Abstract
Ectopic acromegaly represents less than 1% of the reported cases of acromegaly. Although clinical improvement is common after treatment with somatostatin (SMS) analogs, the biochemical response and tumor size of the growth hormone-releasing hormone (GHRH)-producing tumor and its metastases are less predictable. Subject A 36-year-old male was referred because of a 3-year history of acromegaly related symptoms. He had undergone lung surgery in 1987 for a “benign” carcinoid tumor. Endocrine evaluation confirmed acromegaly Plasma IGF-1: 984 ng/ml (63–380), GH: 49.8 ng/ml (<5). MRI showed a large mass in the left cerebellopontine angle and diffuse pituitary hyperplasia. Pulmonary, liver and bone metastases were shown by chest and abdominal CT scans. Ectopic GHRH secretion was suspected. Methods Measurement of circulating GHRH levels by fluorescence immunoassay levels and immunohistochemical study of the primary lung tumor and metastatic tissue with anti-GHRH and anti-somatostatin receptor type 2 (sst2A) antibodies. Results Basal plasma GHRH: 4654 pg/ml (<100). Pathological study of liver and bone biopsy material and lung tissue removed 19 years earlier was consistent with an atypical carcinoid producing GHRH and exhibiting sst2A receptor expression. Treatment with octreotide LAR 20–40 mg q. month resulted in normalization of plasma IGF-1 levels. Circulating GHRH levels decreased dramatically. The size of the left prepontine cistern mass, with SMS receptors shown by a radiolabeled pentetreotide scan, decreased by 80% after 18 months of therapy. Total regression of pituitary enlargement was also observed. No changes were observed in lung and liver metastases. After 24 months of therapy the patient is asymptomatic and living a full and active life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ectopic secretion of growth hormone-releasing hormone (GHRH) is responsible for less than 1% of the reported cases of acromegaly. Bronchial and pancreatic carcinoid tumors are the most frequent sources of ectopic secretion of GHRH [1, 2]. Less frequently, pancreatic islet tumors, pheochromocytomas, medullary carcinomas of the thyroid, and small cell carcinomas of the lung and the thymus, among others, have been reported to cause acromegaly due to GHRH secretion [3–5]. Surgical resection of the primary tumor can cure the disease [6]; however, the diagnosis is frequently not established until the disease is metastatic, and therefore surgical cure is not possible. Although experience is still limited (only 19 patients to our knowledge) [5, 7, 8], somatostatin (SMS) analogs have been used in the treatment of ectopic acromegaly since they may not only control the endocrine hypersecretion but also tumor proliferation. Despite the favorable clinical improvement documented for most of the patients, the significant decrease in GH and insulin-like growth factor 1 (IGF-1) [9–12] and shrinkage of the previously hyperplastic pituitary gland [7, 12], the response of the GHRH-producing tumor and its metastases is less predictable and tumor size can increase despite suppression of the GH axis [5–9].
We describe a patient with ectopic acromegaly secondary to a metastatic bronchial carcinoid tumor with multiple metastases, pituitary hyperplasia and a large extrapituitary intracranial mass in the left cerebellopontine angle cistern with SMS receptors shown by a radiolabeled octreotide scan, likely representing another metastasis from the carcinoid tumor. The size of the pituitary and the left intracranial mass decreased dramatically after 3 months of treatment with long acting octreotide. We also describe the clinical and biochemical evolution of the patient during 2 years of treatment with long acting octreotide.
Subjects and methods
Clinical presentation
A 36-year-old male was referred to the Endocrinology Department at the Buenos Aires Italian Hospital because of acromegaly. The patient had a 3-year history of hyperhidrosis, flushing, headaches and weakness. These symptoms had been recently accompanied by severe arthalgias that affected ambulation. He had undergone lung surgery in 1987 for a “benign” carcinoid tumor which was believed to have been totally removed. No post-operative follow-up was available. The patient had no history of hyperglycemia or hypertension.
In addition to the typical acral and facial changes of acromegaly, physical examination revealed a husky voice, diminished hearing in the left ear, a diffuse goiter of approximately 40 g, and thickened and moist skin.
Biochemical examinations
Blood glucose level: 105 mg/dl, hematocrit: 43%, WBC count: 7,400/mm3, blood urea nitrogen: 36 mg/dl, serum creatinine: 1.1 mg/dl, serum Na+ 145 meq/l, serum K+ 4.3 meq/l, serum Ca++ 10.6 mg/dl (8.5–10.5), liver function test normal. IGF-1: 984 ng/ml (63–380), GH: 49.8 ng/ml (<5), PRL: 23.3 ng/ml (5–20), testosterone: 3.6 ng/ml (3–12), LH: 2.0 mIU/ml (2–8), FSH: 3.9 mIU/ml (2–8), mid-molecule PTH: 31.2 pg/ml (≤125), gastrin: 29 pg/ml (≤90), 5-hydroxyindoleacetic acid: 9 mg/24 h (≤6), serum chromogranin A: 20 nmol/l (<4).
Imaging studies
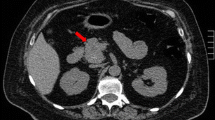
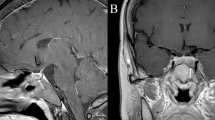
Brain magnetic resonance imaging (MRI) showed a mass of 4.4 cm × 3.8 cm × 3.0 cm (volume 27.1 cc) in the left cerebellopontine angle and prepontine cistern that caused a significant displacement of the cerebellar fissure including the brainstem, and diffuse enlargement of the pituitary gland (Fig. 1A). Chest CT scan revealed a nodular mass of 12 mm in the right parahilar region and a smaller mass of 2 mm in the right upper lobe, with no enlarged mediastinal nodes. Abdominal CT scan showed an enlarged liver and multiple heterogeneous lesions of 5–75 mm in diameter compatible with metastatic disease (Fig. 2A). Multiple osteolytic and osteoblastic images were observed in the axial skeleton, a left rib and the right ilium. The largest lesion was found in L2, with an approximately 40% impigement of the medullary canal.
Based on the presumptive diagnosis of acromegaly secondary to ectopic GHRH secretion, we determined the GHRH plasma concentration, performed pathological and immunohistochemical studies on aspiration of the liver and bone metastases and on the lung tumor tissue surgically removed 19 years previously and performed a radiolabeled octreotide scan.
The patient was subsequently treated with long- acting octreotide 20 mg q. month.
Methods
GHRH measurements
GHRH plasma concentrations were measured by fluorescence immunoassay (FIA) as previously described in detail [13]. In brief, ether isopropanol extracted plasma samples were incubated for 3 days in microtiter plates coated with a polyclonal rabbit-anti-GHRH antiserum. Thereafter, biotinylated GRF 1-44 (Peninsula, San Carlos, CA) was added as a tracer for 24 h. Plates were measured using a DELFIA 1232 fluorometer (Wallac, Turku, Finnland) after addition of streptavidin-europium. Sensitivity was 100 pg/ml, with an intraassay CV of <11% and an interassay CV of <15%.
Histopathologic analysis
Routinely processed paraffin sections were used for hematoxylin–eosin staining. Immunocytochemistry was performed using the streptavidin–biotin–peroxidase complex method. Immunostaining was performed with antibodies to chromogranin, synaptophysin, GH and GHRH, ki67 and sst2A. A characterization of the GHRH antibody has been previously published [14]. Ki67 nuclear antigen immunostaining was performed to evaluate the proliferation rate of tumor cells. Immunohistochemistry for sst2A was performed on sections of primary lung and liver tumors, using a sst2A-specific antibody R2-88 (kindly provided by Dr. A. Schonbrunn, Houston, USA) as well as the commercially available SS-800 (Gramsch, Germany) as previously reported [15].
SMS receptor scintigraphy
Technetium-99 (99mTc) labeled pentetreotide was used for whole body SMS receptor scintigraphy and single photon emission computed tomography (octreo-SPECT) was used to study SMS receptors in the brain image observed on MRI.
Medication
Treatment with 20 mg of long acting octreotide was started after ectopic acromegaly secondary to GHRH-producing metastatic tumor was suspected. The dose of octreotide was titrated to mantain IGF-1 circulating levels in the normal range. After 15 months of treatment the dose was increased to 40 mg q. month.
Results
GHRH measurement
Basal plasma GHRH levels were found to be very high (4,654 pg/ml (<10)).
Pathological studies
The aspiration specimen of the liver lesion revealed atypical cell growth with monomorphic nuclei and homogeneous chromatin. Immunostaining was positive for chromogranin and synaptophysin, consistent with an atypical neuroendocrine carcinoid tumor. The immunohistochemical studies with anti-GHRH antibody were strongly positive (Fig. 3A), while GH antibody staining was negative. Similar results were found in pathological studies of the lumbar lesion (not shown). Interestingly, the pathological study of the lung tissue operated 19 years previously, showed it to be an atypical neuroendocrine GHRH-producing carcinoid tumor (Fig. 3B). Ki67 immunostaining was positive in less than 1% of the tumor cells. Sst2A immunostaining was identified in the majority of tumor cells with both antibodies. Immunostaining was intense in tumors from both sites. The distribution was both cytoplasmic and membranous. Immunostaining was virtually absent in control preabsortion experiments in the presence of peptide antigens (Fig. 4A, B).
Pentetreotide scan
Multiple areas of bone and soft tissue exhibited increased activity (Fig. 5A), including a left intracranial area that corresponded to the left prepontine cistern mass shown by MRI (Fig. 5B).
(A) Whole body somatostatin receptor scintigraphy with 99mTc-pentetreotide. Increased activity is present in multiple areas of soft and bone tissue: brain, neck, left temporomandibular joint, lungs, liver, lumbar rachis and pelvis. (B) 99mTc-pentetreotide SPECT shows increased activity in left and posterior area of the head (+) that corresponds to lesion in left cerebellopontine angle and prepontine cistern seen in MRI (→)
Clinical response and biochemical studies during octreotide treatment
Treatment with octreotide LAR 20 mg q. month was initiated which resulted in symptomatic improvement immediately after the first administration.
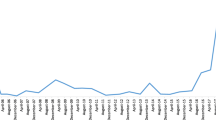
After 3 months of therapy, further regression of the acromegalic symptoms and normalization of serum GH, plasma IGF-1 (Fig. 6B, C) and urine 5-hydroyindoleacetic acid were observed. Although serum cromogranin A levels decreased significantly, they never reached normal values (nadir 8 nmol/l).
Following 15 months of octreotide LAR 20 mg, symptoms of acromegaly recurred, accompanied by an increase in circulating GH and IGF-1 levels. After increasing the dose to 40 mg, serum GH levels diminished significantly and IGF-1 concentrations were again normalized (Fig. 6B, C). Pretreatment plasma GHRH levels were 40 fold above normal values. Although circulating GHRH levels were greatly reduced by octreotide, they did not reach the normal range (Fig. 6A).
Imaging studies during octreotide treatment
The left prepontine cistern mass was suggestive of a chordoma, an invasive acoustic neuroma, or a metastasis. An MRI obtained after 3 months of therapy revealed a significant shrinkage of the pituitary gland and the cerebellopontine angle tumor (Fig. 1B) and further size reduction was observed with continued treatment. The tumor decreased by 80% of its original size after 18 months of therapy (Fig. 1C).
No changes were observed in the size and number of lung, bone and liver metastases (Fig. 2A, B, C).
Although asymptomatic, the vertebral metastasis was successfully treated with radiotherapy because of its infiltration into the medullary canal.
Based on the hormonal, radiologic and histopathologic findings, we confirmed the diagnosis of ectopic acromegaly secondary to a GHRH-secreting bronchial carcinoid tumor with lung, liver and bone metastases.
After 2 years of therapy with long acting octreotide, 20 mg initially and 40 mg subsequently, we observed normalization of IGF-1 levels, regression of the pituitary hyperplasia and a remarkable reduction of the left intracranial tumor, probably another carcinoid metastasis. Liver, bone and lung metastases remained unchanged. The patient is well and is living a full and active life.
Discussion
Acromegaly is caused in over 95% of the cases by a monoclonal GH-secreting pituitary tumor. Pituitary enlargement with the absence of a detectable tumor combined with a history of a pulmonary carcinoid tumor and acromegalic signs and symptoms, as is the case of our patient, are strongly suggestive of acromegaly secondary to ectopic GHRH hypersecretion. Our suspicions were enhanced by the presence of metastases seen on imaging studies. The diagnosis was later confirmed by the elevated plasma GHRH levels and positive GHRH immunoreactivity in tumor tissue.
The patient had undergone pulmonary carcinoid surgery 19 years earlier and never had follow-up evaluation. Carcinoid tumors of the lung are grouped with benign or less aggressive malignant pulmonary tumors and represent the most indolent form of a spectrum of bronchopulmonary neuroendocrine tumors that include small cell carcinoma of the lung as its most malignant member and several other forms of intermediately aggressive tumors, such as atypical carcinoids.
Typical carcinoid tumors characteristically grow slowly and tend to metastasize infrequently. Atypical carcinoid tumors have a more aggressive histologic and clinical picture. They metastasize at a considerably higher rate than do typical carcinoid tumors and, therefore, carry a worse prognosis [16]. Ki67, a molecular marker thoutht to stain only in those cells undergoing active division was low in both the primary lung tumor and peripheral metastases. Ki67 staining is usually low in typical and atypical carcinoid tumors versus small and large cells lung tumors and helps to better characterize neuroendocrine lung tumors [17]. In our patient, histologic review of the lung tissue surgically removed 19 years earlier as well as the histologic study of the metastases and the clinical outcome support the diagnosis of atypical carcinoid. Immunostaining was negative for GH and positive for GHRH, thus supporting the diagnosis of acromegaly due to ectopic secretion of GHRH.
The association of acromegaly and carcinoid tumors had also been recognized in some patients before the characterization of hypothalamic GHRH, whose structure was determined from pancreatic islet tumor tissue [18]. Most of the neoplasms associated with ectopic acromegaly are carcinoid tumors. On the other hand, although overt acromegaly is rare in carcinoid tumors, approximately 25% of such cases express immunoreactivity for GHRH [19].
GHRH also exhibits a wide range of proliferative behavior on the pituitary cells producing GH, and chronic hyperstimulation could lead to somatotroph hyperplasia and possibly to the development of adenomas [20]. MRI of our patient revealed an image typical of pituitary hyperplasia.
Surgical resection of the GHRH-secreting tumor is the treatment of choice in patients with ectopic acromegaly; however, this approach is not indicated in patients with an advanced disease and multiple metastases. SMS analogs appear to be highly effective agents for the treatment of ectopic acromegaly because of their suppressive effects on GH hypersecretion and antiproliferative action on the tumor cells.
Unlike its antisecretory properties, the antiproliferative role of SMS was detected later during subsequent studies with the SMS analog octreotide in the therapy of pancreatic, intestinal and pituitary tumors [21].
These antitumoral effects are produced through direct and indirect pathways. Direct effects involve apoptosis and inhibition of the cell cycle mediated by SMS receptors present in the target tumor cells [22], while the indirect effects involve, both inhibition of angiogenesis and inhibition of secretion of hormones and growth factors in non-tumoral cells [23]. The five SMS receptor subtypes (sst 1–5) are expressed in various normal and tumor cells. Octreotide, binds with high affinity to sst-2 and with less affinity to sst-5 but does not bind to subtypes 1 and 4 [21].
In human somatotroph tumor cells, octreotide promotes apoptosis by activating sst2 [24]. On the other hand, octretotide does not induce apoptosis in nonfunctioning pituitary adenomas which express comparable amount of sst2 [25]. In carcinoid tumors, determination of sst2 status by immunohistochemical investigation showed a strong correlation with the expression of mRNA for sst2 as well as with radiolabeled octreotide uptake and clinical response to SMS analogue treatment. The authors defined a positive clinical response as patients remaining stable or having biochemical response during treatment [26]. Our patient showed a positive biochemical response and although the liver and bone metastases did not shrunk as did the brain mass and the hyperplastic pituitary, the size and number of peripheral metastases did not change during more than 2 years of treatment.
Indirect antiproliferative mechanisms in ectopic secretion of GHRH include, among others, reduction in circulating GHRH, GH and IGF-1 levels, the latter with a known antiapoptotic action [27]. In our patient, all GHRH, GH and IGF-1 levels decreased significantly. It has recently been reported that greater inhibition of GHRH secretion by a bronchial carcinoid in vitro occurred in response to an sst5 specific analog (BIM 23206) than to other specific analogs or octreotide [28]. This observation suggests that this analog, or others with high affinity for sst5, may be more effective than currently available analogs in suppressing GHRH secretion in vivo in patients with ectopic secretion of the hormone.
Plasma IGF-1 levels normalized with octreotide therapy, but GH levels—although reduced 6-fold—remained between 5 and 10 ng/ml. Discrepancies in GH and IGF-1 levels have been previously observed in about 25% of acromegalic patients during treatment with octreotide [29]. Circulating GHRH levels decreased dramatically, although they remained above the normal range. These findings have been reported elsewhere [10, 11]. However, since polyclonal antibodies were used for GHRH measurements, it is possible that part of GHRH levels might represent immunoreactive molecules without biological activity, as has been previously reported [30]. This could explain the total regression of the pituitary hyperplasia in our patient. However, as high performance liquid chromatographic analysis of circulating GHRH was not performed we could not confirm this possibility. On the other hand, it has been previously suggested that inhibition of GH secretion by direct action of SMS analogs on the pituitary is more potent than is suppression of GHRH secretion by tumor cells [31, 32].
One case of normalization of both serum levels of GHRH and chromogranin A was reported in a patient with ectopic acromegaly cured by surgical removal of a bronchial carcinoid tumor [33]. Lower but abnormally elevated levels of serum GHRH and chromogranin A during treatment could be related to the presence of metastatic neuroendocrine tumor tissue.
The experience with SMS analogs in the treatment of ectopic GHRH secretion is limited. As was previously mentioned, fewer than 20 cases have been reported and in most, short half-life analogs were administered. As with our patient, GH and IGF-1 circulating levels decreased significantly [9, 12]. Although infrequent, dramatic reductions of pituitary hyperplasia have been reported elsewhere [12], but the antiproliferative effect of SMS analogs in metastases is uncommon [7]. Barkan et al. were the first to report a significant reduction in the size of liver metastases in a patient who had failed to respond to previous conventional chemotherapy [12]. In other cases, normalization of the GH axis hyperactivity has been associated with an increase in the size and number of metastases [9]. Our patient experienced total regression of the pituitary hyperplasia and a reduction of 84% of the intracranial mass after 18 months of octreotide therapy. However, no reduction was observed in the size of liver and lung metastases. It is possible that SMS receptors subtypes, other than sst2, might vary in the different metastases, and therefore, the development of new analogs that bind to a broader spectrum of SMS receptor subtypes may be useful in these patients [24]. Othman et al. have recently described GHRH receptors in a GHRH-producing pulmonary neuroendocrine tumor which suggests autocrine/paracrine regulation of its own proliferation and endocrine function [34]. Differences in abundance of GHRH receptors and abundance and subtypes of SMS receptors might account for the different responses to treatment observed in the hyperplastic pituitary, the non-pituitary intracranial mass and the peripheral metastases. However, this issue needs further investigation.
Only 1.5% of carcinoid tumors metastasize to the brain [35]. The initial differential diagnosis of the cerebellopontine angle mass involved neoplasms of different etiology such as chordoma and acoustic neurinoma. Acoustic neurinomas lack SMS receptors [36]. Chordomas are rare tumors that arise from the neuraxis and have a poor prognosis when located in the cranial base and tend to recur after surgery and radiotherapy. Interestingly enough, such tumors appear to have SMS receptors, which could be used for diagnostic and therapeutic purposes [37]. A patient with an intracerebral metastasis from a bronchial carcinoid tumor that did not change in size during 6 months of octreotide treatment has been reported [38].
In our patient, a biopsy of the intracranial lesion was not justified in light of the remarkable response to medical treatment. However, radiolabeled octreotide scan and radiological evolution are suggestive of a carcinoid metastasis of unusual location that regressed after octreotide treatment.
References
Faglia G, Arosio M, Bazzoni N (1992) Ectopic acromegaly. Endocrinol Metab Clin North Am 21:575–595
Melmed S (1991) Extrapituitary acromegaly. Endocrinol Metab Clin North Am 20:507–518
Sano T, Asa SL, Kovacs K (1988) Growth hormone-releasing hormone-producing tumors: clinical, biochemical, and morphological manifestations. Endocr Rev 9:357–373
Doga M, Bonadonna S, Burattin A, Giustina A (2001) Ectopic secretion of growth hormone-releasing hormone (GHRH) in neuroendocrine tumors: relevant clinical aspects. Ann Oncol 12 (Suppl. 2):S89–S94
Boix E, Pico A, Pinedo R, Aranda I, Kovacs K (2002) Ectopic growth hormone-releasing hormone secretion by thymic carcinoid tumour. Clin Endocrinol (Oxf) 57:131–134
Reuters VS, Dias EMR, Pupo MRSR, Gadelha MR (2003) Acromegaly secondary to ectopic growth hormone-releasing hormone-secreting bronchial carcinoid cured after pneumectomy. Endocrinologist 13:376–379
Van den Bruel A, Fevery J, Van Dorpe J, Hofland L, Bouillon R (1999) Hormonal and volumetric long term control of a growth hormone-releasing hormone-producing carcinoid tumor. J Clin Endocrinol Metab 84:3162–3169
Altstadt TJ, Azzarelli B, Bevering C, Edmondson J, Nelson PB (2002) Acromegaly caused by a growth hormone-releasing hormone-secreting carcinoid tumor: case report. Neurosurgery 50:1356–1359
Lefebvre S, De Paepe L, Abs R, Rahier J, Selvais P, Maiter D (1995) Subcutaneous octreotide treatment of a growth hormone-releasing hormone-secreting bronchial carcinoid: superiority of continuous versus intermittent administration to control hormonal secretion. Eur J Endocrinol 133:320–324
Drange MR, Melmed S (1998) Long-acting lanreotide induces clinical and biochemical remission of acromegaly caused by disseminated growth hormone-releasing hormone-secreting carcinoid. J Clin Endocrinol Metab 83:3104–3109
Moller DE, Moses AC, Jones K, Thorner MO, Vance ML (1989) Octreotide suppresses both growth hormone (GH) and GH-releasing hormone (GHRH) in acromegaly due to ectopic GHRH secretion. J Clin Endocrinol Metab 68:499–504
Barkan AL, Shenker Y, Grekin RJ, Vale WW (1988) Acromegaly from ectopic growth hormone-releasing hormone secretion by a malignant carcinoid tumor. Successful treatment with long-acting somatostatin analogue SMS 201–995. Cancer 61:221–226
Schopohl J, Losa M, Frey C, Wolfram G, Huber R, Permanetter W et al (1991) Plasma growth hormone (GH)-releasing hormone levels in patiens with lung carcinoma. Clin Endocrinol (Oxf) 34:463–467
Brar A, Brinster R, Frohman LA (1989) Immuhistochemical analysis of human growth hormone-releasing hormone gene expression in transgenic mice. Endocrinology 125:801–809
Korner M, Eltschinger V, Waser B, Schonbrunn A, Reubi JC (2005) Value of immunohistochemistry for somatostatin receptor subtype sst2A in cancer tissues: lessons from the comparison of anti-sst2A antibodies with somatostatin receptor autoradiography. Am J Surg Pathol 29:1642–1651
Warren WH, Gould VE, Faber LP, Kittle CF, Memoli VA (1985) Neuroendocrine neoplasms of the bronchopulmonary tract. A classification of the spectrum of carcinoid to small cell carcinoma and intervening variants. J Thorac Cardiovasc Surg 89:819–825
Rusch VW, Klimstra DS, Venkatraman ES (1996) Molecular markers help characterize neuroendocrine lung tumors. Ann Thorac Surg 62:798–809
Frohman LA, Szabo M, Berelowitz M, Stachura ME (1980) Partial purification and characterization of a peptide with growth hormone-releasing activity from extrapituitary tumors in patients with acromegaly. J Clin Invest 65:43–54
Dayal Y, Lin HD, Tallberg K, Reichlin S, DeLellis RA, Wolfe HJ (1986) Immunocytochemical demonstration of growth hormone-releasing factor in gastrointestinal and pancreatic endocrine tumors. Am J Clin Pathol 85:13–20
Asa SL, Ezzat S (1998) The cytogenesis and pathogenesis of pituitary adenomas. Endoc Rev 19:798–827
Lamberts SWJ, van der Lely AJ, de Herder WW, Hofland LJ (1996) Octreotide. N Eng J Med 334:246–254
Ferjoux G, Bousquet C, Cordelier P, Benali N, Lopez F, Rochaix P, Buscail L, Susini C (2000) Signal transduction of somatostatin receptors negatively controlling cell proliferation. J Physiol (Paris) 94:205–210
Patel YC (1999) Somatostatin and its receptor family. Front Neuroendocrinol 20:157–198
Ferrante E, Pellegrini C, Bondioni S, Peverelli E, Locatelli M, Gelmini P, Luciani P, Peri A, Mantovani G, Bosari S, Beck-Peccoz P, Spada A, Lania A (2006) Octreotide promotes apoptosis in human somatotroph cells by activating somatostatin receptor type 2. Endocr Relat Cancer 13:955–962
Luciani P, Gelmini S, Ferrante E, Lania A, Benvenuti S, Baglioni S, Mantovani G, Cellai I, Ammannati F, Spada A, Serio M, Peri A (2005) Expression of the antiapoptotic gene seladin-1 and octreotide-induced apoptosis in growth hormone-secreting and nonfuncioning pituitary adenomas. J Clin Endocrinol Metab 90:6156–6161
Janson ET, Stridsberg M, Gobl A, Westin JE, Oberg K (1998) Determination of somatostatin receptor subtype 2 in carcinoid tumors by immunohistochemical investigation with somatostatin subtype 2 antibodies. Cancer Res 58:2375–2378
Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M (1998) Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science 279:563–566
Zatelli MC, Maffei P, Piccin D, Martini C, Rea F, Rubello D, Margutti A, Culler MD, Sicolo N, degli Uberti EC (2005) Somatostatin analogs in vitro effects in a growth hormone-releasing hormone-secreting bronchial carcinoid. J Clin Endocrinol Metab 90:2104–2109
Turner HE, Thornton-Jones VA, Wass JA (2004) Systematic dose-extension of octreotide LAR: the importance of individual tailoring of treatment in patients with acromegaly. Clin Endocrinol (Oxf) 61:224–231
Frohman LA, Downs TR, Williams TC, Heimer EP, Pan YE, Felix AM (1986) Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologicall inactive, N-terminally cleaved product. J Clin Invest 78:906–913
Melmed S, Ziel FH, Braunstein GD, Downs T, Frohman LA (1988) Medical managemente of acromegaly due to ectopic production of growth hormone-releasing hormone by carcinoid tumor. J Clin Endocrinol Metab 67:395–399
Moller DE, Moses AC, Jones K, Thorner MO, Vance ML (1989) Octreotide suppresses both growth hormone(GH) and GH-releasing hormone (GHRH) in acromegaly due to ectopic secretion. J Clin Endocrinol Metab 68:499–450
Bolanowski M, Hos-Kudla B, Rzeszutko M, Marciniak M, Zatonska K (2006) Five year remission of GHRH secreting bronchial neuroendocrine tumor with symptoms of acromegaly. Utility of chromogranin A in monitoring of the disease. Endokrynol Pol 57:32–36
Othman NH, Ezzat S, Kovacs K, Horvath E, Poulin E, Smyth HS, Asa SL (2001) Growth hormone-releasing hormone (GHRH) and GHRH receptor (GHRH-R) isoform expression in ectopic acromegaly. Clin Endocrinol (Oxf) 55:135–140
Hlatky R, Suki D, Sawaya R (2004) Carcinoid metastasis to the brain. Cancer 101:2605–2613
Schmidt M, Scheidhauer K, Luyken C, Voth E, Hildenbrandt G, Klug N, Schicha H (1998) Somatostatin receptor imaging in intracranial tumors. Eur J Nucl Med 25:675–686
Di Girolamo S, Ottaviani F, Floris R, Bruno E, Napolitano B, Schillaci O (2005) Indium111 pentetreotide single photon emission computed tomography (In111 pentetreotide SPECT): a new technique to evaluate somatostatin receptors in chordomas. J Laryngol Otol 119:405–408
Ohnsmann A, Sachsenheimer W (1992) Intracerebral metastasis of a bronchial carcinoid tumor. Neurochirurgia (Stuttg) 35:160–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fainstein Day, P., Frohman, L., Garcia Rivello, H. et al. Ectopic growth hormone-releasing hormone secretion by a metastatic bronchial carcinoid tumor: a case with a non hypophysial intracranial tumor that shrank during long acting octreotide treatment. Pituitary 10, 311–319 (2007). https://doi.org/10.1007/s11102-007-0019-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-007-0019-9