Abstract
Cushing’s Syndrome (CS) is associated with an increased mortality, where hypercoagulability seems to have a crucial role in both arterial and venous thrombosis. Parameters of in vitro thrombin generation (TG) such as lag time, peak thrombin and endogenous thrombin potential (ETP), that describe the time until thrombin burst, the peak amount of TG and the total amount of thrombin generated, respectively as well as classical clotting markers were evaluated in 33 CS patients compared to both a group of 28 patients matched for the features of Metabolic Syndrome (MetS) and 31 healthy individuals. CS and MetS patients had shorter lag time (p < 0.0001), higher peak and ETP (p < 0.0001) than healthy controls, though lag time was less shortened in CS (p < 0.0001) respect to MetS group. Prothrombin time (PT) was increased (p < 0.0001) in both CS and MetS patients, while partial thromboplastin time (PTT) was shorter (p < 0.0001) in CS compared to both MetS and healthy group (p < 0.0001). Factor VIII (FVIII), Antithrombin (AT), protein C and S were increased only in CS patients (p < 0.0001). lag time, AT and FVIII correlated to night salivary cortisol (r = + 0.59; p = 0.0005, r = + 0.40; p = 0.003, r = + 0.40; p = 0.04, respectively); PTT correlated inversely to urinary free cortisol (r = −0.45; p = 0.009). BMI correlated negatively to lag time (r = −0.40; p = 0.0001) and positively to peak and ETP (r = + 0.34; p = 0.001, r = + 0.28; p = 0.008, respectively). Obese and diabetic patients had shorter lag time (p = 0.0005; p = 0.0002, respectively), higher ETP (p = 0.0006; p = 0.007, respectively) and peak (p = 0.0003; p = 0.0005, respectively) as well as a more prolonged PT (p = 0.04; p = 0.009, respectively). Hypertensive individuals had higher ETP (p = 0.004), peak (p = 0.0008) and FVIII (p = 0.001). Our findings confirm a prothrombotic state in both CS and MetS patients, though lag time was less shortened in CS. The high levels of endogenous physiological anticoagulants, could possibly represent a protective mechanism against hypercoagulability seen in CS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoid excess is thought to have both direct and indirect effects on vasculature and haemostatic system, the latter due to the long-standing metabolic disorders, potentiating each other and by so contributing to the high risk of arterial and venous thrombosis seen in CS [1–7]. In several recent studies various abnormalities of coagulation have been proposed, most of them focused on determination of a single haemostatic and/or fibrinolytic marker. A few papers have considered the activation of coagulation in vivo [8–10].
Another approach is the analysis of in vitro thrombin generation (TG) which is considered a test of global haemostatic capacity and can reflect both thrombotic (high TG) and haemorrhagic (low TG) phenotype, therefore indicating both the increased risk of thrombosis and bleeding [11–14].
Thrombin is a key enzyme in the coagulation process and leads to the conversion of fibrinogen to fibrin and clot formation. The thrombin activity can be measured in plasma by continuous cleavage of a chromogenic or fluorescent substrate, and can be registered in a thrombin generation curve. From this curve, various parameters can be considered that describe thrombin generation, including the time until thrombin burst (lag phase), the peak amount of thrombin generation (peak thrombin) and the total amount of thrombin generated [area under the curve (AUC), representing the endogenous thrombin potential (ETP)] [15].
To the best of our knowledge TG has rarely been used to study CS. We measured in vitro TG in a group of CS patients compared to both a group of patients matched for the features of Metabolic Syndrome (MetS) and healthy individuals. Single coagulation parameters and conventional clotting tests as well as their possible association with hormonal or metabolic features, if any, has been also reviewed.
Materials and methods
Patients
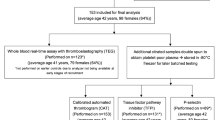
Thirty-three CS patients (24 females and 9 males, aged 52 ± 13 years) referred to our center from 2006 to 2011, had been evaluated: 24 had pituitary-dependent CS, 5 had adrenal-dependent CS, and 4 had CS caused by ectopic ACTH secretion sustained by well-differentiated neuroendocrine tumors with no cellular atypia or signs of invasion of contiguous structures as confirmed by the histological exam obtained during surgical treatment. At the time of entry, assumed as the time of coagulation assessment, all CS patients had active disease based on specific clinical signs and hormonal criteria [1]. Median (range) duration of CS was 24 (8–144) months, assumed as the time between the onset of signs/symptoms and the time of entry into the study.
CS was diagnosed on the basis of its characteristic clinical presentation (moon face, plethora, buffalo hump, truncal obesity with thin limbs and proximal miopathy or purple striae) and standard hormone test results, e.g. increased UFC (mean of 2 samples), loss of plasma cortisol diurnal rhythm (morning and night samples of salivary cortisol were also obtained) as well as failure of plasma cortisol suppression after a low-dose (1 mg) dexamethasone challenge [1]. Measure of ACTH levels, high-dose dexamethasone suppression test and CRH stimulation test, as well as pituitary MRI and chest-abdomen CT, were used to ascertain the origin of the patient’s hypercortisolism. Inferior petrosal sinus catheterization with basal and CRH-stimulated blood sampling was performed in selected cases.
A control group of twenty-eight outpatients (14 females and 14 males, aged 57 ± 14 years) was matched with CS patients for the features of Metabolic Syndrome (MetS patients). In addition, each metabolic feature such as obesity, hypertension, diabetes and dyslipidemia, was present in at least 50 % of MetS group. In five of them high suspicion of hypercortisolism based on clinical signs has been excluded by 1 mg dexamethasone suppression test and UFC determinations.
Moreover a second control group, consisted of thirty-one apparently healthy subjects (25 females and 6 males, aged 54 ± 18 years), was used for comparison.
An informed consent was collected from all participants and the investigation was performed in accordance with the principles of the Declaration of Helsinki.
Anthropometric parameters such as body mass index (BMI), systolic (SBP) and diastolic blood pressure (DBP) as well biochemical tests including fasting blood glucose (FBG), total cholesterol (total-C), low-density lipoproteins (LDL), high-density lipoproteins (HDL) and triglycerides (TG), after overnight fasting, were considered in both CS and controls.
Obesity, Hypertension, Diabetes Mellitus and Dyslipidemia were defined respectively, as follows: BMI >30 kg/m2; SBP of 140 mm Hg or higher and/or DBP of 90 mm Hg or higher and/or current treatment with blood pressure medications; FBG levels greater of 126 mg/dl and/or current antidiabetic drugs or insulin; HDL <50 mg/dl (F) or <40 mg/dl (M) and/or TG >150 mg/dl and/or LDL >100 mg/dl and/or current treatment for lipid disorder. Metabolic Syndrome was defined by the presence of at least three of the above criteria.
Thrombin generation by Calibrated Automated Thrombogram (CAT) method, classical clotting parameters such as Prothrombin Time (PT), Partial Thromboplastin Time (PTT), Fibrinogen, Antithrombin activity (AT), Protein C coagulometric activity (PC), Protein S coagulometric activity (PS) as well plasma Factor VIII (FVIII) were performed in all participants.
None of the CS patients and controls was on estrogen therapy or oral anticoagulants while, in two hospitalized CS patients who were receiving thromboprophylaxis (enoxaparin 4000U once daily) cause at high risk for venous thromboembolism (VTE), LMWH was administered in the morning after blood samples were drawn. VTE not related to surgery occurred in five CS patients, in three of them after 6, 12 and 24 months since enrollment at the study, while in the other two patients such event took place 8–12 months before diagnosis of CS. Four patients suffered from deep vein thrombosis (DVT) and one from pulmonary embolism (PE), all confirmed by objective methods, namely venous compression ultrasound and pulmonary angio-CT, respectively. No control subject had a positive personal or family history of clotting disorders (VTE or bleeding tendency); neither CS patients nor controls had a history of malignancy. Finally, a sudden death occurred to one CS patient due to acute myocardial infarction, confirmed at autopsy.
Laboratory tests
Endocrine analysis
Plasma cortisol and UFC were evaluated by RIA using commercial kits (IMMULITE 2000 Systems, Siemens Healthcare Diagnostics Products Ltd. UK); salivary cortisol was determined by RIA method with an in-house kit (RADIM SpA, Roma- Italia); plasma ACTH levels were measured by IRMA (Nichols Institute Diagnostics, San Juan Capistrano, CA, USA). Total-C, LDL and TG analysis were carried out on an automated analyzer (DAX, Bayer, USA) using commercial reagents (Randox Laboratories, Co. Antrim, N. Ireland), while HDL was measured without a precipitation step (Wako Corp., Japan).
Thrombin generation assay
TG was determined in Platelet Poor Plasma (PPP) by means of CAT method. This assay employs a low-affinity fluorogenic substrate for thrombin. The assay was performed as follows: 80 μl of PPP were pipetted into the well of a microtiter plate together with 20 μl of PPP-Reagent (Thrombinoscope BV, Maastricht, The Netherlands), a mixture of Tissue Factor (TF) (5 pM final concentration in well) and synthetic phospholipids (4 μM final concentration in well). The reaction was started with 20 μl of a mixture composed of a fluorogenic substrate (Z-Gly-Gly-Arg-AMC, Thrombinoscope BV, Maastricht, The Netherlands) and CaCl2 (final concentration 16 mM CaCl2 and 417 μM fluorogenic substrate). Fluorescence was read in a Fluoroscan Ascent® reader (Thermo Labsystems, Helsinki, Finland) and TG curves were calculated using the Thrombinoscope Software (Thrombinoscope BV, Maastricht, The Netherlands). A Thrombin Calibrator (Thrombinoscope BV, Maastricht, The Netherlands) was used in order to compare the measurements of fluorescence for each PPP. Using the Thrombin calibrator as a reference, the concentration of thrombin was calculated at any point during the measurement. TG curves were described in terms of lag time (min), C max (peak, nM) and area under the curve (ETP, nM·min), where shortened lag-times and elevated values of both peak thrombin and ETP are indexes of hypercoagulability.
Coagulation parameters
PT and PTT were measured by conventional clotting methods with Tromborel S and Cephaloplastin, respectively (Dade, Marburg, Germany). FVIII activity was measured using a clotting method on BCT with FVIII-deficient plasma (Siemens Healthcare Diagnostic Products, Marburg, Germany). Fibrinogen was carried out by the Clauss clotting time method using Bovine Thrombin (Biopool, Umeå, Sweden). AT activity, PC and PS coagulometric activity were determined using commercially-available kits (Antithrombin, Boehringer Mannheim, Germany; Berichrom PC, Behring, Scoppito, AQ, Italy; IL Protein S test, IL, Milan, Italy, respectively) [16–19].
Statistical analysis
Quantitative variables are summarized as median, minimum and maximum while categorical ones as percentages. The groups of patients were compared with Fisher‘s exact test for categorical variables and Kruskall-Wallis test in case of quantitative variables. In presence of a statistically significant result at the 5 % level, pairwise comparisons were performed by Fisher’s exact test and the p values were adjusted with the Bonferroni’s method to take into the account the multiplicity of the comparisons for the categorical variables, and with the Dunn method for the quantitative variables. Coagulation parameters were compared between obese and non obese, hypertensive and non hypertensive as well as diabetic and non diabetic patients with Wilcoxon rank sum test. Association between coagulation parameters and hormonal data and between BMI and TG data (lag time, peak, ETP) was expressed as Pearson correlation and p value. Statistical analysis was performed using SAS 9.2 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
The demographic and clinical characteristics of three groups are exposed in Table 1. There was no significant difference in age and gender distribution between CS patients and control groups, however MetS patients were slightly older and there were more males. BMI and the prevalence of obesity, hypertension, diabetes as well as Metabolic Syndrome were higher in both CS and MetS patients compared to healthy controls. More MetS patients tended to be diabetic as well as obese and with a higher BMI, while CS patients were more hypertensive. Hormonal features of CS patients are reported in Table 2.
Analysis of TG (Table 3) showed that both CS and MetS patients had shorter lag time and higher ETP as well as peak values than healthy controls (p = <0.0001). Lag-time was less shortened in CS patients respect to MetS group (p = <0.0001), though ETP and peak did not differ significantly. Similarly, PT was longer in both CS and MetS patients compared to healthy individuals (p = <0.0001). The PTT was significantly shortened in CS compared to healthy individuals (p = <0.0001) as well as between CS and MetS patients (p = <0.0001). Comparison of single haemostatic markers showed a significant increase of FVIII (p = <0.0001) but not of fibrinogen between three groups. Endogenous anticoagulants (AT, PS and PC) were significantly higher in CS patients compared to both healthy controls (p < 0.0001) and MetS patients (p = <0.0001), though there was no difference between MetS patients and healthy individuals (Table 3).
Both lag time and FVIII showed a significant correlation with night salivary cortisol (r = + 0.59; p = 0.0005, r = + 0.40; p = 0.04, respectively), while PTT correlated inversely to UFC (r = −0.45; p = 0.009). AT correlated positively to both night plasma and salivary cortisol (r = + 0.55; p = 0.002, r = + 0.40; p = 0.003, respectively). Finally, BMI correlated negatively to lag time (r = -0.40; p = 0.0001) and positively, thus in a weaker manner, to peak and ETP (r = + 0.34; p = 0.001, r = + 0.28; p = 0.008, respectively). No other correlations were found.
In Table 4 are reported those coagulation parameters that resulted significant when participants were stratified according to the presence of obesity, hypertension and diabetes.
Discussion
In CS patients, the fine-tuned balance between coagulation and fibrinolysis is markedly disturbed versus a prothrombotic state causing a five-fold increase in cardiovascular mortality and more than 10-fold risk of VTE [8, 20]. Measurement of an individual’s capacity to generate thrombin captures the end result of the interaction between proteases and their inhibitors and is therefore more useful as a reflection of a thrombotic (high TG) or hemorrhagic (low TG) phenotype compared to conventional coagulation tests [11–14]. From a clinical perspective it would be worthwhile having a laboratory test that predicts the risk of thrombosis or even bleeding in an individual CS patient due to their cardiovascular risk.
In Both CS and MetS patients, TG values increased earlier and to a higher level compared to healthy individuals confirming a prothrombotic tendency. All parameters of TG correlated to BMI and were significantly higher in obese and diabetic patients with respect to non-obese and non-diabetic ones. ETP and peak height differed significantly between hypertensive and normotensive patients, although no difference was seen in the lag time. Fritsch et al. [21] found a strong correlation between BMI and all parameters of TG in overweight children. The authors concluded that obesity per se rather than insulin resistance/occurrence of the Metabolic Syndrome is associated to the alterations in the coagulation system. On the contrary, some studies reported an association between increased thrombin generation and hyperinsulinemia/diabetes [15, 22, 23]. Increased thrombin-antithrombin (TAT) complexes are reported in CS patients where in one study correlated to BMI [9] and in another were associated to hypertension [10]. In our study dyslipidemia and hypertension were present in 25 and 20 % of healthy controls, respectively which could have been a short of overestimation since no pathological coagulation results emerged among healthy individuals.
Despite the prothrombotic state seen in CS and MetS group, the former had a less shortened lag time and significantly increased AT levels both correlated positively to hormonal parameters. Since AT is a determinant of thrombin generation, it is likely that in CS the increase in AT levels may represent a protective mechanism toward thrombosis. In agreement with our observation, other authors reported increased AT levels in hypercortisolic patients [9, 24–26] and a protective or compensating mechanism against hypercoagulability was proposed [24]. In the present paper it would appear a protective rather than a compensating mechanism since lag time is prolonged under the influence of high—cortisol induced—AT levels. It is well known that thrombin generation is counterbalanced by stoichiometric inhibitors: tissue factor pathway inhibitor (TFPI), AT and by a dynamic anticoagulant process of PC activation and functional expression (PC-thrombomodulin-thrombin APC system), where TFPI is the principal regulator of the initiation phase, AT serves mostly to attenuate thrombin activity and its generation, while PC activated system quenches the propagation phase [27]. However, some questions arise on the role of TFPI. There is only one study in hypercortisolic patients that reports decreased levels of TFPI and prothrombotic state [24]. Previously, Smid et al. [28] reported an increased secretion of TFPI and consequently a slower and lower rate of thrombin formation in patients with unstable atherosclerosis. In our study all participants were clinically stable, although in CS patients silent unstable atherosclerosis cannot be excluded as this is often an unpredictable outcome of the disease. In the present study CS patients were slightly less obese than those in MetS group and the lag time correlated inversely to BMI. This differs from other publications where a positive correlation between TFPI and BMI was found in obese patients [29]. As it has been previously reported [9, 25], we also found increased protein S and C levels in CS patients. Since thrombomodulin was not used in the TG assay we performed, it is unlikely that these proteins could have any influence on our results.
PT was slightly prolonged in both CS and MetS patients compared to healthy individuals, fitting with the results from the TG assay. Although the difference in the lag phase between hypercortisolic and MetS patients was not seen in the PT values. This would be expected as the influence of inhibitors would be better demonstrated using small amounts of tissue factor (TF) as trigger (as used in TG) compared with the much higher concentrations used in the PT assays, masking the effect of inhibitors [30]. In line with our findings, increased PT values were reported in two previous studies in CS patients that also showed no correlation with hormonal parameters [24, 31]. In another paper [32], diabetic and obese patients were shown to have a prolonged PT compared to non-diabetic and non-obese ones, suggesting that in CS this clotting index is rather associated to metabolic disorders than to hypercortisolism.
The slightly shortened PTT seen in CS patients compared to healthy individuals, and between CS and MetS patients probably reflects significantly higher levels of FVIII found in our study. Casonato et al. [33] reported a significant correlation between PTT and FVIII in CS patients. Additionally, as described elsewhere, a correlation of both PTT and FVIII with hormonal parameters has been observed [24, 31]. Recent data in general population report a linear relationship between FVIII level and risk of thrombosis where for FVIII levels ≥150 % there is a 4-fold increased risk of VTE [34, 35]. In CS this VTE risk is thought to be even higher [20, 25, 36]. We believe that the present finding of a prevalence of 15 % VTE is higher than previously reported [36] due to our present inclusion of only those CS patients in whom TG analysis was performed. In the light of the small number of CS patients, no further evaluation was made between patients with VTE and those without such event. On the other hand, TG analysis between patients with ectopic ACTH-secretion CS and those with either pituitary- or adrenal- dependent CS did not differ significantly (data not shown).
One limit of our study is the small number of participants. Nevertheless, the small (less than 10 % for all read-out parameters) intra- and inter-assay variabilities observed makes our data reproducible and consistent with that previously reported in the literature [37]. Other limits are that TFPI was not determined and the lack of previous papers focused on in vitro TG in CS which makes difficult the interpretation of obtained results.
In summary we confirm that in CS a prothrombotic state exists not only on the basis of single coagulation markers and conventional clotting tests but also of TG. Hypercoagulability is also present in patients with MetS, explaining in part the deleterious effects on vasculature and haemostatic system of long-standing metabolic disorders that persist in CS even when remission is achieved. In CS the effect of endogenous physiologic anticoagulants on the initiation phase of TG would reflect a kind of protective mechanism apparently not able to overcome the global prothrombotic state. This finding is rather hypothesis generating that do not allow us do draw definite conclusion and thus we can say that classical clotting indices still remain relevant in the daily clinical practice until further studies on in vitro TG are not available [38]. In CS, thromboprophylaxis should be considered when concomitant prothrombotic risk factors are present, although whether the risk of a latent hypocoagulable state exists must be further investigated.
References
Arnaldi G, Agneli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s Syndrome: a consensus statement. J Clin Endocrinol Metab 88:5593–5602
Extabe J, Vasquez JA (1994) Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol 40:479–484
Lindholm J, Juul S, Jorgensen JOL, Astrup J, Bjerre P, Feldt Rasmussen U, Hagen C, Jorgensen J, Kosteljanetz M, Kristensen LO, Laurberg P, Schmidt K, Weeke J (2001) Incidence and late prognosis of Cushing’s Syndrome: a population based study. J Clin Endocrinol Metab 86:117–123
Dekkers OM, Biermasz NR, Pereira AM, Roelfsema F, Van Aken MO, Voormolen JHC, Romijn JA (2007) Mortality in patients treated for Cushing’s disease is increased compared with patients treated for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab 92:976–981
Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, Marzullo P, Cerbone G, Siciliani M, Lombardi G (1999) Persistence of increased cardiovascular risk in patients with Cushing’s disease after five years of successful cure. J Clin Endocrinol Metab 84:2664–2672
Clayton RN, Raskauskiene D, Reulen RC, Jones PW (2011) Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK. J Clin Endocrinol Metab 96:632–642
Bolland MJ, Holdaway IM, Berkeley JE, Lim S, Dransfield WJ, Conaglen JV, Croxson MS, Gamble GD, Hunt PJ, Toomath RJ (2011) Mortality and morbidity in Cushing’s Syndrome in New Zealand. Clin Endocrinol 75:436–442
Miljic P, Miljic D, Cain JW, Korbonits M, Popovic V (2012) Pathogenesis of vascular complications in Cushing’s Syndrome. Review. Hormones 11:21–30
Manetti L, Bogazzi F, Giovannetti C, Raffaelli V, Genovesi M, Pellegrini G, Ruocco L, Iannelli A, Martino E (2010) Changes of coagulation indexes and occurrence of venous thromboembolism in patients with Cushing’s Syndrome: results from a retrospective study before and after surgery. Euro J Endocrinol 163:783–791
Fatti LM, Bottasso B, Invitti C, Coppola R, Cavanigni F, Mannucci PM (2000) Markers of activation of coagulation and fibrinolysis in patients with Cushing’s Syndrome. J Endocrinol Invest 23:145–150
Hemker HC, Beguin S (1995) Thrombin generation in plasma: its assessment via the endogenous thrombin potential. Thromb Haemost 74:134–138
Kyrle PA, Mannhalter C, Béguin S, Stumpflen A, Hirschl M, Weltermann A, Stain M, Brenner B, Pabinger K, Eichinger S (1998) Clinical studies and thrombin generation in patients homozygous or heterozygous for the G20210A mutation in the prothrombin gene. Arterioscl Thromb Vasc Biol 18:1287–1291
Siegemund T, Petros S, Siegemund A, Scholz U, Engelmann L (2003) Thrombin generation in severe haemophilia A and B: the endogenous thrombin potential in platelet-rich plasma. Thromb Haemost 90:781–786
Kakkar VV, Hoppenstead DA, Fareed J, Kadziola Z, Scully M, Nakov R, Breddin HK (2002) Randomized trial of different regimens of heparins and in vivo thrombin generation in acute deep vein thrombosis. Blood 99:1965–1970
Ay L, Kopp HP, Brix JM, Ay C, Quehenberger P, Schernthaner GH, Pabinger I, Schernthaner G (2010) Thrombin generation in morbid obesity: significant reduction after weight loss. J Thromb Haemost 8:759–765
Casonato A, Pontara E, Sartorello F, Cattini MG, Sartori MT, Padrini R, Girolami A (2002) Reduced von Willebrand factor survival in type vicenza von Willebrand disease. Blood 99:180–184
Sartori MT, Danesin C, Saggiorato G, Tormene D, Simioni P, Spiezia L, Patrassi GM, Girolami A (2003) The PAI-I gene 4G/5G polymorphism and deep vein thrombosis in patients with inherited thrombophilia. Clin Appl Thromb Hemost 9:299–307
Simioni P (1999) The molecular genetics of familial venous thrombosis. Baillieres Best Pract Res Clin Haematol 12:479–503
Rossetto V, Spiezia L, Franz F, Salmaso L, Pozza LV, Gavasso S, Simioni P (2009) The role of antiphospholipid antibodies toward the protein C/protein S system in venous thromboembolic disease. Am J Hematol 84:594–596
Stuijver DJF, Van Zaane B, Feelders RA, Debeij J, Cannegieter SC, Hermus AR, Van den Berg G, Pereira AM, De Herder WW, Wagenmakers MA, Kerstens MN, Zelissen PM, Fliers E, Schaper N, Drent ML, Dekkers OM, Gerdes VE (2011) Incidence of venous thromboembolism in patients with Cushing’s Syndrome: a multicenter cohort study. J Clin Endocrinol Metab 96:3525–3532
Fritsch P, Kleber M, Rosenkranz A, Fritsch M, Muntean W, Mangge H, Reinehr T (2010) Haemostatic alterations in overweight children: associations between Metabolic Syndrome, thrombin generation, and fibrinogen levels. Atherosclerosis 212:650–655
Ay L, Hoellerl F, Ay C, Brix JM, Koder S, Schernthaner GH, Pabinger I, Schernthaner G (2012) Thrombin generation in type 2 diabetes with albuminuria and macrovascular disease. Euro J Clin Invest 42:470–477
Tripodi A, Branchi A, Chantarangkul V, Clerici M, Merati G, Artoni A, Mannucci PM (2011) Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis 31:165–172
Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, Onder Ersoz H (2009) Blood coagulation and fibrinolysis in patients with Cushing’s Syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest 32:169–174
Van der Pas R, De Bruin C, Leebeek FWG, De Maat MPM, Rijken DC, Pereira AM, Romijn JA, Netea-Maier RT, Hermus AR, Zelissen PMJ, De Jong FH, Van der Lely AJ, De Herder WW, Lamberts SWJ, Hofland LJ, Feelders RA (2012) The hypercoagulable state in Cushing’s disease is associated with increased levels of procoagulant factors and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab 97:1303–1310
Kastelan D, Dusek T, Kraljevic I, Polasek O, Giljevic Z, Solak M, Salek SZ, Jelcic J, Korsic M (2009) Hypercoagulability in Cushing’s Syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine 36:70–74
Mann KG, Butenas S, Brummel K (2003) The dynamics of thrombin formation. Arterioscl Thromb Vasc Biol 23:17–25
Smid M, Dielis AW, Winkens M, Spronk HM, Van Oerle R, Hamulyak K, Prins MH, Rosing J, Waltenberger JL, Ten Cate H (2011) Thrombin generation in patients with a first acute myocardial infarction. J Thromb Haemost 9:450–456
Vambergue A, Rugeri L, Gaveriaux V, Devos P, Martin A, Fermon C, Fontaine P, Jude B (2001) Factor VII, tissue factor pathway inhibitor, and monocyte tissue factor in diabetes mellitus: influence of type of diabetes, obesity index, and age. Thromb Res 101:367–375
Cvirn G, Gallistl S, Leschnik B, Muntean W (2003) Low tissue factor pathway inhibitor (TFPI) together with low antithrombin allows sufficient thrombin generation in neonates. J Thromb and Haemost 1:263–268
Boscaro M, Sonino N, Scarda A, Barzon L, Fallo F, Sartori MT, Patrassi GM, Girolami A (2002) Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing’s Syndrome. J Clin Endocrinol Metab 87:3662–3666
Sauls DL, Banini AE, Boyd LC, Hoffman M (2007) Elevated prothrombin level and shortened clotting times in subjects with type 2 diabetes. J Thromb Haemost 5:638–639
Casonato A, Pontara E, Boscaro M, Sonino N, Sartorello F, Ferasin S, Girolami A (1999) Abnormalities in von Willebrand factor are also part of the prothrombotic state of Cushing’s Syndrome. Blood Coagul Fibrinolysis 10:145–151
Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR (1995) Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 345:152–155
Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P (2004) High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol 124:504–510
Koutroumpi S, Daidone V, Sartori MT, Cattini MG, Albiger NM, Occhi G, Ferasin S, Frigo A, Mantero F, Casonato A, Scaroni C (2012) Venous thromboembolism in patients with Cushing’s Syndrome: need of a careful investigation of the prothrombotic risk profile. Pituitary. doi:10.1007/s11102-012-0398-4
Spronk HM, Dielis AW, De Smedt E, Van Oerle R, Fens D, Prins MH, Hamulyák K, Ten Cate H (2008) Assessment of thrombin generation II: validation of the Calibrated Automated Thrombogram in platelet-poor plasma in a clinical laboratory. Thromb Haemost 100(2):362–364
Castoldi E, Rosing J (2011) Thrombin generation tests. Thromb Res 127:S21–S25
Acknowledgments
We thank Professor Franco Mantero (University of Padova) for his continuous encouragement and advice as well as Doctor Barry Woodhams for his critical reading of the manuscript.
Conflict of interest
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Koutroumpi and L. Spiezia equally contributed to this work.
Rights and permissions
About this article
Cite this article
Koutroumpi, S., Spiezia, L., Albiger, N. et al. Thrombin generation in Cushing’s Syndrome: do the conventional clotting indices tell the whole truth?. Pituitary 17, 68–75 (2014). https://doi.org/10.1007/s11102-013-0467-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-013-0467-3