After treatment, this association did not persist and a lower IGFBP3 levels was observed in patients carrying the GHR-d3 genotype (P = 0.009 and P = 0.033, according addictive and dominant model, respectively; Table 3). However, multiple linear regression analysis did not confirm these associations (R = 0.3/P = 0.057 and R = 0.7/P = 0.075, for GHR-d3 vs. serum GH and IGFBP3, respectively) but showed, as expected, a strong correlation between log10 GH and ULNR of IGF-1 (R = 0.3/P = < 0.001) and log10 GH and age (R = 0.3/P = 0.005).
In relation to IGFBP3, polymorphism -202A/C was detected in homozygosis in 38 (20 %) and heterozygosis in 95 (51 %), whereas 53 (28 %) of patients were homozygous for wild type C allele. After treatment, a lower basal serum GH concentration was observed in patients carrying allele A (P = 0.040 and P = 0.018, according addictive and dominant model, respectively (Table 4).
The proportion of patients having comorbidities and requiring multiple forms of therapy (surgery, radiotherapy and pharmacological therapy) were similar between three genotypes for both GHR-exon3 and -202 A/C IGFBP3 variants (data not shown).
All polymorphisms evaluated followed Hardy–Weinberg equilibrium.
Discussion
The association of the GHR-exon 3 and -202 A/C polymorphisms on the clinical features, comorbidities and responses to medical treatment were evaluated in a large cohort of acromegalic patients (n = 186). The genotype frequency of GHR-exon 3 polymorphism found in the present study are similar to that observed in healthy subjects [10, 12] and in other reports of acromegalics patients [16, 17, 22–29] where, homozygous for GHR-fl is the predominant genotype and almost half of the individuals tested are carriers of at least one d3-GHR allele (Table 5).
The presence of d3 allele was found to be associated with a tendency of lower GH concentrations at diagnosis (P = 0.007) and lower IGFBP3 levels after treatment (P = 0.033). However, when we used multiple linear regression to combine log10 GH and IGFBP3 concentrations, % ULNR-IGF-1, age and GHR-exon 3 genotypes, these associations does not persist but display almost significant tendency (P = 0.057 and 0.075, respectively). In this analysis, the only variable that affected basal GH was age (P = 0.005) whereas % ULNR-IGF-1 was influenced by log10 GH (P < 0.001).
Schmid et al. [16] first demonstrated the influence of GHR-exon 3 polymorphism on endogenous GH concentrations, evaluating untreated acromegalic patients at diagnosis. These authors reported that basal GH was significantly higher in the GHR-fl than in the GHR-d3 group, whereas IGF1 concentrations were comparable in both groups; thus, it was suggested that lower GH concentrations are required for patients carrying d3 allele to produce a given increase in serum IGF-1 concentrations and to develop acromegalic symptoms due to higher sensitivity of GHR-d3 isoform [16]. GHR-d3 showed an increased rate of GH and IGF-I levels (high IGF-I levels and GH ≤2 ng/ml) after somatostatin analogs treatment.
The relationship between GH and IGF1 levels appear to be linear in patients with active disease [1, 2], but discrepant results may be observed in many acromegalic patients either at diagnosis either after treatment [3–8]. Bianchi et al. [22] described that treated acromegalic patients carrying the GHR-d3 allele were likely to have post treatment GH values lower than those fl/fl homozygous but with an increased rate of GH and IGF-I levels discordancy (high IGF-I levels and GH ≤2 ng/ml) after SA [22]. On the other hand, Kamenicky et al. [24] observed that the GHR-exon 3 genotype did not affect the positive relationship between GH and IGF-I levels. In our cohort of acromegalic patients, the discrepancy between GH and IGF-1 levels at diagnosis, after surgery and after medical treatment did not correlate to GHR-exon3 genotypes.
There are also contrasting findings about GHR-d3 polymorphism influence on the outcome of acromegaly treatment. Mercado et al. [17] observed that subjects carrying GHR-fl allele had greater probability of achieving IGF-I normalization after either surgical or medical therapy. In other two studies, GHR-d3 allele was associated to lower pegvisomant dose of and fewer months of treatment to normalize IGF-I [23, 26]. In contrast, another study reported that GHR-d3 was not a predictor of both dose and efficacy of mono- and combined pegvisomant therapies in 111 patients with acromegaly [27]. In our cohort, the post-treatment GH levels were not significantly lower in patients carrying the GHR-d3 despite similar IGF-1 levels. Also, no influence of GHR-exon 3 genotypes on treatment outcome was found in our study. The mean number of treatments to which patients underwent was similar among the GHR-d3 genotypes. This is in contrast with previous studies that observed a higher number of treatments in GHR-d3 compared to GHR-fl genotype [17, 25].
In addition, it has been hypothesized that patients who carried GHR-d3 would be more susceptible to long-term complications of acromegaly. Mercado et al. [17] demonstrated an increased prevalence of type 2 DM in active acromegaly patients with the d3 genotype [17]. Wassenaar et al. [28] observed that the GHR-d3 was associated with a higher prevalence of colonic polyps and dolichocolon and osteoarthritis and Turgut et al. [29] observed that systolic blood pressure was significantly increased in homozygote GHR-d3 genotype group compared to d3/fl subjects. However, our results did not find any consistent results on percentages of patients with comorbidities among GHR-exon 3 genotypes.
IGFPB3 displays growth inhibitory/proapoptotic action and counteracts the IGF-1 tumor-promoting effects by down regulating its bioavailability [21]. To our knowledge, only one study has evaluated the role of IGFBP-3 polymorphisms in acromegaly to date. Akin et al. [30] examined 34 patients with acromegaly and 37 healthy subjects and compared -202 A/C IGFBP3 genotypes with serum levels of glucose, insulin, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, growth hormone (GH), Insulin-like growth factor I (IGF-I) and IGFBP-3 [30]. They observed that the frequency of -202 A/C IGFBP3 genotypes was significantly different between control and patients but with no difference in the clinical presentation of acromegalic patients [30]. In our evaluation, no association between -202 A/C IGFBP3 and clinical/hormonal characteristics and response to therapy was found, with the exception of GH levels after treatment that were significantly lower in patients carrying A allele.
In conclusion, our results did not found any consistent evidence for the influence of GHR-exon 3 or -202 A/C IGFBP3 polymorphisms in the variability on phenotypic expression of GH hypersecretion in terms of clinical features, co-morbidities and response to treatment and its peripheral biological effects in patients with acromegaly.
References
Dobrashian RD, O’Halloran DJ, Hunt A, Beardwell CG, Shalet SM (1993) Relationships between insulin-like growth factor-1 levels and growth hormone concentrations during diurnal profiles and following oral glucose in acromegaly. Clin Endocrinol (Oxf) 38:589–593
Parkinson C, Ryder WD, Trainer PJ, Group SAS (2001) The relationship between serum GH and serum IGF-I in acromegaly is gender-specific. J Clin Endocrinol Metab 86:5240–5244
Dimaraki EV, Jaffe CA, DeMott-Friberg R, Chandler WF, Barkan AL (2002) Acromegaly with apparently normal GH secretion: implications for diagnosis and follow-up. J Clin Endocrinol Metab 87:3537–3542
Freda PU, Nuruzzaman AT, Reyes CM, Sundeen RE, Post KD (2004) Significance of “abnormal” nadir growth hormone levels after oral glucose in postoperative patients with acromegaly in remission with normal insulin-like growth factor-I levels. J Clin Endocrinol Metab 89:495–500
Mercado M, Espinosa de los Monteros AL, Sosa E et al (2004) Clinical–biochemical correlations in acromegaly at diagnosis and the real prevalence of biochemically discordant disease. Horm Res 62:293–299
Alexopoulou O, Bex M, Abs R, T’Sjoen G, Velkeniers B, Maiter D (2008) Divergence between growth hormone and insulin-like growth factor-i concentrations in the follow-up of acromegaly. J Clin Endocrinol Metab 93:1324–1330
Machado EO, Taboada GF, Neto LV et al (2008) Prevalence of discordant GH and IGF-I levels in acromegalics at diagnosis, after surgical treatment and during treatment with octreotide LAR. Growth Horm IGF Res 18:389–393
Ho PJ, Jaffe CA, Friberg RD, Chandler WF, Barkan AL (1994) Persistence of rapid growth hormone (GH) pulsatility after successful removal of GH-producing pituitary tumors. J Clin Endocrinol Metab 78:1403–1410
Walenkamp MJ, Wit JM (2006) Genetic disorders in the growth hormone—insulin-like growth factor-I axis. Horm Res 66:221–230
Pantel J, Machinis K, Sobrier ML, Duquesnoy P, Goossens M, Amselem S (2000) Species-specific alternative splice mimicry at the growth hormone receptor locus revealed by the lineage of retroelements during primate evolution. J Biol Chem 275:18664–18669
Buzi F, Mella P, Pilotta A, Prandi E, Lanfranchi F, Carapella T (2007) Growth hormone receptor polymorphisms. Endocr Dev 11:28–35
Dos Santos C, Essioux L, Teinturier C, Tauber M, Goffin V, Bougnères P (2004) A common polymorphism of the growth hormone receptor is associated with increased responsiveness to growth hormone. Nat Genet 36:720–724
Sobrier ML, Duquesnoy P, Duriez B, Amselem S, Goossens M (1993) Expression and binding properties of two isoforms of the human growth hormone receptor. FEBS Lett 319:16–20
Urbanek M, Russell JE, Cooke NE, Liebhaber SA (1993) Functional characterization of the alternatively spliced, placental human growth hormone receptor. J Biol Chem 268:19025–19032
Bass SH, Mulkerrin MG, Wells JA (1991) A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci USA 88:4498–4502
Schmid C, Krayenbuehl PA, Bernays RL, Zwimpfer C, Maly FE, Wiesli P (2007) Growth hormone (GH) receptor isoform in acromegaly: lower concentrations of GH but not insulin-like growth factor-1 in patients with a genomic deletion of exon 3 in the GH receptor gene. Clin Chem 53:1484–1488
Mercado M, González B, Sandoval C et al (2008) Clinical and biochemical impact of the d3 growth hormone receptor genotype in acromegaly. J Clin Endocrinol Metab 93:3411–3415
Costalonga EF, Antonini SR, Guerra-Junior G, Mendonca BB, Arnhold IJ, Jorge AA (2009) The -202 A allele of insulin-like growth factor binding protein-3 (IGFBP3) promoter polymorphism is associated with higher IGFBP-3 serum levels and better growth response to growth hormone treatment in patients with severe growth hormone deficiency. J Clin Endocrinol Metab 94:588–595
van der Kaay DC, Hendriks AE, Ester WA et al (2009) Genetic and epigenetic variability in the gene for IGFBP-3 (IGFBP3): correlation with serum IGFBP-3 levels and growth in short children born small for gestational age. Growth Horm IGF Res 19:198–205
Jernström H, Deal C, Wilkin F et al (2001) Genetic and nongenetic factors associated with variation of plasma levels of insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in healthy premenopausal women. Cancer Epidemiol Biomarkers Prev 10:377–384
Deal C, Ma J, Wilkin F et al (2001) Novel promoter polymorphism in insulin-like growth factor-binding protein-3: correlation with serum levels and interaction with known regulators. J Clin Endocrinol Metab 86:1274–1280
Bianchi A, Giustina A, Cimino V et al (2009) Influence of growth hormone receptor d3 and full-length isoforms on biochemical treatment outcomes in acromegaly. J Clin Endocrinol Metab 94:2015–2022
Bianchi A, Mazziotti G, Tilaro L et al (2009) Growth hormone receptor polymorphism and the effects of pegvisomant in acromegaly. Pituitary 12:196–199
Kamenicky P, Dos Santos C, Espinosa C et al (2009) D3 GH receptor polymorphism is not associated with IGF1 levels in untreated acromegaly. Eur J Endocrinol 161:231–235
Montefusco L, Filopanti M, Ronchi CL et al (2010) d3-Growth hormone receptor polymorphism in acromegaly: effects on metabolic phenotype. Clin Endocrinol (Oxf) 72:661–667
Bernabeu I, Alvarez-Escolá C, Quinteiro C et al (2010) The exon 3-deleted growth hormone receptor is associated with better response to pegvisomant therapy in acromegaly. J Clin Endocrinol Metab 95:222–229
Filopanti M, Olgiati L, Mantovani G et al (2012) Growth hormone receptor variants and response to pegvisomant in monotherapy or in combination with somatostatin analogs in acromegalic patients: a multicenter study. J Clin Endocrinol Metab 97:E165–E172
Wassenaar MJ, Biermasz NR, Pereira AM et al (2009) The exon-3 deleted growth hormone receptor polymorphism predisposes to long-term complications of acromegaly. J Clin Endocrinol Metab 94:4671–4678
Turgut S, Akın F, Ayada C, Topsakal S, Yerlikaya E, Turgut G (2012) The growth hormone receptor polymorphism in patients with acromegaly: relationship to BMI and glucose metabolism. Pituitary 15:374–379
Akin F, Turgut S, Cirak B, Kursunluoglu R (2010) IGF(CA)19 and IGFBP-3-202A/C gene polymorphism in patients with acromegaly. Growth Horm IGF Res 20:399–403
Acknowledgments
We thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support. This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP (Grant Number 2010/11718-1).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jallad, R.S., Trarbach, E.B., Duarte, F.H. et al. Influence of growth hormone receptor (GHR) exon 3 and -202A/C IGFBP-3 genetic polymorphisms on clinical and biochemical features and therapeutic outcome of patients with acromegaly. Pituitary 18, 666–673 (2015). https://doi.org/10.1007/s11102-014-0629-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0629-y