Abstract
Lycopodium is a genus of the family Pteridophytes, which is widely distributed in temperate and tropical climates and tropical mountains. Plants of genus Lycopodium are ancient medicinal plants which have been used in different traditional medicinal system to treat many diseases, mainly focus on central nervous system and inflammation-related diseases. Rigorous pharmacological and clinical studies conducted in recent decades have demonstrated their special efficacy in the treatment of Alzheimer's disease (AD). Furthermore, secondary metabolites and extracts from these plants have been proven to possess neuroprotective, anti-tumor, anti-inflammatory, anti-microbial, and antiviral effects, which supports most of traditional medicinal uses of Lycopodium plants. To date, a total of 508 secondary metabolites have been reported from the 46 species belonging to genus Lycopodium. Among those metabolites, Lycopodium alkaloids and serratene triterpenoids represent two major classes of bioactive ingredients. Notably, huperzine A, a Lycopodium alkaloid originally isolated from L. serratum, was licensed in China as a drug for the treatment of AD and in the United States as a dietary supplement. Besides, serratane-type triterpenoids may be potential candidates for the development of anticancer drugs. This review covers the literatures available from 1947 to 2020 and mainly discusses knowledge on ethnopharmacology, secondary metabolites, pharmacological activities, clinical trials, toxicology, and quality control of Lycopodium species. In addition, the present review also draws attention to the gaps that still exist in the scientific studies on Lycopodium plants, which would accelerate the contemporary development of this traditional medicinal plant.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Lycopodium, widely distributed in temperate and tropical climates and tropical mountains, contains about 40–50 species characterized by low, evergreen, coarsely moss-like and club-shaped strobili at the tips of mosslike branches. Given the wide distribution of club mosses, it is no wonder that various species of this genus have been used to treat multiple diseases dominated by central nervous system and inflammation-related ailments in traditional medicine around the world. In Europe and America, Hildegard of Bingen recorded different recipes and formulas with Lycopodium clavatum L. for the treatment of skin irritations and acne, nosebleed, inflammation of the liver, irritation of the intestinal tract, and kidney disorders (Siengalewicz et al. 2013). In addition, Lycopodium saururus Lam. is a specie well known in Argentina for its traditional use as an aphrodisiac and for memory improvement (Ortega et al. 2004a). In traditional Chinese medicine, five Lycopodium species all named Shi Song have been used as a traditional herbal medicine for the treatment of arthritic pain, quadriplegia, contusions, dysmenorrhea, and other health problems since Tang dynasty (Zhang et al. 2014). Lycopodium plants were also used to treat various mental conditions such as amnesia, anxiety, and fatigue in other Asian countries (Banerjee et al. 2014).
The classification of Lycopodiaceae has always been in a confused situation, because of the uncertain boundaries between the genera, which has caused a long-term discussion and research by botanists. The genus Lycopodium was established on the basis of Lycopodium clavatum L. by Linnaeus in 1753. During the period since its establishment, all Lycopodiaceae plants have been members of this group except Phylloglossum. With the development of techniques and methods, this family is divided into 2 to 16 genera by researchers with different viewpoints (Schuettpelz et al. 2016; Christenhusz et al. 2011; Field and Bostock 2013; Holub 1985, 1991; llgaard 1987). Therefore, the scientific name of the genus Lycopodium also produced confusion (for instance, Lycopodium serratum Thunb. is a synonym of Huperzia serrata (Thunb. ex Murray) Trevis.; Lycopodium phlegmaria L. is a synonym of Phlegmariurus phlegmaria (L.) Holub).
The earliest studies of secondary metabolites of genus Lycopodium dates back to the 1940s. Canadian scientists have studied structures of Lycopodium alkaloids, and W. A. Ayer classified theses alkaloids into four structural classes (Ayer et al. 1994). Until 1980s, some Lycopodium alkaloids represented by huperzine A (Hup A) were found to possess potent acetylcholinesterase (AChE) inhibition activity by Chinese scientists. Since then, a new wave of research on Lycopodium alkaloid has been rising. To date, researches on plants of genus Lycopodium revealed that it was not only a known source of Lycopodium alkaloids, but also as a source of serratene-type triterpenoids. Up to now, a total of 508 secondary metabolites have been isolated and identified from the Lycopodium plants. Modern pharmacological studies have revealed that secondary metabolites or extracts from plants of genus Lycopodium exhibit an extensive range of biological activities including anti- Alzheimer's disease (AD), neuroprotection, anti-tumor, anti-inflammation. Hup A has been approved by FDA as a dietary supplement in 1997. As a second-generation AChE inhibitor, Hup A has also been used for the treatment of AD in China after numerous clinical studies. Besides, serratane-type triterpenoids may be potential candidates for the development of anticancer drugs for cancer therapy.
Scientists have never stopped the study of Lycopodium plants because of the unique and abundant biological activities of Lycopodium alkaloids. However, there is hardly any systematic in-depth review of genus Lycopodium. In this review, we intend to comprehensively present the advances achieved in the aspects of ethnopharmacology, secondary metabolites, pharmacological and biological activity, toxicology, and quality control of Lycopodium species. Plant names were also validated by “The Plant List” (www.theplantlist.org). The relationship between traditional uses and modern pharmacological activities is also discussed, aiming to outline existing research gaps and further research directions.
Traditional uses
The plants of the genus Lycopodium were utilized ethnobotanically in mainly temperate and tropical regions, such as most of European and Asian countries, the United States, Argentina, and Brazil, to treat various diseases including central nervous system disease, inflammation-related ailments including hepatitis, arthritis, rheumatism, muscle swelling, pain, fever and dermatosis (Siengalewicz et al. 2013; Halldorsdottir et al. 2015; Namsa et al. 2009; Zhang et al. 2014).
In traditional European medicine (TEM), herbs from genus Lycopodium were generally reputed to be nontoxic and were occasionally used for preparing a salubrious tea which was employed to medicate inflammation of the liver, nosebleed, irritation of the intestinal tract and kidney disorders in different recipes and formulas. For example, skin irritations and acne were treated with a tea brewed from L. clavatum and couch grass (Agropyron repens L.). In Pliny the Elder’s report, the Druids of Gaul have pretended that L. clavatum should be carried about the person as a preservative against accidents of all kinds, and that the smoke of it is extremely good for all maladies of the eyes (Siengalewicz et al. 2013). The herbal drug made from L. clavatum has been used to cure ailments of the kidney and bladder, while the similar-looking plant Lycopodium selago L. was used as a drastic emetic and a cathartic. Moreover, L. selago was known to be toxic and has been used for centuries to induce abortion. Reportedly, the high efficacy of this drug was well-known to prostitute when committing infanticide (Felgenhauer et al. 2000).
In native American tribes, the standard treatment for injuries and lesions was the application of spores of L. clavatum in the open wound. Members of the Blackfoot tribe used Lycopodium complanatum L. to treat pulmonary disease, and Iroquois believed in the ability of the plant to induce pregnancy (Siengalewicz et al. 2013). In addition, Upper Tanana Indians often used the whole plant of L. selago in a poultice applied to the head for headaches. In Argentinian traditional medicine, the leaves and stems of L. clavatum were used as carminative, expectorant and diuretic (Goleniowski et al. 2006). Further, the use of an infusion of aerial parts of L. saururus as an aphrodisiac and for memory improvement was widely known (Ortega et al. 2004a). Equally important, the decoction of the entire Lycopodium thyoides Humb. and Bonpl. ex Willd was used by the Quechua ethnic group in Ecuador to treat disorders of childbirth and used in Brazilian traditional medicine as a nerve tonic for central nervous system (CNS)-related conditions (Halldorsdottir et al. 2015; Konrath et al. 2012).
In India, Indian Ocean Islanders used the fresh plant infusion of Lycopodium cernuum L. to treat rickets of children and intestinal infection (Jain et al. 2005). Locally, the young shoot powder of L. clavatum known as Luanha mixed with seeds powder of Sesamum indicum Linn. was used for body massaging to relieve muscle pain and fatigue (Namsa et al. 2009). In Anatolia, Turkey, L. clavatum, the most common specie of Lycopodium has been used in form of powder against baby skin irritation caused by nappy, therefore, also called “belly powder”. Additionally, the spores of this plant have been stated to be protective for tender skin (Orhan et al. 2013). Moreover, the whole plant powder of L. clavatum was also used by Nepalese especially rural people to treat burn and headache (Hasan et al. 2013). In Bangladesh, L. clavatum was used for the treatment of kidney stone, urinary tract infection and digestive aliment and imperfect erection in aged person by various tribes (Banerjee et al. 2014).
In traditional Chinese medicine (TCM), a series of Lycopodium species named Shi Song including Lycopodium japonicum Thunb., Lycopodium annotinum L., Lycopodium obscurum L., L. complanatum, and Lycopodium serratum Thunb. have been used to treat various conditions such as arthritic pain, rheumatic numbness, contusion, blood stasis, muscle pain, dysmenorrheal, digestive problems, recognitive disorder and inflammation states. (Ma et al. 2007). The earliest record of medicinal usage of Lycopodium species can be traced back to an ancient Chinese pharmacopeia “Bencao Shiyi” (《本草拾遗》), which was written by Cangqi Chen in A.D. 739 (during the Tang Dynasty). It was recorded that L. serratum could be used to relieve rheumatism and cold, relax muscle and tendon, and promote blood circulation. This herb can also be found in “Compendium of Materia Medica” (《本草纲目》) by Shizhen Li in A.D. 1578 (during the Ming Dynasty) and “Chih-wu ming-shih t'u kao” (《植物名实图考》) by Qijun Wu in A.D. 1848 (during the Qing Dynasty) with a different name “Qian Ceng Ta”. Besides, According to the Great Dictionary of Chinese Medicine (《中药大辞典》), fresh “Qian Ceng Ta” mixed with distiller's grains and brown sugar are mashed and heated to treat bruise, sprain, swelling and pain by external application. The dried whole plant of L. japonicum also named “Lycopodii Herba” was mentioned in “Gleaning Herb” to be able to dispel wind, dehumidify, activate collaterals, and used for the treatment of inconvenient flexing and stretching, wind dampness arthralgia syndrome, bruises, and other diseases (Zhang et al. 2014; Cai et al. 2015). In the coastal areas, Chinese often cure shingles by using the powder of L. japonicum, mixing it into paste with the seed oil of Sapium sebiferum (L.) Roxb. and smearing the affected area several times a day. In the traditional Yao communities of Yunnan Province, southwest of China, the aerial part of L. japonicum and L. complanatum were used to treat rheumatalgia, skin diseases and activate the blood circulation with medicinal baths, an important traditional way of adding proper herbal medicines to water, decocting them for a proper time, and then using the decocted liquid to bathe in proper temperature (Li et al. 2006a; Lee et al. 2008).
Overall, Lycopodium species were highly valued herbal remedies in several early cultures all over the world. Table 1 includes the detailed uses of different species in the diverse traditional medicines.
Secondary metabolites from Lycopodium species
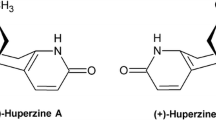
Lycopodium species have been investigated since 1840s. Review of literature reveals the presence of altogether 508 secondary metabolites from around 46 Lycopodium species. we categorize them into alkaloids (1–443), triterpenoids (444–503), glycosides (504–508). Their structures are shown in Fig. 1 and chemical names, chemical class and the corresponding plant sources are summarized in Table 2. Detailed and extensive chemical investigation of quite a few species, exactly used in common traditional medicine, such as L. serratum, L. complanatum and L. japonicum, led to the characterization of a large number of bioactive constituents. Hup A (172), originally isolated from L. serratum, is the main focus of numerous studies concerning the biological activities represented by anti-AD, of the genus Lycopodium.
Alkaloids
Lycopodium alkaloids are a family of structurally diverse natural products with complex polycyclic skeletons. They are a kind of nitrogen heterocyclic compounds with novel skeleton, which is a tricyclic or tetracyclic compound composed of the basic skeleton C16N and C16N2, as well as a small amount of C11N, C15N2, C22N2 and C27N3 alkaloids. A common feature in all Lycopodium alkaloids is a polycyclic carbon skeleton with varying levels of oxidation (Hirasawa et al. 2004).
W. A. Ayer was an outstanding chemist who spent most of his professional career investigating Lycopodium alkaloids and published many important articles and reviews on them. According to the structure characteristics of Lycopodium alkaloids, Ayer divided them into four structural classes: the lycopodine class, the lycodine class, the fawcettimine class and the miscellaneous group, with lycopodine, lycodine, fawcettimine and phlegmarine as representative compounds, respectively (Ayer et al. 1994).
Lycopodine class
A total of 157 Lycopodium alkaloids (1–157) have been isolated from the whole plant of Lycopodium species that belong to this class. This is the largest group of known Lycopodium alkaloids, and appears to be the most widely distributed.
This class is characterized by four connected six-membered rings, with rings A and C being a cis quinolizidine ring system (C-4 and C-13 are connected). Most of the ring B have carbonyl groups at C-5 and a few at C-6. Examples are huperzines E (26), F (91) and O (107) which were isolated from L. serratum (Ma et al. 2004). Lycopodine (1) is a representative compound of this class, which was also the first identified and belongs to this class (Ma et al. 2004). And its C-4, C-6 of the carbonyl alpha position and H atoms at tertiary C-7 are easily oxidized to hydroxyl groups, such as 4α,8β-dihydroxylycopodine (10), 8β-hydroxylycoposerramine K (21), 11β-hydroxy-12-epilycodoline (14) (Cai et al. 2015). Besides, its N atom also can be oxidized, as miyoshianine C (23). Lannotinidine H (113) is the first Lycopodium alkaloid possessing a lycopodine skeleton with an additional C3 unit (Ishiuchi et al. 2009a, b). The rings A, B and C of these compounds are relatively stable, and the changes of the skeleton are mainly concentrated in the D ring. For example, C-15 of the D ring related to C-12 in lannotinidine E (43) after breaking with C-8. At the same time, C-8 formed an epoxide ring with C-5. It is worth mentioning that it can enhance the mRNA expressions for nerve growth factor (NGF) (Koyama et al. 2005).
Lycodine class
A total of 67 Lycopodium alkaloids (158–224) have been identified from the whole plant of Lycopodium species that belong to this class. So far, it is found that most of the Lycopodium alkaloids with AChE inhibition activity comes from this class, most notably: Hup A (172), huperzine B (173), N-methyl-huperzine B (191) and huperzinine (176). Generally, the character of structure is also four-ring. The difference with lycopodine class is that the A ring is opened and rearranged to form a pyridine or pyridone A ring, and the C ring is converted into separate hexahydropyridine C ring. Its representative compound is lycodine (179). Intriguingly, complanadine E (165) was a new unsymmetrical dimeric alkaloid, which was the first example forming a piperidine ring at C-1–C-5 and N-1 of lycodine-type alkaloids (Ishiuchi et al. 2011).
Among the Lycopodium alkaloids, Hup A (172) isolated from L. serratum in 1986 has been shown to have highly specific and potent inhibitory activity against AChE and to improve memory disorders in AD patients (Liu et al. 1986). It has greatly stimulated interest in this group of alkaloids. Relevant investigation has been conducted to study their pharmacology in depth.
Fawcettimine class
144 of the Lycopodium alkaloids belong to the fawcettimine class (225–368). This class of compound can be regarded as the result of C4-C13 bond breaking and forming C4-C12 bond in lycopodine class. The C-13 connected with N atom is unstable and easy to be oxidized to hydroxyl group or break the C13-N bond to form C13-carbonyl group. Therefore, a characteristic of this class not shared with the other classes is the presence of a five-membered ring B. Its representative compound, fawcettimine (251), has been proved to be an equilibrium mixture between the alcohol-amine type and keto-amine type, and thus evolved into two major groups of the carbinolamine form and the keto-amine form. In the carbinolamine form, N atom is linked to C-13, such as lycopoclavamine A (245); the keto-amine form does not have N-C13 bond, such as palhinine A (272).
Obscurumines H (306) and I (307) represent rare naturally occurring structures based on the fawcettimine-type skeleton, including a new C17N2 skeleton that is formed a ring via the linkage of C-9–N-2′, which is rarely present in Lycopodium alkaloids (Jiang et al. 2015). Moreover, it was found that three new skeleton alkaloids, named lycojaponicumins A (297), B (298) and C (277), were isolated from L. japonicum. They represented a unique heterocyclic skeleton formed by the new linkage C4-C9. Notably, lycojaponicumins A and B are the first examples of natural products possessing a 5/5/5/5/6 pentacyclic ring system with a 1-aza-7-oxabicyclo[2.2.1]heptane moiety. Lycojaponicumin C is composed of a 6/5/5/6 tetracyclic skeleton, except that C-3 is connected to C-13. Biological testing in vitro showed that lycojaponicumins A-C inhibited lipopolysaccharide (LPS)-induced pro-inflammatory factors in BV2 microglia and macrophages (Wang et al. 2012a, b). Interestingly, lycojapodine A (310), a novel alkaloid with an unprecedented 6/6/6/7 tetracyclic ring system, was also isolated from the club moss L. japonicum. Meanwhile, it exhibited anti-human immunodeficiency virus (HIV)-1 activity, showing an EC50 value of 85 µg/mL (He et al. 2009).
Miscellaneous group
There is no fixed uniform skeleton for this group. In addition to the first three classes, the rest of the Lycopodium alkaloids can be classified into this group, and represent quite a diversity of structural motifs. 75 Lycopodium alkaloids (369–443) are included in this group. Among these, phlegmarine type have the largest number of this group. In all of the miscellaneous group compounds, the C-4 is unconnected to C-12 or C-13. The C-4 of phlegmarine (425) always forms a C–C bond with either C-13 or C-12 during the C–C coupling reaction, which leads to the formation of the other three classes. So phlegmarine (425) can be regarded as the precursor of the first three kinds of alkaloids.
It was reported that lyconadin B (395) is a novel type of alkaloid consisting of five fused rings (one five-membered and four six-membered rings), were able to enhance NGF mRNA expression and production in human glial cells (Ishiuchi et al. 2006a). Zhang et al. found a new C16N skeleton molecule, lycopladine A (301), from L. complanatum in 2017. The structure of this compound was characterized as 6/9/5 tricyclic skeleton with a γ-lactone ring. And the skeleton is constructed from an unusual 1-oxa-6-azaspiro[4.4]nonane moiety and an unprecedented 3-azabicyclo[6.3.1]dodecane unit. As an aside, it is interesting to note that the inhibitory activity of the Cav3.1 T-type calcium channel of lycoplanine A was comparable to that of Mibefradil, which approved by the FDA for the treatment of hypertension (Zhang et al. 2017). It may be a lead compound for the treatment of diseases associated with T-type calcium channel, such as Parkinson's disease, pain, tumor, sleep disorder, epilepsy, and other central or peripheral neurological diseases. This discovery broadens the understanding of the activity of Lycopodium alkaloids and opens new research fields.
Triterpenoids
A total of 59 triterpenoids (444–479,481–503) have been found in Lycopodium species. Serratene triterpenoids, the most common terpenoids isolated from the extracts of Lycopodium plants, are the widely concerned chemical constituents in addition to alkaloids of genus Lycopodium. Moreover, triterpenoids of the serratane type appear to be characteristic of this genus, which could be used as a valuable chemotaxonomical criterion to distinguish from the related genera such as Selaginella and Isoetes. (Miller et al. 1972). Serratenes, a kind of pentacyclic triterpenoids which come from single protonation of α-onocerin (503) that rich in genus Lycopodium (Zhao et al. 2010), possess unusual skeletons with a seven-membered C-ring, seven tertiary methyl groups (one or two may be oxygenated), as well as usually a double bond between C-14 and C-15 and oxygen functionalities at C-3 and C-21. Further, there should be an existing singly protonated enzyme so that α-onocerin triterpenoid could be converted to serratenediol triterpenoid, and both belong to serratenes (Inubushi et al. 1967b). In recent years, the bioactivity of serratenediol (496) that first isolated from the Japanese club moss, L. serratum has been examined. It can induce apoptosis via regulating the ratio of Bax/Bcl-xL in HL-60 cell lines. Therefore, serratane triterpenoids might be potential candidates for the development of anti-cancer drugs (Ham et al. 2012). Besides, according to Zhang et al. (2014), regarding the serratenediol derivatives, the introduction of a polar hydroxyl and carboxyl group at C-24 led to the increase in the antitumor activity. The presence of the carbonyl group in ring B was also more favorable for enhancing the antitumor activity among the α-onocerin (503) derivatives. In addition, a variety of significant biological activities including anti- AChE, inhibition of aspartate protease secreted by Candida albicans and DNA anti-topoisomerase II of serratane triterpenoids have been found in many Lycopodium plants, especially L. japonicum (Yan et al. 2005a, b). Lately, Wang et al. (2014) isolated two new triterpenoids, named 3β-hydroxyserrat-14-en-21β-yl-formate (473) and 21β-hydroxyserrat-14-en-3β-yl-formate (458), for the first time from an ethanol extract of the entire plant of L. japonicum. Their structures were elucidated by means of spectroscopic analysis to be both the formates of serratenediols, which were reported rarely in serratene-type terpenoids.
Glycosides
In the continual search for biologically active substances active substance, Tori et al. (2005) investigated Japanese L. clavatum, and have isolated three new glycosides (505, 506, 508) as well as known apigenin-4′-O-(2′′,6′′-di-O-p-coumaroyl)-β-D-glucopyranoside (504) and apigenin-7-O-β-D-(2",6"-di-O-p-coumaroyl)glucoside (507) (Rao et al. 1983). Notably, all belong to flavones glycosides except compound 508, a new class of glucoside from Lycocpodium species so far, having a benzoate group instead of the flavone unit. Additionally, in the Candida-secreted aspartic proteases (SAP) inhibition assay of an ethanol extract of L. cernuum, apigenin-4′-O-(2′′,6′′-di-O-p-coumaroyl)-β-D-glucopyranoside (504) was identified and found to be active with IC50 of 8.4 µg/mL (Zhang et al. 2002a). Reports on glycosides in Lycopodium are very rare, making them an interesting topic for further studies.
Others
A small amount of other compounds including one monoterpene (480), aliphatic alcohol, anthraquinone and two phytosterols have been found in Lycopodium species (Li et al. 2006b; Cai et al. 2015; Teng et al. 2010). Insufficiently, few reports of biological and pharmacological activity are available on these compounds of genus Lycopodium.
Biological and pharmacological activities of drugs made from Lycopodium species
Secondary metabolites from plants of genus of Lycopodium have many pharmacological effects, including anti-AD, neuroprotection, anti-tumor, anti-inflammatory and so on. Details of biological and pharmacological activities are shown in Tables 3, 4, 5, 6 and 7. Triterpenoids mainly showed anti-tumor effect, and alkaloids possess pharmacological effect on cholinesterase inhibition, neuroprotection and anti-inflammatory. Among them, Hup A is commonly used to improve memory and mental function in people with Alzheimer’s disease, other types of dementia, age-related memory loss, and other conditions.
Anti-AD activity
AD is a neurodegenerative disease and the most frequent and predominant cause of dementia among the elderly. Although the pathogenesis of AD is complicated and involves numerous pathways, two major hypotheses are currently under consideration regarding the molecular mechanism: the cholinergic hypothesis and the amyloid cascade hypothesis (Chuong et al. 2014). There is considerable evidence that the memory deficits associated with AD are due to impairment of cholinergic neurotransmission in the central nervous system (Ma et al. 2004). As a consequence, the enhancement of cholinergic neurotransmission has been considered as one potential therapeutic approach against AD. The inhibition of cholinesterase may be related to the traditional use of Lycopodium in the treatment of mental disorders such as memory impairment and anxiety. At the same time, the curative effect on mild nausea caused by stimulation of the central nervous system may be the reason why people in some regions traditionally use these herbs as expectorants and emetics. Therefore, it is imperative to evaluate the cholinesterase inhibition of secondary metabolites of Lycopodium in vitro and in vivo. Until now, nearly 400 Lycopodium alkaloids have been reported. As a result, some of them have been proven to be good AChE inhibitors such as Hup A, huperzine B, huperzine C, and N-methylhuperzine B belonging to the lycodine class. Among them, Hup A was licensed in China as a drug for the treatment of AD and in the United States as a dietary supplement. It could be seen that the main activity of Hup A is anti-AChE.
Since Hup A was discovered from L. serratum in the 1980s, it has been extensively evaluated by the Chinese for its bioactivity, or its inhibitory activity towards cholinesterase and for treatment of AD (Liu et al. 1986). Before 2000, many cholinesterase inhibitory tests have shown that Hup A is the most selective AChE inhibitor, although its activity is not the strongest in vitro and the IC50 value of Hup A relative to other AChE inhibitors is: donepezil < Hup A < tacrine < physostigmine < galantamine(Gal) (Wang et al. 1986; Tang et al. 1988; Tang et al. 1999). This result can be illustrated by the specific data below. The ratios of Hup A, E2020 and tacrine for butyrylcholinesterase (BChE) (rat serum): AChE (rat cortex homogenate) determined by a colorimetric method were 884.57, 489.05 and 0.80, respectively. (Cheng et al. 1996). In contrast to the AChE inhibition in vitro, the relative inhibitory effect of oral Hup A on cortical AChE was found to be about 24-fold and 180-fold potent in molar terms than donepezil and tacrine, respectively (Wang et al. 1998). In a later similar experiment, when the effects of Hup A, donepezil and rivastigmine on cortical acetylcholine levels and AChE activity in rats were compared, Hup A was still the most potent and had longer lasting effect (Liang et al. 2004). In addition, Hup A injected intraperitoneally exerted similar efficacy of AChE inhibition in rats as observed following oral administration (Wang et al. 1998), but the inhibitory potency of it on brain AChE was less than that of donepezil after the intraventricular injection (Cheng et al. 1998). These findings indicated that Hup A has higher bioavailability and penetrates the blood brain barrier more easily. Moreover, repeated doses of Hup A showed no significant difference on the AChE inhibition compared with that of single dose, indicating no tolerance to Hup A (Wang et al. 1998; Laganiere et al. 1991). In 1997, The X-ray crystal structure of the (-)-Hup A-AChE complex showed that the three-carbon bridge, the prerequisite structure for the AChE inhibitor activity in Hup A (Kozikowski et al. 1991), was inserted into the hydrophobic area of AChE surrounded by aromatic residues (Raves rt al. 1997). Notably, the other AChE inhibitors function in a similar manner, but the Hup A-AChE complex has a longer half-life than these and other prophylactic agents (Ma et al. 2004). Before this, a Lineweaver-Burke plot for Hup A indicated a pattern of AChE inhibition of the mixed competitive type, as the intersection of the lines occurred in the second quadrant (Cheng et al. 1996). In particular, the AChE activity recovered to 94 ± (SD)5% of the control after 4 times washing with 5–6 fold volume of 0.01 mol/L pH 7.4 Tris–HCl buffer, indicating the inhibitory manner of Hup A was reversible (Tang et al. 1988). Overall, Hup A is a potent, reversible and selective AChE inhibitor, which has been approved as the drug for treatment of AD in China, and is marketed in USA as a dietary supplement (as powdered L. serratum in tablet or capsule format). In addition, Hup A is now used to prevent organophosphorus poisoning and clinically to treat myasthenia gravis due to its AChE inhibitory activity.
Although all of the other Lycopodium alkaloids identified to date have either not shown any AChE inhibition activity or possessed activity that is significantly lower than that of Hup A, one interesting thing to note is that there may be a synergistic effect among the alkaloids. For example, L. saururus, an important commercial item in Argentina, was widely used as a stimulant and for memory improvement traditionally. Until now, studies on it showed the presence of nine different alkaloids in a purified alkaloid extract, with sauroine (Ortega et al. 2004b), being the predominant one. According to the reported by Ortega et al., the purified alkaloid extract of L. saururus was evaluated in relation to its effect on the AChE and exhibited a strong inhibitory effect (IC50 = 0.58 µg/mL) (Ortega et al. 2004a). However, hitherto evaluations of purified alkaloids showed that the major alkaloid (sauroine) showed no inhibitory effect and sauroxine had the strongest inhibition with an IC50 = 8.9 ± 0.4 µg/mL (Vallejo et al. 2013), that is, no compound has similar inhibitory activity to the alkaloid extract.
The residues responsible for the catalytic activity of AChE, the catalytic triad (Ser203, His447, Glu334), are found at the bottom of a 20 Å deep gorge or cavity that constitutes the active site (AS). Outside the gorge, next to the “entrance”, there is another site called the peripheral anionic site (PAS), where the precursors of the amyloid β (Aβ) are proposed to deposit before aggregation (Ferrari et al. 2001; Giacobini et al. 2000). Previous studies have shown that molecules able to interact with both sites of AChE could prevent the aggregating activity of AChE toward Aβ as well as the hydrolysis of acetylcholine (Bartolini et al. 2003). On this basis, the fact is that alkaloids with different degrees of affinity for the AS and the PAS exhibit different inhibitory abilities. The molecular dynamics simulation for the AChE complexes with Lycopodium alkaloids highlighted that the compounds like Hup A and sauroxine with higher AChE inhibition activity presented higher affinities for the AS than the PAS, but sauroine had no activities due to similar binding energy for the AS and the PAS (Puiatti et al. 2013).
Except to alkaloids, triterpenoids from plants of this genus are also found to be active against AD. For example, the inhibitory activity of α-onocerin, a well-known serratene-type triterpenoid isolated from the chloroform extract of L. clavatum, was determined by the Ellman method at 1, 3 and 5 mg/mL. Surprisingly, in a dose-independent manner, α-onocerin (IC50 = 5.2 μM) inhibited AChE better than donepezil at 1 and 3 mg/mL concentrations and almost the same inhibition rate at 5 mg/mL (Orhan et al. 2003). In the same way, only lycoclavanol and α-onocerin showed inhibition activities (20.0 and 39.0%, resp) among seven triterpenoids isolated from L. japonicum. Gal was used as the standard drug (inhibition 63.3%) (Yan et al. 2005a, b). These findings could suggest that α-onocerin may be a candidate compound in the treatment of AD for further research. In addition, it is worth noting that most of the literature we collected used Ellman’s reagent to determine the anti-AChE activity of secondary metabolites or extracts. Although Elman’s method is still an appropriate method for the determination of cholinesterase activity, it is prone to false-positive. To solve this problem, Rhee developed a method using thin layer chromatography (TLC) assay based on Ellman’s method, and the true enzyme inhibition could be distinguished from the false-positive chemical inhibition (Rhee et al. 2003). In further, anti-AChE activity of secondary metabolites should be tested by Ellman based TLC assay.
The amyloid hypothesis for the pathogenesis of AD was proposed, by which the accumulation of Aβ aggregates triggers a cascade of neurotoxic events in the brain eventually leading to a widespread neuronal degeneration and hence to dementia (Inestrosa et al. 1996; Alvarez et al. 1995). Accordingly, new anti-Alzheimer drug candidates focused on this problem, in order to modify the stage of the diseases (Muñoz-Torrero et al. 2008). In an in vitro study, lycopodine alkaloids annotinolides A-C were evaluated for their inhibitory effects against the aggregation of Aβ1-42 peptide, a key factor in AD’s pathogenesis, using thioflavin T fluorescence. All exhibited considerable antiaggregating activities at 50 μM with inhibitory ratios of 42.4, 38.1, and 36.1%, respectively. Epigallocatechin-3-gallate was used as a positive control (inhibitory ratio: 86.6% at 10 μM). Additionally, they were also tested for their anti-AChE but showed no activities. As an initial protease that processes APP in the pathway leading to the production of Aβ, β-site amyloid precursor protein-cleaving enzyme 1(BACE1) has long been regarded as a therapeutic target for the reduction of Aβ formation (Ghosh et al. 2008). In recent years, ten diterpenes from L. complanatum were assessed for their cholinesterase and BACE1 inhibitory potential. All of them did not inhibit AChE and BChE, but did inhibit BACE1. Based on fluorescence resonance energy transfer, an in vitro study showed that compounds (21β,24-dihydroxyserrat-14-en-3α-yl acetate, 16-oxo-21β-hydroxyserrat-14-en-3α-yl acetate, 16-oxo-21β,24-dihydroxyserrat-14-en-3α-yl acetate, 21β-hydroxyserrat-14-en-3α-yl acetate) could be interesting leads for BACE1–inhibiting molecules, thanks to the α-acetoxy moiety at C-3 that is one of the key factors of inhibition ability. Among them, 21β,24-dihydroxyserrat-14-en-3α-yl acetate and 16-oxo-21β-hydroxyserrat-14-en-3α-yl acetate showed a potent effect with IC50 values of 2.79 ± 0.28 and 2.49 ± 0.12 µM, respectively, indicating higher potency than quercetin, the positive control (IC50 value of 6.75 ± 0.54 µM). Notably, 16-oxo-21β-hydroxyserrat-14-en-3α-yl acetate was found to be a mixed-type inhibitor by using the Lineweaver–Burk and Dixon plot, and had the lowest BACE1 inhibition Ki values of 5.4. Therefore, these results revealed that 16-oxo-21β-hydroxyserrat-14-en-3α-yl acetate may be a promising BACEl inhibitor and a promising lead for further research on compounds for AD treatment (Nguyen et al. 2017).
Neuroprotective activity
The Lycopodium alkaloids dominated by Hup A have been recently found to exert versatile neuroprotective effects in cell and animal models under various pathological conditions by antagonizing neurotoxicity, inhibiting oxidative stress injury and apoptosis, inducing secretion of neurotrophic factors and hippocampal neurogenesis other than affecting the hydrolysis of synaptic acetylcholine (ACh) and the aggregation of Aβ described directly above. Notably, these multiple neuroprotective effects of Hup A are important in AD treatment.
A series of studies was launched investigating the link between Aβ toxicity and oxidation, which demonstrated that the production of free radicals and oxidative stress were involved in Aβ insults (Behl et al. 1994). Some antioxidants have been proved to rescue cells from Aβ toxicity. Fragment 25–35 of Aβ is the functional part that mediates the toxicity of whole-length Aβ (Pike et al. 1993). An in vitro study showed that Hup A (0.01–1.0 μM) protected rat pheochromocytoma line PC12 cells against Aβ25–35 (1 μM) insult through an antioxidant pathway. Compared with no Hup A treatment, an elevation in activities of catalase and glutathione peroxidase, the enhanced cell survival as well as a decrease in the level of malondialdehyde and superoxide dismutase (SOD) activity were observed (Xiao et al. 2000). Interestingly, SOD activity increased after the exposure of the cells to Aβ25–35, which might be a direct induction or otherwise a compensatory reaction against Aβ insult (Pappolla et al. 1998). Subsequently, Xiao et al. provided the first direct evidence that the protection of Hup A against Aβ25-35-induced apoptosis is related to inhibiting reactive oxygen species (ROS) formation and caspase-3 activation in primary rat cortical cultures. In this study, ROS-based fluorescence, caspase-3-like fluorogenic cleavage, and Western blot analysis demonstrated that Hup A (0.01–10 μM) reduced Aβ25-35-induced (20 μM) ROS formation in a dose-dependent manner, and 1 μM of Hup A attenuated caspase-3 activity at 6, 12, 24, and 48 h posttreatment (Xiao et al. 2002). Moreover, the concentration required to produce maximal attenuation in ROS formation was not quite correlated with its neuroprotective dose, possibly because ROS formation is a very early event in the Aβ cascade (Mattson et al. 1995). Dahlgren et al. reported that Aβ (1–42) oligomers inhibit neuronal viability tenfold more than fibrils and approximately 40-fold more than unaggregated peptide (Dahlgren et al. 2002). In an in vitro study, Hup A was added with a concentration of 10 μM for a 2-h preincubation before exposure to 5 μM Aβ (1–42) oligomer. The direct protein–protein interaction network analysis showed that Hup A protects neuroblastoma N2a cells against Aβ oligomer-induced cell death by downregulation of cellular tumor antigen p53 expression (Tao et al. 2013).
It is known that glutamate is the most toxic excitatory amino acid in the brain, which can induce degeneration of hippocampal neurons and cause damage to neurons. Previous studies have suggested that Hup A act on glutamate receptors to exert its neuroprotective effects and reckoned antagonism of Hup A at N-methyl-D-aspartate (NMDA) receptor as one of mechanisms for ameliorating abnormal lipid peroxidation (Ved et al. 1997; Shang et al. 1999; Wang et al. 1999). Pretreatment of cultured brain neurons with Hup A (100 μM) reduced glutamate-induced neuronal cell death and calcium mobilization, but did not affect the increase in intracellular free calcium channel induced by exposure to high KCl or a calcium activator Bay-K-8644 (Ved et al. 1997). Further, in another in vitro study, Hup A (0.1–300 μM) reversibly inhibited NMDA (100 μM)-induced current in acutely dissociated hippocampus pyramidal neurons in a concentration-dependent manner with IC50 of 0.49 μM (Wang et al. 1999). These results demonstrated that Hup A acted directly on NMDA receptor to achieve a neuroprotective effect.
The neuroprotective effect of Hup A was also realized by preventing apoptosis of neuron cells. An in vitro study showed that Hup A can attenuate staurosporine-induced apoptosis of primary cortical neurons by upregulation of bcl-2, downregulation of bax and blockade of decrease in immunoreactive caspase-3 proenzyme. MTT-reduction was decreased to 68% of the control level after 24 h exposure of the primary cortical neurons to 0.5 μM staurosporine. This effect was significantly attenuated by incubation with Hup A (0.1–100 mM) starting 2 h before staurosporine. Hup A had the maximal neuroprotective effect at a concentration of 1 μM, where it increased MTT signal to 84% of the control (Zhang et al. 2003). Similarly, Hup A (1 μM) exerted significant protection against H2O2-induced (100 μM) apoptosis through improving expression of apoptosis related genes (Wang et al. 2001). In addition, an in vivo study was performed to explore the effects of Hup A on relieving the acute hypobaric hypoxic-induced apoptosis of hippocampal neurons in rats. Compared with high altitude group, lower rate of hippocampal neurons apoptosis, down-regulated expression of Bax and up-regulated expression of Bcl-2 in the hippocampus tissues were found in the high altitude + Hup A group rats that given intragastrically with Hup A suspension (10 mg/mL) in a dose of 0.1 mg/kg before one day of the decompression simulation experiment (Shi et al. 2013). However, the positive group were absent in above studies.
Although Hup A has been shown to exert multiple beneficial effects in brain, few of its actions on adult hippocampal neurogenesis have been established. Hup A can activate mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) signaling pathway, which is a well-known regulator of biological processes including cell proliferation and differentiation. Recent studies have shown the modulating role of the MAPK/ERK pathway in neurogenesis (Lu et al. 2011; Hao et al. 2004). In vivo and in vitro studies carried out by Ma et al. suggested that Hup A enhances neurogenesis through promoting cell proliferation by a MAPK/ERK-dependent mechanism in cultured hippocampal neural stem cells (NSCs). An in vitro study showed that Hup A (0.01–100 μM) promoted the proliferation of cultured mouse embryonic hippocampal NSCs. Besides, intraperitoneal injection of Hup A (0.2 mg/kg/day for 4 weeks) increased the newly generated cells in the subgranular zone (SGZ) of the hippocampus in adult mice (Ma et al. 2013).
Alkaloids (complanadine A, complanadine B, lyconadin A, lyconadin B) isolated from L. complanatum have been detected to induce secretion of neurotrophic factors from human astrocytoma cells. In an in vitro study, human astrocytoma cells (glial cell line) were incubated for 2 days with 1–10 μM complanadine A, and then rat pheochromocytoma (PC-12) cells were cultivated for 2 days in the conditioned 1321N1 culture medium. Phorbol 12-myristate 13-acetate (100 μM), an activator of neurotrophic factor biosynthesis, was used as a positive control. As a result, the culture medium showed to contain neurotrophic factors synthesized in 1321N1 cells, which promote the differentiation of PC-12 cells in a does dependent manner (Morita et al. 2005a). Additionally, releasing activities of neurotrophic factors from 1321N1 human astrocytoma cells by complanadine B, lyconadins A and B were examined in a subsequent study using a semiquantitative RT-PCR method, in which enhancement of the mRNA expression for NGF was observed (Ishiuchi et al. 2006a). However, according to the organized Table 4, almost all the experiments did not set up a positive control. Therefore, it is necessary to add positive controls to verify the neuroprotective activity of the extracts or secondary metabolites of plants of genus Lycopodium.
Anti-tumor activity
Traditional herbal medicines have attracted a great deal of attention as alternative cancer therapy due to their low toxicity and costs. Many plant-derived bioactive constituents, such as camptothectin (from Camptotheca acuminata), paclitaxel (from Taxus brevifolia), and vinblastine (from Catharanthus roseus), or plant-based semisynthetic compounds (e.g., cabazitaxel, docetaxel, vinorelbine), have been discovered and commercialized as cancer therapeutics (Vickers et al. 2002). Extracts and secondary metabolites from plants of genus Lycopodium also show obvious antitumor effects, even though their antitumor effects are rarely recorded in traditional usage. The mechanisms include reducing migration and inducing apoptosis of tumor cells. Detailed information about these activities is listed in Table 5.
The results of an in vitro study clearly showed that ethanol extract of L. serratum (ELS) (5, 10, 25, 50 μg/mL) could decrease the ability of wound healing of LPS-treated C6 glioma cells in a dose-dependent manner, compared with the untreated control. It also indicated that the mechanisms of ROS scavenging and suppressing matrix metalloproteinase-9 (MMP-9) expression are involved (Park et al. 2018). However, there was no positive group in this study, and anti-tumor activity in vivo was not evaluated either. At present, few studies on the reduction of tumor cell migration of Lycopodium species have been reported, which is worthy of further investigation in the future.
Another major mechanism of antitumor effect of Lycopodium species is the induction of tumor cells apoptosis. It was reported that the crude ethanol extract of L. clavatum contains certain alkaloids, in which lycopodine is the major active principle. In an in vitro study, the effect of lycopodine on viability of HeLa cells was measured by MTT assay. Lycopodine (50, 100, 150, 200 µg/mL) reduces the viability of HeLa cells in a dose-dependent manner and time-dependent. However, IC50 value was not presented. Further studies have shown that lycopodine can inhibit the proliferation of HeLa cells through induction of apoptosis via caspase-3 activation (Mandal et al. 2010). Lycopodine also had a good inhibitory effect on hormone sensitive (LnCaP) and refractory prostate cancer cells (PC3). Lycopodine (74, 148, 222 mM) could down-regulate the expression of 5-lipoxygenase and the 5-oxo-ETE receptor (OXE receptor1) and epidermal growth factor (EGF) receptor, thus effectively causing up-regulation of cytochrome c with depolarization of mitochondrial inner membrane potential, eventually leading to cell apoptosis. However, the tested three doses of lycopodine are too large, and the IC50 value is not specified. Concomitantly, circular dichroism (CD) spectroscopic analysis showed that lycopodine could be inserted into DNA molecules to inhibit the synthesis and replication of DNA (Bishayee et al. 2013). An overall analysis of results showed that lycopodine is a promising candidate suitable for therapeutic use as an anti-tumor drug. But these studies lack positive controls and data from in vivo studies, which is a concern for subsequent studies of lycopodine. Lycopodine also has anti-AChE activity and anti-inflammatory activity, but less research has been done. The main effect of lycopodine is still anti-tumor activity, and other pharmacological activities of lycopodine could be further studied.
In another in vitro study, L. serratum (6–100 μg/ml) significantly inhibited cell viability, in which 100 μg/ml was found to be the most active dose. At this dose, L. serratum induced apoptosis of SK-Hep1 (75.7%), HT-29 (71.7%), A549 (53.8%) and HL-60 (89.2%) cell. Among these, HL-60 cells were highly sensitive to growth inhibition and apoptosis induced by L. serratum. Subsequent investigations indicated that serratenediol, a triterpenoid obtained from the methylene chloride fraction of L. serratum, strongly inhibited the proliferation of HL-60 cells with IC50 = 12.9 μΜ. An analysis of the mechanism indicated that serratenediol (6.25, 12.5 and 25 μM) treated HL-60 cells had hallmarks of apoptotic events, including increased ratio of Bax/Bcl-xL, released the cytochrome c, activated caspase-9, -3, and cleaved poly-ADP-ribose polymerase (PARP) (Ham et al. 2012). Nevertheless, the IC50 values of L. serratum-induced apoptosis in these cells were not presented, preventing comparison of the sensitivities among different cells.
As reports show, the new skeleton secondary metabolites including lycojaponicumin A, lycojaponicumin B and lycojaponicumin C all had significant inhibitory effects on the growth of ileocecal cancer cells HCT-8 with IC50 = 1.15 ± 0.17, 1.33 ± 0.11 and 2.04 ± 0.21 μM, respectively (Jiang et al. 2013a; b; Wu et al. 2013a). Meanwhile, lycojaponicumin A (0.625, 1.25, 2.5, 5.0, 10.0 μg/ml) can inhibit the proliferation and induce apoptosis of leukemia HL-60 cells in a dose-dependent manner (Jiang et al. 2013c), and lycojaponicumin C significantly inhibited the growth of gastric cancer cell lines (HGC-27, MGC-803, BGC-823, SGC-7901) with IC50 = 3.21 ± 0.33, 1.12 ± 0.17, 0.87 ± 0.07 and 1.33 ± 0.19 μM and liver cancer cell lines (HepG2, MHCC-LM3, Bel-7402, HuH-7) with IC50 = 2.26 ± 0.32, 1.46 ± 0.21, 2.41 ± 0.11 and 3.21 ± 0.37 μM, respectively. (Wu et al. 2013b; Wu et al. 2013c). In addition, lycophlegmarin, a serratane-type triterpene, exhibited modest growth-inhibitory activity in vitro against human hepatoma (BEL-7402) cells (Shi et al. 2005). Therefore, several of the secondary metabolites mentioned above may be used to prepare as anti-tumor drugs and have great prospects for development and application. However, none of these secondary metabolites have been evaluated for their antitumor activity in vivo, and their detailed mechanism of action has not been reported either.
However, positive control was lack in all of the experiments (Table 5), and positive controls should be added to confirm the anti-tumor activity of these secondary metabolites.
Anti-inflammatory activity
Secondary metabolites from plants of genus Lycopodium have shown notable anti-inflammatory activities. More information related to these activities is present in Table 6. The main effective substances associated with this effect are demonstrated to be Hup A and the extract of Lycopodium japonicum Thunb. L. japonicum has been used as a TCM, which has a long history of treating the inconvenient flexing and stretching, wind dampness arthralgia syndrome, bruises, and other diseases with definitely curative effects. In addition, it has been used in traditional medicine around the world to treat inflammation related diseases such as dermatitis, enteritis and hepatitis, which also strongly relate to the anti-inflammatory activity of Lycopodium plants.
LPS-induced rat microglial cells were treated with various doses of Hup A (0.1, 1, 10 μmol/L) in vitro, and the results suggested that this secondary metabolite decreased the releasing of major inflammatory factors such as IL-6, TNF-α and NO (Huang et al. 2017). But the information of positive control group was absent in this study. An in vivo investigation revealed that different doses of Hup A (0.1, 0.3, 0.9 mg/kg, i.p.) had certain inhibitory effects on foot swelling caused by egg white in mice (Shi et al. 2014). Nevertheless, the study did not establish a positive control group. Before this study, Gu et al. intended to establish the model of presbycusis rats by subcutaneous injection of D-galactose to simulate the aging mechanism of the body. The result showed that Hup A (0.1 mg/kg) blocked Schwann cells, inhibited the activation of NF-κB, and then inhibited the release of inflammatory factors (such as IL-1β, IL-6, TNF-α), which played a local anti-inflammatory effect on cochlear tissue (Gu et al. 2014). However, there was only one dose in the study that could not adequately reflect its anti-inflammatory strength and the benefit of Hup A for the treatment of presbycusis needs to be confirmed by further clinical research.
In an in vivo study, Zeng et al. compared the anti-inflammatory effect of different extracts (chloroform extract, n-butanol extract and water extract) of L. japonicum (40 g/kg) by using rat ear dimethyl benzene-induced inflammation method, acetic acid induced abdominal inflammation method and rat metatarsal edema method. The result showed that L. japonicum has significant anti-inflammatory pharmacological effects, and its effective components are concentrated in chloroform extract (Zeng et al. 1999). However, there was no analysis of phytochemical composition and positive control. Furthermore, another study showed that the alkaloids of L. japonicum (30, 60, 120 mg/kg) significantly inhibited the swelling of arthritis induced by Complete Freund's Adjuvant (CFA) in rats, and improved the synovial lesions of ankle joint in rats with a positive control (etoricoxib, 1 mg/kg) and a vehicle group. The mechanism may be related to the decrease of IL-1β and TNF- α levels in vivo (Liu et al. 2019). Besides, Orhan et al., from Turkey, found that chloroform extract of L. clavatum (LC) and the alkaloid fraction could inhibit acetic acid induced increasing in capillary permeability and had an obvious anti-inflammatory effect at a dose of 500 mg/kg having percentage of inhibition 24.3 and 32.1, respectively. Indomethacin (10 mg/kg) was used as the positive control. Among them, the anti-inflammatory activity of the aerial parts of LC are primarily due to the alkaloidal components, which might most probably be lycopodine (84.5%) as the major compound (Orhan et al. 2007a). However, only one dose was tested in above study.
In addition, acetyldihydrolycopodine and stigmastane-3-oxo-21-oic acid isolated from L. obscurum and methyl p-coumarate isolated from L. japonicum could significantly inhibit nitric oxide production in dose-dependent manner (6.25, 12.5, 25, 50 μM), using dexamethasone (10 μM) as a positive control. It was suggested that they had potential anti-inflammatory activities with IC50 = 46.84 ± 9.64 for acetyldihydrolycopodine and 10.73 ± 4.12 μM for methyl p-coumarate, respectively. (Yang et al. 2015). Nevertheless, the IC50 value of stigmastane-3-oxo-21-oic acid was not mentioned. An in vitro study showed that six alkaloids (8β-hydroxylycodoline, 11β-hydroxy-12-epilycodoline, lycopodine, deacetylfawcettiine, acetyllycofawcine, α-lofoline) isolated from L. japonicum displayed moderate activities against LPS-induced pro-inflammatory factors in BV2 macrophages with IC50 in the range of 4.23–49.03 μM. Among them, α-lofoline showed the strongest activity with an IC50 value of 4.23 μM. Untreated culture cells were used as a negative control and curcumin was used as the positive control (IC50 = 3.12 μM) (Wang et al. 2009). Reportedly, obscurumine O (1.25, 2.5, 5, 10, 20, 40 μM), a lycopodine-type Lycopodium alkaloid from L. obscurum, exhibited inhibition of the secretion of IL-2 in phytohemagglutinin (PHA) and phorbol myristate acetate (PMA) stimulated Jurkat cells with dexamethasone as positive control, and the IC50 value of obscurumine O was 17.2 μM (Jiang et al. 2016). But the dose of positive control was not presented. As above data show, a series of hydrophobic alkaloids from plants of genus Lycopodium exhibited potential anti-inflammatory effect in vitro. However, there is a lack of in vivo investigation and mechanism report of these secondary metabolites, and thus related study on anti-inflammation in vivo is expected to offer new evidence for the clinical use of Lycopodium species.
Other activities
The in vitro antimicrobial properties of the petroleum ether, chloroform, ethyl acetate, methanol extracts and alkaloid fraction of L. clavatum (0.25–512 μg/mL) were evaluated against two Gram-positive, five Gram-negative bacteria, as well as two yeast-like fungi using micro-well dilution method. Results revealed that Staphylococcus aureus (ATCC 25,923) is the most susceptible bacteria with a minimum inhibitory concentration (MIC) of 4 μg/ml and all the extracts possess noteworthy activity against it. Ampicilline (MIC < 0.12 μg/ml) and ofloxacine (MIC = 0.5 μg/ml) were served as positive controls for the antibacterial tests. As for the antifungal tests using ketoconazole (MIC = 1 μg/ml) and fluconazole (MIC = 4 μg/ml) as positive controls, ethyl acetate, methanol extracts and alkaloid fraction inhibited Candida albicans (ATCC 10,231) and C. parapsilosis (ATCC 22,019) (MIC = 16 μg/ml) moderately but better than petroleum ether and chloroform extracts (MIC = 32 μg/ml) (Orhan et al. 2007b). The SAP of Candida albicans have been shown to be a major virulence factor in Candida infections (Hoeǵl et al. 1999). Several secondary metabolites isolated from L. cernuum were tested in a Candida- SAP inhibition assay at three concentrations (50, 10, 2 μg/ml). Only a serratene triterpene (3β, 14α, 15α, 21β, -29-pentahydroxyserratane-24-oic acid) and a flavone glycoside (apigenin-4′-O-(2′′, 6′′-di-O-p-coumaroyl)-β-D-glucopyranoside) were active, with IC50 value of 20 and 8.5 µg/mL, respectively. The aspartic protease inhibitor pepstatin A was used as a positive control (IC50 = 0.0015 µg/mL) (Zhang et al. 2002a). In addition, the chloroform extract of L. clavatum exerted good antiviral effect towards the DNA virus HSV (8–16 μg/ml). The maximum nontoxic concentration (MNTC) is 16 μg/ml, which is similar to that of acyclovir (< 0.25 to 16 μg/ml), except for its therapeutic range was narrower. In particular, the alkaloid fraction showed quite similar anti-PI-3 effect and MNTC value to that of oseltamivir (< 0.25 to 32 μg/ml) (Orhan et al. 2007b). Encouraged by the records on traditional use of several Lycopodium species as anti-infective in Turkey, Orhan et al. assessed in vitro growth-inhibitory activity of different extracts (0.123–90 μg/mL) of L. clavatum and L. complanatum subsp. Chamaecyparissus against clinically relevant stages of Trypanosoma brucei rhodesiense (bloodstream forms), Trypanosoma cruzi (intracellular amastigotes in L6 rat skeletal myoblasts), Leishmania donovani (axenic amastigotes) and Plasmodium falciparum (blood stage forms of K1 strain resistant to chloroquine and pyrimethamine). Both fern species showed anti-protozoan activity, indicating that their extracts may have the potential to provide novel antiprotozoal agents and deserve further phytochemical investigations (Orhan et al. 2013). This discovery confirmed the traditional uses of the treatment of some infectious diseases, like dysentery, rubella, intestinal infection and viral hepatitis caused by bacteria or viruses. It has also been reported that Lycopodium alkaloids have cardiovascular and auditory function protective activities (Jiang et al. 2015; Ishiuchi et al. 2016; Kong et al. 2013). The ethanol or aqueous extracts of L. clavatum and L. serratum showed some anti-diabetes and wound healing activities (Tam et al. 2011; Manjunatha et al. 2007) (see Table 7). However, these studies reported in the literature are too preliminary and seriously suffer from important experimental shortcomings, such as single dose-testing and absence of appropriate positive and negative controls.
Clinical trials
Clinical trials with Lycopodium alkaloids have been carried out since the 1990s after the anti-AChE activity of Hup A was detected. In 1996, Hup A was approved as a second-generation AChE inhibitor for the treatment of AD in China according to the second class of new drugs (Wang et al. 2006a). It was approved for listing by the FDA of the United States as a dietary supplement in 1997. In recent years, many clinical trials have been published, which confirm that Hup A has a good therapeutic effect on patients with early and middle AD, and also has a certain therapeutic effect on cognitive dysfunction associated with vascular dementia (VaD), schizophrenia, Parkinson's disease, diabets and other diseases.
Treatment of AD
Most of clinical trials of Hup A in the treatment of AD have been performed in China, where an estimated more than 100,000 people have been treated by Hup A. Results of these studies indicate that Hup A is a safe and effective drug that improves cognitive function. In 2002, Zhang et al. conducted a placebo controlled, randomized, and multicenter study with 202 AD patients. One group of 100 patients was administered 400 μg/day Hup A for 12 weeks and 102 patients received placebo. The treatment group displayed improvements in cognition measured on the Alzheimer's disease Assessment Scale (ADAS-Cog) as well as an increase in behavior and mood (ADAS non-Cog) and improvement in the ability to do activities of daily living (ADL). Hup A had good safety, with mild and transient adverse events (insomnia and edema of bilateral ankles) occurring in 3% of Hup A treated patients (Zhang et al. 2002b). Later, Qiu et al. and Li et al. got similar results in their researches (Qiu et al. 2009; Li et al. 2011). A study of the safety and efficacy of Hup A in the United States observed dose-related improvements with higher Mini-Mental State Examination (MMSE) scores at higher dosage, and no serious side effects. 26 patients met the Diagnostic and Statistical Manual of Mental Disorders—Fourth Revision (DSM IV-R) and the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS-ADRDA) criteria for uncomplicated AD and probable AD. An oral dose of 50 μg Hup A was given to 22 patients twice a day, and the 4 other patients received a dose of 100 μg twice daily. This study lasted 3 months. A mean dementia baseline score of 22.6 was measured with the MMSE. The changes in this score, for the 50 μg group and for the 100 μg group, respectively, were 0.5 and 1.5 points at 1 month; 1.2 and 1.8 points at 2 months, and 1.1 and 1.0 points at 3 months (Mazurek et al. 2000). In addition, a multicenter randomized controlled study was conducted in the Department of Neurology, University of California School of Medicine. 210 patients with mild to moderate AD were randomly divided into three groups. 70 patients in each group were given placebo, Hup A 200 or 400 μg twice a day, respectively, for a period of not less than 16 weeks. The results showed that the cognitive score of AD rating scale (ADAS-Cog) in Hup A 200 μg group was not improved, but the ADAS-Cog score in 400 μg group was increased by 2.27 points, and that in the corresponding placebo group was decreased by 0.29 points (Rafii et al. 2011).
In view of the fact that AD is a neurodegenerative disease with multiple etiologies and complicated pathogenesis, it has become a new therapeutic strategy to explore multi-target drugs or combination drugs for the treatment of AD. The multi-target effect and the good efficacy of Hup A in combination with other drugs indicating a broad prospect in clinical application. In the future, it still needs to be further expanded and further studied in clinical practice in terms of indications, therapeutic dose, combination method and treatment course. The clinical study of stricter design, larger sample size, longer treatment cycle and adjusting dose within the range of dosage will have more guiding significance for the rational application of Hup A in the treatment of AD.
Treatment of VaD
The pathological conditions of ischemia and hypoxia in the brain of the patient can also cause damage to the central cholinergic system, and the clinical application proves that the Hup A can also relieve the dementia symptoms of the patients. After 6 to 48 weeks of twice-daily oral Hup A (0.1–0.2 mg) treatment, hundreds of patients with VaD showed significant improvement in MMSE, Clinical Dementia Rating (CDR) and ADL compared to conventional treatment (Zhao et al. 2007; Zhang et al. 2011). The patients taking both the Hup A and the medicine that improve cerebral circulation and dilate blood vessels, such as Ginkgo biloba preparations and Nimodipine Tablets, had better therapeutic effect (Ma et al. 2007a; Fan et al. 2009; Zhang et al. 2007).
Treatment of other cognitive disorders
In patients with schizophrenia, taking antipsychotic drugs (such as cloproterol, clozapine, etc.) at the same time, twice-daily treatment with Hup A (0.1–0.2 mg) for 4 to 12 weeks could help to improve the cognitive function of patients (Chen et al. 2007; Liang et al. 2009). Yao et al. used Hup A (0.1 mg) combined with cognitive function rehabilitation training to treat patients with Parkinson's disease. After 6 weeks of treatment three times a day, the results of WMS score showed that the cognitive function of the patients was improved (Yao et al. 2006). Besides, Wang et al. injected 0.3 mg Hup A intravenously 30 min before anesthesia in 15 patients under general anaesthesia. It was found that the level of ACh in the brain of the patients during recovery was increased, which was helpful to the recovery of cholinergic nerve function in the brain (Wang et al. 2006b). After taking Hup A (0.1 mg) three times a day for 8 weeks, the MMSE score, latency and amplitude of diabetic patients were improved before treatment (Zhang et al. 2008). The deficiency was that most of the trial treatment courses were short, the number of samples was small, and the positive controls were lacking.
Toxicity and side effects
There are few data about the toxicity or side effect of species of the genus Lycopodium. But the plants such as L. serratum, L. japonicum and L. clavatum still have a certain level of toxicity.
The Great Dictionary of Chinese Medicine (《中药大辞典》) points out that the use of L. serratum to take care of the overdose, which could lead to poisoning, dizziness, nausea, vomiting and other symptoms (Wang et al. 2012a, b). Especially for pregnant women, it could cause fetal stunting, deformities and miscarriages in severe cases. This indicated that the L. serratum has certain toxicity. Meanwhile, Hup A isolated from L. serratum is important in AD treatment. The LD50 of Hup A mice was given intravenously at 10.2 μmol/kg and intraperitoneally at 7.4 μmol/kg, respectively. The experimental results showed that the acute toxicity of Hup A to mice and rats was significantly lower than that of physostigmine (Phys), neostigmine (Neos) and Gal. The therapeutic indexes of Hup A in mice and rats were 23.1 and 72.9 respectively, which were 7–10 times larger than Phys and most of the Lycopodium alkaloids have certain toxicity, among which the toxicity of lycopodine is relatively high. The LD50 of lycopodine injected into mice by intraperitoneal and intravenous injection is 78 mg/kg and 28 mg/kg, respectively. The main symptoms of poisoning in mice, frogs and rabbits are hyperexcitability, rigidity and paroxysmal convulsion, asphyxia, paralysis and death. However, the toxicity of clavatine and clavatoxine is slightly less than that of lycopodine. The LD50 of clavatine is 50 mg/kg for cats and 100–200 mg/kg for rabbits and rats, while clavatoxine has excitatory effects on the respiration of mammals and can paralyze the central and peripheral nervous system of frogs.
The chloroform, n-butanol, and water extracts of L. japonicum after acute toxicity test were intragastrically administrated to NIH mice at 0.4 mL/10 g body weight respectively. The results showed that the maximum tolerance dosage of the three extracts was more than 80 g crude drug/kg body weight, which indicated that the herb had low toxicity (Zeng et al. 1999). It is not safe to use L. japonicum externally. A case of contact dermatitis caused by L. japonicum was diagnosed and treated by Li Suping in 1995. External application after mashing the plant, the local skin felt burning pain after about two hours. Twelve hours later, the external skin appeared the phenomenon of red swelling, blisters and severe pain. It was diagnosed as contact dermatitis. After the use of L. japonicum was stopped, it was cured by anti-allergy treatment within a week (Li et al. 1995).
Sharma et al. conducted a toxicity assessment with Denio rerio (Zebrafish) model in vivo. This experiment using homeopathy, Zebrafish embryos were treated at different concentration (1, 5 and 10%) of L. clavatum mother tincture for 120 h and was examined these developmental defects at 24, 48 and 72 hpf (hour post fertilisation). These results indicate that 10% of L. clavatum mother tincture was found to be highly toxic as mortality rates of 80% at 24 hpf and 100% at 48 hpf. Whereas 5% of L. clavatum mother tincture showed 50% mortality rate of 24 hpf and remained constant at 48 and 72 hpf with moderate toxicity and side effects. However, 1% of L. clavatum mother tincture is considered completely safe and non-toxic to zebrafish embryos. Therefore, L. clavatum mother tincture affects the normal growth and development of zebrafish embryos in a dose-dependent manner (Sharma et al. 2019).
At present, the study on the toxicity of the genus Lycopodium is insufficient. Further studies are required to reveal the possible toxicity and side effect of Lycopodium species in order to better explain the safety of them and provide security for clinical medication.
Quality control
The most common used medicinal herb of this genus is the whole plant of L. japonicum. In Chinese Pharmacopoeia 2015 edition, only microscopic and TLC identification was required for the quality standard of this herb. In order to make a comprehensive evaluation on the quality of L. japonicum, Zou et al. used HPLC method to determine the content of α-obscurine in 10 batches of L. japonicum at different altitudes. The chromatographic separation was conducted at 25 °C on Hypersil ODS and Spherigel C18 Columns (200 × 4.6 mm, 5 μm), with methanol and water (65:35, V/V) as the mobile phase at a flow rate of 1.0 mL/min, and the wavelength for detection is at 248 nm. The content of α-obscurine was between 0.083 and 0.215%, which was related to the origin but not to the altitude. α-Obscurine is an effective component with anti-inflammatory and anti-platelet aggregative activities, which can be used as a marker for the quality control of L. japonicum (Zou et al. 2010). You et al. successfully identified flavonoids, triterpenoids and alkaloids in L. japonicum by TLC with high specificity and sensitivity. Meanwhile, HPLC was used to determine the content of α-obscurine, with good reproducibility and high accuracy. However, the contents of flavonoids and triterpenoids were not determined (You et al. 2012). Furthermore, an HPLC fingerprint analysis was established by Yang for the analysis of L. japonicum. A total of 19 mutual peaks were indicated, and the similar degrees of the ten batches were between 0. 923–0. 985, which provided a basis for effective control and scientific evaluation of the quality of this herb. However, none of the indicated peaks was identified (Yang et al. 2013). Except for α-obscurine, there is no specific study implemented for the content of other alkaloids in L. japonicum, thus more research is needed to improve the quality standard of L. japonicum.
Hup A isolated from L. serratum is a reversible AChE inhibitor, which has significant effects on Alzheimer's disease, simple memory impairment, myasthenia gravis, etc. (Liu et al. 1986; Zhang et al. 1991). Thus, it is a representative marker for quality standard of medicinal species of the genus Lycopodium. Determination of Hup A in different parts of L. serratum from 12 producing areas revealed that the highest content of Hup A was 0.0900%, the lowest content was 0.0014%, and the content in leaves was generally higher than that in stems and roots. The analytical conditions were carried out as follows: using an UItimate XB—C18 column (4.6 mm × 250 mm, 5 μm), with the mobile phase of acetonitrile—methanol—water (10:55:240) containing 0. 32% phosphoric acid and 0. 5% triethylamine (Yuan et al. 2012). Wang et al. found that the average content of Hup A in the seedlings and young plants was significantly higher than that in mature plants, and there were significant differences in the average content in different areas. The content of Hup A ranged from 0.0447% to 0.066%, which was seriously affected by habitat factors. Among them, the annual rainfall was negatively correlated with the content of Hup A, whereas elevation and annual average temperature had no significant effect (Wang et al. 2014). Then Lai et al., found that the dynamic variation of Hup A in the plant was December > September > June > March, and the highest was 325.9 μg/g. But only two regions of plants were measured, and the rule was not fully representative (Lai et al. 2014). In addition to the commonly used HPLC method, Li et al. used the liquid chromatography tandem mass spectrometry (LC–MS) method to determine the content of Hup A in different parts of plants from four producing areas. The chromatographic separation was conducted at 28 °C on an ZORBAX SB—C18 column (250 × 4.6 mm, 5 μm), and the mobile phase at a flow rate of 0.5 mL/min was composed of methanol and water (90:10, V/V). The results showed that the content in the leaves and stems was between 0.02% and 0.06%, and the content in leaves was greater than that in stems. However, the content of Hup A of the whole plant was not determined (Li et al. 2017).
The above results showed that the content of Hup A in L. serratum is relatively low, and the content of Hup A in the plant in different regions also varies greatly under the influence of environment.
To sum up, the quality evaluation research of plants of this genus is too few, and there is no ideal standard for qualitative identification and content determination of active components or indicator components. Therefore, it is necessary to apply various advanced quality control methods and establish scientific and comprehensive quality control methods on the basis of in-depth and systematic study on chemical components of those herbs.
Conclusion and further scope
In the present review, we summarize knowledge on traditional uses, secondary metabolites, pharmacology, toxicity, clinical trials and quality control of the Lycopodium plants. In view of the wide distribution of club-mosses, extensive literature survey revealed that most of the species are used ethnopharmacologically in different Europe, America and Asia countries. Since 1840s, the studies have resulted in isolation of 508 secondary metabolites including alkaloids, triterpenoids, glycosides and others. Many pharmacological studies were carried out using various in vivo and in vitro biological techniques after the detection of anti-AChE activity of Hup A in 1990s. In terms of current research, Lycopodium alkaloids and serratene triterpenoids as the main active ingredients exhibit a variety of biological activities including anti-AD, neuroprotection, anti-tumor, anti-inflammation and so on, which supports most of traditional medicinal uses of Lycopodium plants. For example, L. saururus and L. serratum have been popularly used in Chinese and Europe traditional medicine for its memory-enhancing effect since centuries. It indicated that these plants may treat central nervous system-related diseases. And now available studies have showed neuroprotective activity and a certain therapeutic effect on AD of alkaloids dominated by Hup A and few triterpenoids isolated from plants of genus Lycopodium. Moreover, bioassay-guided fractionation of the alkaloid fraction of Lycopodium species revealed that the alkaloids are responsible for the anti-inflammatory activity of the extract, which supports the traditional uses of treating inflammation-related ailments such as dermatitis and rheumatic arthritis. On the other hand, pharmacological studies on Lycopodium species have also explored the effects such as antitumor and cardiovascular protection not found in ancient times. However, gaps exist in the scientific studies on Lycopodium plants. therefore, we provide a summary several topics that should have priority for detailed investigation.
Firstly, the use of an infusion of Lycopodium species is widely known in traditional medicine around the world, and the most common form in traditional medicine is water decoction. However, modern secondary metabolites and pharmacological researches focus on alkaloids, triterpenoids and some hydrophobic extracts (chloroform, ethanolic, ethyl acetate, petroleum ether). Further, a hydrophilic secondary metabolite [apigenin-4′-O-(2′′,6′′-di-O-p-coumaroyl)-β-D-glucopyranoside] has been found to exhibit remarkable antifungal activity. Thus, much more effort should be paid to investigate the bioactivity of water-soluble components.
Secondly, most of the investigations regarding anti-AD, anti-tumor, anti-microbial, antiviral and cardiovascular protection currently collected were limited to the in vitro studies without a detailed exploration of the exact molecular mechanism of action, which may be due to the complex pathogenesis, involving a variety of pathways such as Alzheimer's disease. Besides, the in vitro studies of the hydrophobic extracts of Lycopodium species showed anti-microbial and antiviral activity, but the extracts were not standardized and the results require confirmation in vivo. Therefore, mechanism-based in vitro and in vivo investigations should be performed to further understand the underlying mechanisms linked to ethnopharmacological uses.
Thirdly, anti-microbial and antiviral activity shown in pharmacological studies mainly come from various extracts (petroleum ether, chloroform, ethyl acetate, and methanol) and alkaloids fraction. From a limited number of studies, alkaloid fraction might be considered worthy of advance phytochemical investigation to find out the active ingredients, although lycopodine, the major alkaloid of L. clavatum and L. complanatum, seems to be possibly responsible, which needs to be confirmed. Plant extracts are known to have a quite complex mixture and therefore, high probability of competing or synergistic interactions within the same extract may exist for any biological activity. Thus, further study should be directed toward bio-assay-guided isolation of the bioactive metabolites, studying their mechanisms of action individually or in combination.
Fourthly, there has been quite a number of researches on the structure–activity relationship of the Lycopodium alkaloid against AChE, but the exploration of structure–activity relationship in terms of other pharmacological activities is still lacking. Stimulating cholinergic anti-inflammatory pathway (CAP), a neuroscience and immunology pathway discovered back in 2002, can suppress local and systematic inflammatory responses. But so far, it is not clear about the structure–activity relationship of alkaloids and how they activate the cholinergic anti-inflammatory pathway. Thus, more in-depth investigation of structure–activity relationships should be performed.
Fifthly, in traditional medicine, herbs from the genus Lycopodium was generally reputed to be nontoxic except for L. selago that was known to be toxic and has been used for centuries to induce abortion. In addition, from the Argentinian ethnomedical references, the infusion is indicated to be the safest method, while the decoction can cause severe adverse reactions such as vomiting, drunkenness, convulsions, diarrhea, abortion in pregnant women and even death, which was deduced that the way in which it is prepared has an important significance for the achievement of the effects that are attributed to it. Today, some Lycopodium plants and extracts are not commonly employed as herbal remedies as the side effects often exceed the benefits. According to the existing toxicity studies, most of the Lycopodium alkaloids have certain toxicity, among which lycopodine is highly toxic, and it can be speculated that the toxicity may mainly come from lycopodine class and lycodine class alkaloids. Therefore, it is essential to comprehensively study the potential toxicity and toxicity target organs of genus Lycopodium, formulate the safe starting dose for human use and safety parameters for monitoring and better serve for clinical safe drug use.
Sixthly, as a kind of fern, the spores are the main breeding methods of genus Lycopodium. The application of club moss spores directly to wounds and rashes is well known from natives in North America and Europe. In TCM, these spores namely “Shi Song Zi” are used as powder to treat skin erosion. Unluckily, there have been almost no documented studies on it.
Finally, among the ferns, many species have been widely used in medicine since ancient times to treat various diseases for the people, and genus Lycopodium is no exception. With the acceleration of the world aging population, the demand of Hup A is increasing. The wild resources of the Lycopodium species, especially L. serratum are exhausting, but it is extremely difficult to reproduction. Although some experiments such as the success of stem tip culture of L. selago bring hope to overcome this problem (Wojciech et al. 2005), there is a long way to go for mass production economically. Therefore, the increasing biotechnological research aimed at rapid reproduction of Lycopodium species is an urgent need of society.
Overall, we reviewed the traditional uses and studies of secondary metabolites, pharmacology, toxicity, clinical trials and quality control in recent 80 years. The research characteristics of Lycopodium plants are clearly understood. There is a good correspondence between traditional uses and pharmacological activities. The activities not shown in traditional uses found in modern pharmacological studies suggest that further research on active ingredients is worthwhile. Large quantities of alkaloids have been isolated from Lycopodium species, which showed abundant bioactivities in pharmacological studies due to their unique structures. Hup A also has been clinically used for quite a period of time because of its proven potent, reversible, and selective anti-AChE activity. Future research should focus on the molecular mechanism and structure–activity relationship of Lycopodium alkaloids. Last but not the least, other active ingredients (water-soluble and liposoluble) have rarely been purified and identified due to past emphasis on alkaloids and some triterpenoids. Therefore, studies of other types of secondary metabolites and possible mechanisms of synergy cannot be overlooked.
Abbreviations
- Aβ :
-
Amyloid β
- ACh:
-
Acetylcholine
- AChE:
-
Acetylcholinesterase
- AD:
-
Alzheimer's disease
- ADAS-Cog:
-
Alzheimer’s disease assessment scale
- ADL:
-
Activities of daily living
- AS:
-
Active site
- BACE1:
-
β-Site amyloid precursor protein-cleaving enzyme 1
- BChE:
-
Butyrylcholinesterase
- CAP:
-
Cholinergic anti-inflammatory pathway
- CDR:
-
Clinical dementia rating
- CNS:
-
Central nervous system
- DSM IV-R:
-
Diagnostic and statistical manual of mental disorders—fourth revision
- DMSO:
-
Dimethylsulfoxide
- EGF:
-
Epidermal growth factor
- ELS:
-
Extract of Lycopodium serratum Thunb.
- ERK:
-
Extracellular signal-regulated kinase
- Gal:
-
Galantamine
- HIV:
-
Human immunodeficiency virus
- Hup A:
-
Huperzine A
- LC:
-
Lycopodium clavatum L.
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MIC:
-
Minimum inhibitory concentration
- MNTC:
-
Maximum nontoxic concentration
- MMSE:
-
Mini-mental state examination
- MMP-9:
-
Matrix metalloproteinase-9
- MS:
-
Murashige and skoog medium
- NGF:
-
Nerve growth factor
- NINCDS-ADRDA:
-
National Institute of Neurological and Communicative Disorders and Stroke
- NMDA:
-
N-Methyl-D-aspartate
- NSC:
-
Neural stem cell
- OXE receptor1:
-
5-Oxo-ETE receptor
- PAS:
-
Peripheral anionic site
- PARP:
-
Poly-ADP-ribose polymerase
- PHA:
-
Phytohemagglutinin
- Phys:
-
Physostigmine
- PMA:
-
Phorbol myristate acetate
- ROS:
-
Reactive oxygen species
- SAP:
-
Secreted aspartic proteases
- SGZ:
-
Subgranular zone
- SOD:
-
Superoxide dismutase
- TCM:
-
Traditional Chinese medicine
- TEM:
-
Traditional European medicine
- VaD:
-
Vascular dementia
References
Alvarez A, Bronfman F, Pérez CA, Vicente M, Garrido J, Inestrosa NC (1995) Acetylcholinesterase, a senile plaque component, affects the fibrillogenesis of amyloid-β-peptides. Neurosci Lett 201(1):49–52. https://doi.org/10.1016/0304-3940(94)12127-C
Anet FAL, Eves CR (1958) Lycodine, a new alkaloid of Lycopodium annotinum. Can J Chem Eng 36:902–909. https://doi.org/10.1139/v58-130
Anet FAL, Khan NH (1959) Alkaloids of Lycopodium annotinum part II. Isolation of four new alkaloids. Can J Chem Eng 37(9):1589–1596. https://doi.org/10.1139/v59-230
Anet FAL, Ahmad M, Khan NH (1962) The alkaloids of Lycopodium annotinum. Can J Chem Eng 40:236–239. https://doi.org/10.1139/v62-040
Anet FAL, Haq MZ, Khan NH, Ayer WA, Hayatsu R, Lopez SV, Deslongchsmps P, Riess W, Ternbah M, Valanta Z, Wiesner K (1964) The structure of lyconnotine: a novel Lycopodium alkaloid. Tetrahedron Lett 5(14):751–757. https://doi.org/10.1016/0040-4039(64)83031-8
Ansari FR, Ansari WH, Rahrnan W, Seligmann O, Chari VM, Wagner H, Osterdahl BGA (1979) A new acylated apigenin 4’–O–β–D–glucoside from the Leaves of Lycopodium clavatum L. Planta Med 36:196–199. https://doi.org/10.1055/s-0028-1097269
Ayer WA (1973) Lycopodium alkaloids including synthesis and biosynthesis. Ser Org Chem 9:1–25
Ayer WA (1991) The Lycopodium alkaloids. Nat Prod Rep 5:455–463. https://doi.org/10.1039/NP9910800455
Ayer WA, Altenkipk B (1969) Alkaloids of Lycopodium alopecuroides. Part 3. Structure of alolycopine. Can J Chem Eng 47(13):2457–2459. https://doi.org/10.1139/v69-400
Ayer WA, Altenkirk B (1969) Structure of lucidioline. An alkaloid of Lycopodium lucidulum. Can J Chem Eng 47(3):499–502. https://doi.org/10.1139/v69-074
Ayer WA, Iverach GG (1962) The structure and stereochemistry of lycodoline (lycopodium alkaloid L.8). Tetrahedron Lett 3(3):87–92. https://doi.org/10.1016/S0040-4039(00)71105-1
Ayer WA, Iverach GG (1964) Lycopodium alkaloids VII. lycopoline (alkaloid L.8). Can J Chem Eng 42(11):2514–2522. https://doi.org/10.1139/v64-369
Ayer W, Kasitu GC (1989) Some new Lycopodium alkaloids. Can J Chem Eng 67:1077–1086
Ayer WA, Law DA (1962) Lycopodium alkaloids IV. The constitution and stereochemistry of lycoclavine, an alkaloid of Lycopodium clavatum var. megastachyon. Can J Chem Eng 40:2088–2100. https://doi.org/10.1139/v62-321
Ayer WA, Masaki N (1971) Alkaloids of Lycopodium alopecuroides. Part 4. The structure of lycopecurine. Can J Chem Eng 49(3):524–527. https://doi.org/10.1139/v71-083
Ayer WA, Piers K (1967) The alkaloids of Lycopodium cernuum L. III. The synthesis of dihydrodeoxyepiallocernuine. Can J Chem Eng 45(5):451–459. https://doi.org/10.1139/v67-079
Ayer WA, Berezowsky JA, Iverach GG (1961) Lycopodium alkaloids-II. Tetrahedron 18(5):567–573. https://doi.org/10.1016/S0040-4020(01)92707-3
Ayer WA, Berezowsky JA, Law DA (1963) Lycopodium alkaloids vs the bromination of lycopodine and the structure of alkaloid L.20. Can J Chem Eng 41(3):649–657. https://doi.org/10.1139/v63-092
Ayer WA, Hogg AN, Soper AC (1964) Lycopodium alkaloids. VI the nature of alkaloid L.9. Can J Chem Eng 42(4):949–951. https://doi.org/10.1139/v64-140
Ayer WA, Habgood TE, Deulofeu V, Juliani HR (1965) Lycopodium alkaloids: Sauroxine. Tetrahedron 21(8):2169–2172. https://doi.org/10.1016/S0040-4020(01)98352-8
Ayer WA, Jenkins JK, Piers K, Valverde-lopez S (1967a) The alkaloids of Lycopodium cernuum L. I. The stereochemistry of cernuine and lycocernuine. Can J Chem Eng 45(5):433–443. https://doi.org/10.1139/v67-077
Ayer WA, Jenkins JK, Piers K, Valverde-lopez S (1967b) The alkaloids of Lycopodium cernuum L. II. The stereochemistry of cernuine and lycocernuine. Can J Chem Eng 45(5):445–450. https://doi.org/10.1139/v67-078
Ayer WA, Altenkir B, Lopez SV, Douglas B, Raffauf RF, Weisbach JA (1968a) The alkaloids of Lycopodium alopecuroides L. Can J Chem Eng 46(1):15–20. https://doi.org/10.1139/v68-003
Ayer WA, Masaki N, Nkunika DS (1968b) Luciduline: a unique type of Lycopodium alkaloid. Can J Chem Eng 46(23):3631–3642. https://doi.org/10.1139/v68-602
Ayer WA, Altenkirk B, Burnell RH, Moinas M (1969a) Alkaloids of Lycopodiumlucidulum Michx. Structure and properties of alkaloid L.23. Can J Chem Eng 47(3):449–455. https://doi.org/10.1139/v69-065
Ayer WA, Altenkirk B, Masakt N, Valvered-Lopezs S (1969b) Alkaloids of Lycopodium alopecuroides. Part 2. Alopecurine, a new type of Lycopodium alkaloid. Can J Chem Eng 47:2449–2455. https://doi.org/10.1139/v69-399
Ayer WA, Browne LM, Nakahara Y, Tori M, Delbaere LTJ (1979) A new type of Lycopodium alkaloid. The alkaloids from Lycopodium lucidulum. Can J Chem Eng 57(35):1105–1107. https://doi.org/10.1139/v79-180
Ayer WA, Ball LF, Browne LM, Tori M, Delbaere LTJ, Stlverberg A (1984) Spirolucidine, a new Lycopodium alkaloid. Can J Chem Eng 62:298–302. https://doi.org/10.1139/v84-051
Ayer WA, Browne LM, Orszans H, Valenta Z, Liu JS (1989) Alkaloids of Lycopodium selago on the identity of selagine with huperzine A and the structure of a related alkaloid. Can J Chem 67(10):1538–1540
Ayer WA, Browne LM, Elgersma AW, Singer PP (1990) Identification of some L-numbered Lycopodium alkaloids. Can J Chem 68(8):1300–1304. https://doi.org/10.1139/v90-200
Ayer WA, Ma YT, Liu JS, Huang MF, Schulz W, Clardy J (1994) Macleanine, a unique type of dinitrogenous Lycopodium alkaloid. Can J Chem 72(1):128–130. https://doi.org/10.1139/v94-020
Bai DL (1993) Traditional Chinese medicines and new drug development. Pure Appl Chem 65(6):1103–1112. https://doi.org/10.1351/pac199365061103
Banerjee J, Biswas S, Madhu NR, Karmakar SR, Biswas SJ (2014) A better understanding of pharmacological activities and uses of phytochemicals of Lycopodium clavatum: a review. J Pharmacogn Phytochem 3(1):207–210
Bartolini M, Bertucci C, Cavrini V, Andrisano V (2003) β-Amyloid aggregation induced by human acetylcholinesterase:inhibition studies. Biochem Pharmacol 65(3):407–416. https://doi.org/10.1016/S0006-2952(02)01514-9
Behl C, Davis JB, Lesley R, Schubert D (1994) Hydrogen peroxide mediates amyloid β protein toxicity. Cell 77(16):817–827. https://doi.org/10.1016/0092-8674(94)90131-7
Bennert HW, Suksathan P, Horn K (2007) Diphasiastrum multispicatum (J.H. Wilce) Holub (Lycopodiaceae) in Thailand. Am Fern J 97(3):155–165. https://doi.org/10.1640/0002-8444(2007)97[155:DMJWHL]2.0.CO;2
Betts EE, Maclean DB (1958) Lycopodium alkaloids VI. reactions of the diphenyl derivative of desoxodihydroannotinine. Can J Chem 36(3):473–479. https://doi.org/10.1139/v58-067
Bishayee K, Chakraborty D, Ghosh S, Boujedaini N, Khuda-Bukhsh AR (2013) Lycopodine triggers apoptosis by modulating 5-lipoxygenase, and depolarizing mitochondrial membrane potential in androgen sensitive and refractory prostate cancer cells without modulating p53 activity: signaling cascade and drug–DNA interaction. Eur J Pharmacol 698:110–121. https://doi.org/10.1016/j.ejphar.2012.10.041
Borloz A, Marstion A, Hostettmann K (2006) The determination of huperzine A in European Lycopodiaceae species by HPLC-UV-MS. Phytochem Anal 17(5):332–336. https://doi.org/10.1002/pca.922
Braekman JC, Nyembo L, Bourdoux P, Kahindo N, Hootele C (1974) Distribution des alcaloides dans le genre Lycopodium. Phytochemistry 13(11):2519–2528. https://doi.org/10.1016/S0031-9422(00)86930-7
Braekman JC, Hootele C, Miller N, Declercq JP, Germain G, Meerssche MV (1979) Megastachine, a new alkaloid from Lycopodium megastachyum. Can J Chem Eng 57(13):1691–1693. https://doi.org/10.1139/v79-271
Burnell RH, Mootoo BS (1961) Lycopodium alkaloids part IV. alkaloids of jamican Lycopodium clavatum Linn. Can J Chem Eng 39(5):1090–1093. https://doi.org/10.1139/v61-135
Burnell RH, Mootoo BS, Taylor DR (1960) Alkaloids of Lycopodium fawcettii. part II. Can J Chem Eng 38(10):1927–1932. https://doi.org/10.1139/v60-259
Burnell RH, Chin CG, Mootoo BS, Taylor DR (1963) Lycopodium alkaloids: part viii new alkaloids from Jamaican Lycopodium species. Can J Chem Eng 41(12):3091–3094. https://doi.org/10.1139/v63-452
Burnell RH, Mo L, Moinas M (1972) Le lycoxanthol, nouveau diterpenoide de Lycopodium lucidulum. Phytochemistry 11(9):2815–2820. https://doi.org/10.1016/S0031-9422(00)86518-8
Cai X, Pan DJ, Xu GY (1989) Studies on the tetracyclic triterpenoids from Lycopodium obscurum L. Acta Chim Sinica 47:1025–1028
Cai X, Pan DJ, Chen YS, Wu WL, Liu XZ (1991) The isolation and identification of the anthraquinnones from Lycopodium japonicum L. and Lycopodium obscurunm L. Acta Acad Med Shanghai 18(5):383–385
Cai ZY, Zhou ZG, Li P, Qin Y (2015) Advances in studies on chemical constituents in Lycopodii herba and their pharmacological activities. Chin Tradit Herb Drugs 46(2):297–304. https://doi.org/10.7501/j.issn.0253-2670.2015.02.026
Casttllo M, Morales G, Loyola LA (1976) The alkaloids of L. paniculatum and the structure of paniculatine. Can J Chem Eng 54(18):2900–2908. https://doi.org/10.1139/v76-410
Chee YC, Hirasawa Y, Karimata C, Koyama K, Sekiguchi M, Kobayashid J, Morita H (2007) Carinatumins A–C, new alkaloids from Lycopodium carinatum inhibiting acetylcholinesterase. Bioorg Med Chem 15:1703–1707. https://doi.org/10.1016/j.bmc.2006.12.005
Chen FX (2007) Effects of huperzine A on memory function in chronic psychosis. Mod Pract Med 19(5):370–371. https://doi.org/10.3969/j.issn.1671-0800.2007.05.018
Chen Y, He HW, Mei ZN, Yang GZ (2014) Lycopodium alkaloids from Lycopodium obscurum L. Helv Chim Acta 97:519–523. https://doi.org/10.1002/hlca.201300243
Cheng DH, Tang XC (1998) Pharmacol Biochem Behav 60:377–386. https://doi.org/10.1016/S0091-3057(97)00601-1
Cheng DH, Ren H, Tang XC (1996) Huperzine A, a novel promising acetylcholinesterase inhibitor. NeuroReport 8(1):97–101. https://doi.org/10.1097/00001756-199612200-00020
Cheng JT, Liu F, Li XN, Wu XD, Dong LB, Peng LY, Huang SX, He J, Zhao QS (2013) Lycospidine A, a new type of Lycopodium alkaloid from Lycopodium complanatum. Org Lett 15(10):2438–2441. https://doi.org/10.1021/ol400907v
Choo CY, Hirasawa Y, Karimata C, Koyama Y, Sekiguchi M, Kobayashid J, Morita H (2007) Carinatumins A–C, new alkaloids from Lycopodium carinatum inhibiting acetylcholinesterase. Bioorg Med Chem 15:1703–1707. https://doi.org/10.1016/j.bmc.2006.12.005
Christenhusz MJM, Zhang XC, Schneider H (2011) A linear sequence of extant families and genera of lycophytes and ferns. Phytotaxa 19:7–54. https://doi.org/10.11646/phytotaxa.19.1.2
Chuong NN, Huong NTT, Hung TM, Luan TC (2014) Anti-cholinesterase activity of Lycopodium alkaloids from vietnamese Huperzia squarrosa (Forst.) Trevis. Molecules 19:19172–19179. https://doi.org/10.3390/molecules191119172
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, Du MJ (2002) Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem 277:32046–32053. https://doi.org/10.1074/jbc.M201750200
Das S, Das J, Samadder A, Boujedaini N, Khuda-Bukhsh AR (2012) Apigenin-induced apoptosis in A375 and A549 cells through selective action and dysfunction of mitochondria. Exp Biol Med 237:1433–1448. https://doi.org/10.1258/ebm.2012.012148
Deng FM, Luo XD, Lang HL (2018) Role of amyloid beta-protein in postoperative cognitive dysfunction and effect of huperzine A on cognitive dysfunction in aged rats. Chin J Gerontol 38(24):6034–6036. https://doi.org/10.3969/j.issn.1005-9202.2018.24.057
Fan WN (2009) Clinical observation of huperzine A combined with nimodipine in the treatment of mild and moderate vascular dementia. Prevent Treat Cardiovasc Cerebrovasc Dis 9(3):216–217. https://doi.org/10.3969/j.issn.1009-816X.2009.03.031
Felgenhauer N, Zilker T, Worek F, Eyer P (2000) Intoxication with huperzine A, a potent anticholinesterase found in the fir club moss. Clin Toxicol 38(7):803–808. https://doi.org/10.1081/CLT-100102396
Ferrari GVDE, Canales MA, Shin I, Weiner LM, Silman I, Inestrosa NC (2001) A structural motif of acetylcholinesterase that promotes amyloid β-peptide fibril formation. Biochemistry 40(35):10447–10457. https://doi.org/10.1021/bi0101392
Field AR, Bostock PD (2013) New and existing combinations in Palaeotropical Phlegmariurus (Lycopodiaceae) and lectotypification of the type species Phlegmariurus phlegmaria (L.) T. Sen & U. Sen. Phytokeys 20:33–51. https://doi.org/10.3897/phytokeys.20.4007
Gerard RV, Maclean DB, Fagianni R, Lock CJ (1986) Fastigiatine, a Lycopodium alkaloid with a new ring system. Can J Chem 64(5):943–949. https://doi.org/10.1139/v86-157
Ghosh AK, Gemma S, Tang J (2008) β-Secretase as a therapeutic target for Alzheimer’s disease. Neurotherapeutics 5(3):399–408. https://doi.org/10.1016/j.nurt.2008.05.007
Giacobini E (2000) Cholinesterase inhibitors stabilize Alzheimer disease. Neurochem Res 25(9):1185–1190. https://doi.org/10.1023/A:1007679709322
Goleniowski ME, Bongiovanni GA, Palacio L, Nuñez CO, Cantero JJ (2006) Medicinal plants from the “Sierra de Comechingones.” Argentina J Ethnopharmacol 107(3):324–341. https://doi.org/10.1016/j.jep.2006.07.026
Gu J, Li T, Ao HF, Ruan QW, Kong DQ (2014) Effects of huperzine A on local anti-inflammatory of presbycusis rats induced by D-galactose. J Med Res 43(2):76–79
Halldorsdottir ES, Palmadottir RH, Nyberg NT, Olafsdottir ES (2013) Phytochemical analysis of alkaloids from the Icelandic club moss Diphasiastrum alpinum. Phytochem Lett 6:355–359. https://doi.org/10.1016/j.phytol.2013.04.004
Halldorsdottir ES, Kowal NM, Olafsdottir ES (2015) The genus Diphasiastrum and its Lycopodium alkaloids. Planta Med 81:995–1002. https://doi.org/10.1055/s-0035-1546182
Ham YM, Yoon WJ, Park SY, Jung YH, Kim D, Jeon YJ, Wijesinghe WAJP, Kang SM, Kim KN (2012) Investigation of the component of Lycopodium serratum extract that inhibits proliferation and mediates apoptosis of human HL-60 leukemia cells. Food Chem Toxicol 50:2629–2634. https://doi.org/10.1016/j.fct.2012.05.019
Hao Y, Creson T, Zhang L, Li P, Du F, Yuan P, Gould TD, Manji HK, Chen G (2004) Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J Neurosci 24(29):6590–6599. https://doi.org/10.1523/JNEUROSCI.5747-03.2004
Hasan MK, Gatto P, Jha PK (2013) Traditional uses of wild medicinal plants and their management practices in Nepal-A study in Makawanpur district. Int J Med Arom Plants 3(1):102–112
He J, Chen XQ, Li MM, Zhao Y, Xu G, Cheng X, Peng LY, Xie MJ, Zheng YT, Wang YP, Zhao QS (2009a) Lycojapodine A, a novel alkaloid from Lycopodium japonicum. Org Lett 11(6):1397–1400. https://doi.org/10.1021/ol900079t
Hirasawa Y, Morita H, Kobayashi J (2002) Lyconesidines A–C, new alkaloids from Lycopodium chinense. Tetrahedron Lett 58(2002):5483–5488. https://doi.org/10.1016/S0040-4020(02)00520-3
Hirasawa Y, Morita H, Shiro M, Kobayashi J (2003) Sieboldine A, a novel tetracyclic alkaloid from Lycopodium sieboldii, inhibiting acetylcholinesterase. Org Lett 5(21):3991–3993. https://doi.org/10.1021/ol035560s
Hirasawa Y, Morita H, Kobayashi J (2004) Nankakurine A, a novel C16N2-type alkaloid from Lycopodium hamiltonii. Org Lett 6(19):3389–3391. https://doi.org/10.1021/ol048621a
Hirasawa Y, Kobayashi J, Morita H (2006) Lycoperine A, a Novel C27N3-type pentacyclic alkaloid from Lycopodium hamiltonii, inhibiting acetylcholinesterase. Org Lett 8(1):123–126. https://doi.org/10.1021/ol052760q
Hirasawa Y, Kato E, Kobayashi J, Kawahara N, Goda Y, Shiro M, Morita H (2008a) Lycoparins A–C, new alkaloids from Lycopodium casuarinoides inhibiting acetylcholinesterase. Bioorg Med Chem 16:6167–6171. https://doi.org/10.1016/j.bmc.2008.04.044
Hirasawa Y, Tanaka T, Kobayashi J, Kawahara N, Goda Y, Morita H (2008b) Malycorins A–C, new Lycopodium Alkaloids from Lycopodium phlegmaria. Chem Pharm Bull 56(10):1473–1476. https://doi.org/10.1248/cpb.56.1473
Hirasawa Y, Tanaka T, Koyama K, Morita H (2009) Lycochinines A–C, novel C27N3 alkaloids from Lycopodium chinense. Tetrahedron Lett 50:4816–4819. https://doi.org/10.1016/j.tetlet.2009.05.072
Hirasawa Y, Mitsui C, Uchiyama N, Hakamatsuka T, Morita H (2018) Hupercumines A and B, Lycopodium alkaloids from Huperzia cunninghamioides, inhibiting acetylcholinesterase. Org Lett 20(5):1384–1387. https://doi.org/10.1021/acs.orglett.8b00152
Holub J (1985) Transfers of Lycopodium species to Huperzia: with a note on generic classification in Huperziaceae. Folia Geobotanica et Phytotaxonomica 20(1):67–80. https://doi.org/10.1007/BF02856466
Holub J (1991) Some taxonomic changes within the Lycopodiales. Folia Geobot Phytotx 26:81–94. https://doi.org/10.1007/BF02912943
Hu P, Cross ML, Yuan SQ, Wei TT, Lu YQ (1992) Mass spectrometric differentiation of huperzinine, N, N-dimethylhuperzine A and N-methylhuperzine B. Org Mass Spectrom 27(2):99–104. https://doi.org/10.1002/oms.1210270206
Hu TM, Chandler RF, Hanson AW (1987) Obscurinine: a new Lycopodium alkaloid. Tetrahedron Lett 28(48):5993–5996. https://doi.org/10.1016/S0040-4039(00)96845-X
Huang JZ (2017) Huperzine A can weaken the lipopolysaccharide induce inflammatoryreaction of microglia in rat. Pharm Today 27(4):251–254. https://doi.org/10.12048/j.issn.1674-229X.2017.04.009
Inubushi Y, Harayama T, Akatsu M (1968) The structure of serratidine. Chem Commun 18:1138–1139. https://doi.org/10.1039/C19680001138
Inestrosa NC, Alvarez A, Pérez CA, Moreno RD, Vicente M, Linker C, Casanueva OI, Soto C, Garrido J (1996) Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron 16(4):881–891. https://doi.org/10.1016/S0896-6273(00)80108-7
Inubushi Y, Sano T, Tsuda Y (1964) Serratenediol: a new skeletal triterpenoid containing a seven membered ring. Tetrahedron Lett 5(21):1303–1310. https://doi.org/10.1016/S0040-4039(00)90472-6
Inubushi Y, Sano T, Price JR (1967a) Triterpene constituents of Lycopodium complanatum from new guinea. Aust J Chem 20(2):387–388. https://doi.org/10.1071/CH9670387
Inubushi Y, Tsuda Y, Sano T, Konita T, Suzuki S, Ageta H, Otake Y (1967b) The structure of serratenediol. Chem Pharm Bull 15(8):1153–1168. https://doi.org/10.1248/cpb.15.1153
Inubushi Y, Harayama T, Hibino T (1970) Phlegmanol A, dihydrocaffeic acid ester of the triterpene serratenediol. Chem Commun 17:1118–1119. https://doi.org/10.1039/C29700001118
Inubushi Y, Hibino T, Harayama T, Hasegawa T (1971) Triterpenoid constituents of Lycopodium phlegmaria L. J Chem Soc. https://doi.org/10.1039/J39710003109
Ishiuchi K, Kubota T, Hoshino T, Obara Y, Nakahata N, Kobayashi J (2006a) Lycopladines B–D and lyconadin B, new alkaloids from Lycopodium complanatum. Bioorg Med Chem 14:5995–6000. https://doi.org/10.1016/j.bmc.2006.05.028
Ishiuchi K, Kubota T, Mikami Y, Obara Y, Nakahata N, Kobayashi J (2006b) Complanadines C and D, new dimeric alkaloids from Lycopodium complanatum. Bioorg Med Chem 15(1):413–417. https://doi.org/10.1016/j.bmc.2006.09.043
Ishiuchi K, Kubota T, Morita H, Kobayashi J (2006c) Lycopladine A, a new C16N alkaloid from Lycopodium complanatum. Tetrahedron Lett 47(19):3287–3289. https://doi.org/10.1016/j.tetlet.2006.03.027
Ishiuchi K, Kubota T, Hayashi S, Shibata T, Kobayashi J (2009a) Lycopladines F and G, new C16N2-type alkaloids with an additional C4N unit from Lycopodium complanatum. Tetrahedron Lett 50:4221–4224. https://doi.org/10.1016/j.tetlet.2009.04.139
Ishiuchi K, Kubota T, Hayashi S, Shibata T, Kobayashi J (2009b) Lycopladine H, a novel alkaloid with fused-tetracyclic skeleton from Lycopodium complanatum. Tetrahedron Lett 50:6534–6536. https://doi.org/10.1016/j.tetlet.2009.09.035
Ishiuchi K, Kubota T, Ishiyama H, Hayashi S, Shibata T, Mori K, Obara Y, Nakahata N, Kobayashi J (2011) Lyconadins D and E, and complanadine E, new Lycopodium alkaloids from Lycopodium complanatum. Bioorg Med Chem 19:749–753. https://doi.org/10.1016/j.bmc.2010.12.025
Ishiuchi K, Jiang WP, Fujiwara Y, Wu JB, Kitanaka S (2016) Serralongamines B–D, three new Lycopodium alkaloids from Lycopodium serratum var. longipetiolatum, and their inhibitory effects on foam cell formation in macrophages. Bioorg Med Chem Lett 26:2636–2640. https://doi.org/10.1016/j.bmcl.2016.04.019
Jain SK, Srivastava S (2005) Traditional uses of some Indain plants among islanders of the Indian Ocean. Indian J Tradit Know 4(4):345–357
Jiang CP, Wang H (2013c) Application of lycojaponicumin A in the treatment of leukemia drugs. CN 103446134 A, 18 Dec 2013
Jiang CP, Wang ZG (2013b) Application of lycojaponicumin B in the medicine of ileocecal bowel cancer. CN 103463022 A, 25 Dec 2013
Jiang CP, Wang ZZ (2013a) Application of lycojaponicumin A in the medicine of ileocecal bowel cancer. CN 103463054 A, 25 Dec 2013
Jiang WP, Ishiuchi K, Wu JB, Kitanaka S (2014) Serralongamine a, a new Lycopodium alkaloid from Lycopodium serratum var. longipetiolatum. Heterocycles 89:747–752. https://doi.org/10.3987/COM-13-12928
Jiang WW, Liu F, Gao X, He J, Cheng X, Peng LY, Wu XD, Zhao QS (2014) Huperserines A–E, Lycopodium alkaloids from Huperzia serrata. Fitoterapia 99:72–77. https://doi.org/10.1016/j.fitote.2014.09.005
Jiang SG, Xu L, Chai DM, Zhu HW, Lin Z (2015) Protect effects of huperzine A on methylglyoxal induced injury in the cultured human brain microvascular endothelial cell in vitro experimental study. Chin J Mod App Pharm 32(3):277–281. https://doi.org/10.13748/j.cnki.issn1007-7693.2015.03.005
Jiang WW, Liu YC, Zhang ZJ, He J, Su J, Xiao C, Li YP, Shao LD, Wu XD, Yang JH, Zhao QS (2016) Obscurumines H–P, new Lycopodium alkaloids from the club moss Lycopodium obscurum. Fitoterapia 109:155–161. https://doi.org/10.1016/j.fitote.2015.12.017
Jiang FF, Qi BW, Ding N, Yang HY, Jia FF, Luo Y, Wang J, Liu X, Wang XH, Tu PF, Shi SP (2019) Lycopodium alkaloids from Huperzia serrata. Fitoterapia 137:104277. https://doi.org/10.1016/j.fitote.2019.104277
Johns SR, Lamberton JA, Sioumts AA (1969) Alkaloids of Lycopodium volubile (Lycopodiaceae). Aust J Chem 22(6):1317–1318. https://doi.org/10.1071/ch9691317
Katakawa K, Kitajima M, Aimi N, Seki H, Yamaguchi K, Furihata K, Harayama T, Takayama H (2004) Structure elucidation and synthesis of lycoposerramine-B, a novel oxime-containing Lycopodium alkaloid from Lycopodium serratum Thunb. J Org Chem 70(2):658–663. https://doi.org/10.1021/jo0483825
Katakawa K, Kitajima M, Yamaguchi K, Takayama H (2006) Three new phlegmarine-type Lycopodium alkaloids, lycoposerramines-X, -Yand -Z, having a nitrone residue, from Lycopodium serratum. Heterocycles 69(1):223–229
Katakawa K, Nozoe A, Kogure N, Kitajima M, Hosokawa M, Takayama H (2007) Fawcettimine-related alkaloids from Lycopodium serratum. J Nat Prod 70(6):1024–1028. https://doi.org/10.1021/np0700568
Katakawa K, Kogure N, Kitajima M, Takayama H (2009) A New Lycopodium alkaloid, lycoposerramine-R, with a novel skeleton and three new fawcettimine-related alkaloids from Lycopodium serratum. Helv Chim Acta 92(3):445–452. https://doi.org/10.1002/hlca.200800327
Katakawa K, Mito H, Kogure N, Kitajima M, Wongseripipatana S, Arisawa M, Takayama H (2011) Ten new fawcettimine-related alkaloids from three species of Lycopodium. Tetrahedron 67:6561–6567. https://doi.org/10.1016/j.tet.2011.05.107
Kobayashi J, Hirasawa Y, Yoshida N, Morita H (2000) Complanadine A, a new dimeric alkaloid from Lycopodium complanatum. Tetrahedron Lett 41:9069–9073. https://doi.org/10.1016/S0040-4039(00)01630-0
Kobayashi J, Hirasawa Y, Yoshida N, Morita H (2001) Lyconadin A, a Novel alkaloid from Lycopodium complanatum. J Org Chem 66(17):5901–5904. https://doi.org/10.1021/jo0103874
Kobayashi J, Hirasawa Y, Yoshida N, Morita H (2002) Complanadine A, a new alkaloid from Lycopodium complanatum. Tetrahedron Lett 41:9069–9073. https://doi.org/10.1016/S0040-4039(00)01630-0
Kogure N, Maruyama M, Wongseripipatana S, Kitajima M, Takayama H (2016) New lycopodine-type alkaloids from Lycopodium carinatum. Chem Pharm Bull 64(7):793–799. https://doi.org/10.1248/cpb.c16-00171
Kong DQ, Gu J, Li C (2013) Effect of huperzine A on auditory function of presbycusis rats induced by D-galactose. Chin J Otorhinolaryngol Skull Base Surg 19(3):199–203. https://doi.org/10.11798/j.issn.1007-1520.201303005
Konrath EL, Neves BM, Lunardi PS, Passos CS, Simoes-Pires A, Ortega MG, Goncalves CA, Cabrera JL, Moreira JCF, Henriques AT (2012) Investigation of the in vitro and ex vivo acetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extracts. J Ethnopharmacol 139(1):58–67. https://doi.org/10.1016/j.jep.2011.10.042
Koyama K, Morita H, Hirasawa Y, Yoshinaga M, Hoshino T, Obara Y, Nakahata N, Kobayashi J (2004) Lannotinidines A–G, new alkaloids from two species of Lycopodium. Tetrahedron 61(15):3681–3690. https://doi.org/10.1016/j.tet.2005.02.016
Koyama K, Hirasawa Y, Kobayashi J, Morita H (2007) Cryptadines A and B, novel C27N3-type pentacyclic alkaloids from Lycopodium cryptomerinum. Bioorg Med Chem 15:7803–7808. https://doi.org/10.1016/j.bmc.2007.08.043
Kozikowski AP, Miller CP, Yamada F, Pang YP, Miller JH, McKinney M, Ball RG (1991) Delineating the pharmacophoric elements of huperzine A: importance of the unsaturated three-carbon bridge to its AChE inhibitory activity. J Med Chem 34(12):3399–3402. https://doi.org/10.1021/jm00116a010
Kubota T, Yahata H, Yamamoto S, Hayashi S, Shibata T, Kobayashi J (2009) Serratezomines D and E, new Lycopodium alkaloids from Lycopodium serratum var. serratum. Bioorg Med Chem Lett 19:3577–3580. https://doi.org/10.1016/j.bmcl.2009.04.146
Laemmerhold KM, Breit B (2010) Total synthesis of (+)-clavolonine,(-)-deacetylfawcettiine, and (+)-acetylfawcettiine. Angew Chem Int Ed 49(13):2367–2370. https://doi.org/10.1002/anie.200907248
Laganiere S, Coray J, Tang XC, Wlfer E, Hanin I (1991) Acute and chronic studies with the anticholinesterase huperzine-a-effect on central-nervous-system cholinergic parameters. Neuropharmacology 30(7):763–768. https://doi.org/10.1016/0028-3908(91)90184-D
Lai Z, Li XX, Wang Y, Yan RM, Zhang ZB, Zhu D (2014) The determination of huperzine A and temporal and spatial variation of Huperzia serrata. J Jiangxi Normal Univ (Nat Sci Ed) 38(5):489–495. https://doi.org/10.3969/j.issn.1000-5862.2014.05.012
Lee SW, Xiao CJ, Pei SJ (2008) Ethnobotanical survey of medicinal plants at periodic markets of Honghe Prefecture in Yunnan Province, SW China. J Ethnopharmacol 117(2):362–377. https://doi.org/10.1016/j.jep.2008.02.001
Li SP (1995) A case of contact dermatitis caused by Lyccopodium japonicum. Chin J Dermatovenereol 9(1):37
Li GY, Su YY (2017) Determination of huperzine A in Lycopodium serratum Thunb. from different habitats by LC-MS method. Zhejiang Agric Sci 58(7):1186–1189. https://doi.org/10.16178/j.issn.0528-9017.20170729
Li WN, Yu HY (2011) Efficacy of huperzine A in the treatment of senile patients with mild and moderate alzheimer’s disease. Chin J Gerontol 31(11):2096–2097. https://doi.org/10.3969/j.issn.1005-9202.2011.11.074
Li S, Long CL, Liu FY, Lee S, Guo Q, Li R, Liu YH (2006) Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol 108(1):59–67. https://doi.org/10.1016/j.jep.2006.04.014
Li XL, Zhao Y, Cheng X, Peng LY, Xu G, Zhao QS (2006) Japonicumins A-D: four new compounds from Lycopodium japonicum. Helv Chim Acta 89(7):1467–1473. https://doi.org/10.1002/hlca.200690148
Li C, Gu J, Ao HF, Ruan QW, Kong DQ (2014) Protective effect of Huperzine A on nerve fibers of presbycusis rats induced by D-galactose. Chin J Ophthalmol Otorhinolaryngol 14(3):177–180. https://doi.org/10.14166/j.issn.1671-2420.2014.03.017
Liang YQ, Tang XC (2004) Comparative effects of huperzine A, donepezil and rivastigmine on cortical acetylcholine level and acetylcholinesterase activity in rats. Neurosci Lett 361(1):56–59. https://doi.org/10.1016/j.neulet.2003.12.071
Liang XJ, Gan JL, Zhang W, Zhang WD, Wang JW (2009) Improvement effect of huperzine A combined with MECT on the cognitive function of patients with principal schizophrenia. Prac J Med Pharm 26(12):16–18. https://doi.org/10.3969/j.issn.1671-4008.2009.12.008
Liu JS, Huang MF (1994) The alkaloids huperzines C and D and huperzinine from Lycopodiastrum casuarinoides. Phytochemistry 37(6):1759–1761. https://doi.org/10.1016/S0031-9422(00)89606-5
Liu JS, Zhu YL, Yu CM, Zhou YZ, Han YY, Wu FW, Qi BF (1986) The structures of huperzine A and B, two new alkaloids exhibiting marked anticholinesterase activity. Can J Chem 64(4):837–839. https://doi.org/10.1139/v86-137
Liu F, Wu XD, He J, Deng X, Peng LY, Luo HL, Zhao QS (2013) Casuarines A and B, Lycopodium alkaloids from Lycopodium casuarinoides. Tetrahedron Lett 54:4555–4557. https://doi.org/10.1016/j.tetlet.2013.06.083
Liu F, Dong LB, Gao X, Wu XD, He J, Peng LY, Cheng X, Zhao QS (2014) New Lycopodium alkaloids from Phlegmariurus squarrosus. J Asian Nat Prod Res 16(6):1–7. https://doi.org/10.1080/10286020.2014.920010
Liu J, Nian H, Xu Y, Wu TJ, Sun YC, Shao YT, Hunag J (2019) Anti-inflammatory effect and mechanism of shenjincaoalkaloids on adjuvant arthritis rats. Drug Eval Res 42(5):869–872. https://doi.org/10.7501/j.issn.1674-6376.2019.05.010
Loyola LA, Morales G, Castillo M (1979) Alkaloids of Lycopodium magellanicum. Phytochemistry 18(10):1721–1723. https://doi.org/10.1016/0031-9422(79)80193-4
Lu KT, Sun CL, Wo PY, Yen HH, Tang TH, Ng MC, Huang ML, Yang YL (2011) Hippocampal neurogenesis after traumatic brain injury is mediated by vascular endothelial growth factor receptor-2 and the Raf/MEK/ERK cascade. J Neurotrauma 28(3):441–450. https://doi.org/10.1089/neu.2010.1473
Ma XQ, Gang DR (2004) The Lycopodium alkaloids. RSC 21:752–772. https://doi.org/10.1039/b409720n
Ma SP, Zhang X (2007) Effects of huperzine A tablet combined with Ginkgo biloba preparations on cognitive ability and hemorheology in patients with vascular dementia. Chin Community Doc 9(6):13
Ma XQ, Tan CH, Zhu DY, Gang DR (2006) A survey of potential huperzine A natural resources in China: the Huperziaceae. J Ethnopharmacol 104(1):54–67. https://doi.org/10.1016/j.jep.2005.08.042
Ma XQ, Tan CH, Zhu DY, Gang DR, Xiao P (2007) Huperzine A from Huperzia species-an ethnopharmacolgical review. J Ethnopharmacol 113(1):15–34. https://doi.org/10.1016/j.jep.2007.05.030
Ma T, Gong K, Yan Y, Zhang LH, Tang PF, Zhang XF (2013) Huperzine A promotes hippocampal neurogenesis in vitro and in vivo. Brain Res 1506:35–43. https://doi.org/10.1016/j.brainres.2013.02.026
Maclean DB (1963) Lycopodium alkaloids xiii. mass spectra of representative alkaloids. Can J Chem Eng 41(10):2654–2670. https://doi.org/10.1139/v63-387
Maclean DB, Curcumelli-Rodostamo M (1966) Lycopodium alkaloids xvii. mass spectra of annotine and some annotine derivatives. Can J Chem Eng 44(5):611–620. https://doi.org/10.1139/v66-082
Mandal SK, Biswas R, Bhattacharyya SS, Paul S, Dutta S, Pathak S, Khuda-Bukhsh AR (2010) Lycopodine from Lycopodium clavatum extract inhibits proliferation of HeLa cells through induction of apoptosis via caspase-3 activation. Eur J pharmacol 626:115–122. https://doi.org/10.1016/j.ejphar.2009.09.033
Manjunatha BK, Krishna V, Vidya DM, Mankani KL, Manohara YN (2007) Wound healing activity of Lycopodium serratum. Indian J Pharm Sci 69(2):283–287. https://doi.org/10.4103/0250-474X.33159
Manske RHF (1953) The alkaloids of Lycopodium species xiii. Lycopodium densum Labill. Can J Chem Eng 31(10):894–895. https://doi.org/10.1139/v53-119
Manske RH, Marion L (1947) The alkaloids of Lycopodium species. IX. Lycopodium annotinum var. acrifolium, Fern. and the structure of annotinine. J Am Chem Soc 69(9):2126–2129. https://doi.org/10.1021/ja01201a019
Mao XY, Cao DF, Li X, Yin JY, Wang ZB, Zhang Y, Mao CX, Zhou HH, Liu ZQ (2014) Huperzine A ameliorates cognitive deficits in streptozotocin-Induced diabetic rats. Int J Mol Sci 15:7667–7683. https://doi.org/10.3390/ijms15057667
Marion L, Manske RHF (1948) The alkaloids of Lycopodium species x. Lycopodium cernaum L.I. Can J Res 26(1):1–2. https://doi.org/10.1139/cjr48b-001
Mattson MP, Goodman Y (1995) Different amyloidgenic peptides share a similar mechanism of neurotoxicity involving reactive oxygen species and calcium. Brain Res 676(1):219–224. https://doi.org/10.1016/0006-8993(95)00148-J
Michael JP (2004) Indolizidine and quinolizidine alkaloids. Nat Prod Rep 21:625–649. https://doi.org/10.1039/b310689f
Michael JP (2007) Indolizidine and quinolizidine alkaloids. Nat Prod Rep 24:191–222. https://doi.org/10.1039/b509525p
Miller N, Braekman F (1971) Lycopsida Lycopodiaceae Alcaloïdes De Lycopodium Alpinum. Phytochemistry 10(8):1931–1934. https://doi.org/10.1016/S0031-9422(00)86462-6
Miller N, Hootele C, Braekman JC (1972) Triterpenoids of Lycopodium megastach yum. Phytochemistry 12(7):1759–1761. https://doi.org/10.1016/0031-9422(73)80398-X
Morales G, Loyola LA, Castillo M (1979) Alkaloids of Lycopodium paniculatum: The structure of paniculine. Phytochemistry 18(10):1719–1720. https://doi.org/10.1016/0031-9422(79)80192-2
Morel S, Kerzaon I, Azaroual N, Sahpaz S, Joseph H, Bailleul F, Hennebelle T (2012) A new cernuane-type alkaloid from Lycopodium cernuum. Biochem Syst Ecol 45:188–190. https://doi.org/10.1016/j.bse.2012.07.026
Morita H, Arisaka M, Yoshida N, Kobayashi J (2000) Serratezomines A-C, new alkaloids from Lycopodium serratum var. serratum. J. Org. Chem 65(19):6241–6245. https://doi.org/10.1021/jo000661e
Morita H, Hirasawa Y, Yoshida N, Kobayashi J (2001) Senepodine A, a novel C22N2 alkaloid from Lycopodium chinense. Tetrahedron Lett 42:4199–4201. https://doi.org/10.1016/S0040-4039(01)00688-8
Morita H, Hirasawa Y, Kobayashi J (2003) Himeradine A, a novel C27N3-type alkaloid from Lycopodium chinense. J Org Chem 68(11):4563–4566. https://doi.org/10.1021/jo034294t
Morita H, Hirasawa Y, Shinzato T, Kobayashi J (2004) New phlegmarane-type, cernuane-type, and quinolizidine alkaloids from two species of Lycopodium. Tetrahedron 60(33):7015–7023. https://doi.org/10.1016/j.tet.2003.09.106
Morita H, Ishiuchi K, Haganuma A, Hoshino T, Obara Y, Nakahata N, Kobayashi J (2005) Complanadine B, obscurumines A and B, new alkaloids from two species of Lycopodium. Tetrahedron 61:1955–1960. https://doi.org/10.1016/j.tet.2005.01.011
Morita H, Hirasawa Y, Kobayashi J (2005) Lycopodatines A–C, C16N alkaloids from Lycopodium inundatum. J Nat Prod 68(12):1809–1812. https://doi.org/10.1021/np050389+
Muñoz-Torrero D (2008) Acetylcholinesterase inhibitors as disease-modifying therapies for Alzheimers disease. Curr Med Chem 15(24):2433–2455. https://doi.org/10.2174/092986708785909067
Nakayama W, Fujiwara Y, Kosuge Y, Monthakantirat O, Fujikawae K, Watthanaf S, Kitanakag S, Makinoa T, Ishiuchi K (2019) Phlenumdines D and E, new Lycopodium alkaloids from Phlegmariurus nummulariifolius, and their regulatory effects on macrophage differentiation during tumor development. Phytochem Lett 29:98–103. https://doi.org/10.1016/j.phytol.2018.11.010
Namsa ND, Tag H, Mandal M, Kalita P, Das AK (2009) An ethnobotanical study of traditional anti-inflammatory plants used by the Lohit community of Arunachal Pradesh, India. J Ethnopharmacol 125(2):234–245. https://doi.org/10.1016/j.jep.2009.07.004
Nguyen VT, Zhao BT, Seong SH, Kim JA, Woo MH, Choi JS, Min BS (2017) Inhibitory effects of serratene-type triterpenoids from Lycopodium complanatum on cholinesterases and β-secretase 1. Chem Biol Interact 274:150–157. https://doi.org/10.1016/j.cbi.2017.07.006
Nguyen HT, Doan HT, Ho DV, Raal A, Morita H, Pham KT (2018) Huperphlegmines A and B, two novel Lycopodium alkaloids with an unprecedented skeleton from Huperzia phlegmaria, and their acetylcholinesterase inhibitory activities. Fitoterapia 129:267–271. https://doi.org/10.1016/j.fitote.2018.07.016
Nilsu T, Thorroad S, Ruchirawat S, Thasana T (2016) Squarrosine A and pyrrolhuperzine A, new Lycopodium alkaloids from Thai and Philippine Huperzia squarrosa. Planta Med 82(11–12):1046–1050. https://doi.org/10.1055/s-0042-106904
Nilsua T, Thaisaeng W, Thamnarak W, Eurtivong C, Jumraksac A, Thorroad S, Khunnawutmanotham N, Ruchirawat S, Thasana N (2018) Three Lycopodium alkaloids from Thai club mosses. Phytochem 156:83–88. https://doi.org/10.1016/j.phytochem.2018.09.001
Nyembo L, Goffin A, Hootele C, Braekman JC (1978) Phlegmarine, a likely key intermediate in the biosynthesis of the Lycopodium alkaloids. Can J Chem 56(30):851–856. https://doi.org/10.1002/chin.197830352
Øllgaard B (1987) A revised classification of the Lycopodiaceae s.l. Oper Bot 92:153–178
Orhan I, Terzioglu S, Sener B (2003) α-Onocerin: an acetycholinesterase inhibitor from Lycopodium clavatum. Planta Med 69:265–267. https://doi.org/10.1055/s-2003-38489
Orhan I, Özçelik B, Aslan S, Kartal M, Karaoglu T, Şener B, Terzioglu S, Choudhary MI (2007) Antioxidant and antimicrobial actions of the clubmoss Lycopodium clavatum L. Phytochem Rev 6:189–196. https://doi.org/10.1007/s11101-006-9053-x
Orhan I, Kupeli E, Sener B, Yesilada E (2007) Appraisal of anti-inflammatory potential of the clubmoss, Lycopodium clavatum L. J Ethnopharmacol 109:146–150. https://doi.org/10.1016/j.jep.2006.07.018
Orhan IE, Sener B, Brun M, Tasdemir D (2013) Antiprotozoal activity and cytotoxicity of Lycopodium clavatum and Lycopodium complanatum subsp chamaecyparissus extracts. Turk J Biochem 38(4):403–408. https://doi.org/10.5505/tjb.2013.07379
Orito K, Manske RH, Rodrigo R (1972) The triterpenes of Lycopodiumlucidulum Michx. Can J Chem Eng 50(20):3280–3282. https://doi.org/10.1139/v72-525
Ortega MG, Agnese AM, Cabrera JL (2004) Anticholinesterase activity in an alkaloid extract of Huperzia saururus. Phytomedicine 11:539–543. https://doi.org/10.1016/j.phymed.2003.07.006
Ortega MG, Agnese AM, Cabrera JL (2004) Sauroine-a novel Lycopodium alkaloid from Huperzia saururus. Tetrahedron Lett 45:7003–7005. https://doi.org/10.1016/j.tetlet.2004.07.149
Pan K, Luo JG, Kong LY (2013) Two new Lycopodium alkaloids from Lycopodium obscurum. Helv Chim Acta 96:1197–1201. https://doi.org/10.1002/hlca.201200505
Pan K, Luo JG, Kong LY (2013) Three new Lycopodium alkaloids from Lycopodium obscurum. J Asian Nat Prod Res 15(5):441–445. https://doi.org/10.1080/10286020.2013.780045
Pappolla MA, Chyan YJ, Omar RA, Hsiao K, Perry G, Smith MA, Bozner P (1998) Evidence of oxidative stress and in vivo neurotoxicity of beta-amyloid in a transgenic mouse model of Alzheimer’s disease: a chronic oxidative paradigm for testing antioxidant therapies in vivo. Am J Pathol 152(4):871–877
Park JY, Kim H, Lim DW, Kim JE, Park WH, Park SD (2018) Ethanol extract of Lycopodium serratum Thunb. attenuates lipopolysaccharide-induced C6 glioma cells migration via matrix metalloproteinase-9 expression. Chin J Integr Med 24(11):860–866. https://doi.org/10.1007/s11655-017-2923-9
Perry GS, Maclean DB (1956) Lycopodium alkaloids. Cam J Chem 34:1189–1199
Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13(4):1676–1687. https://doi.org/10.1523/JNEUROSCI.13-04-01676.1993
Pongpamorn P, Wan-erlor S, Ruchirawat S, Thasana N (2016) Lycoclavatumide and 8α,11α-dihydroxylycopodine, a new fawcettimine and lycopodine-type alkaloid from Lycopodium clavatum. Tetrahedron 72:7065–7069. https://doi.org/10.1016/j.tet.2016.09.046
Puiatti M, Borioni JL, Vallejo MG, Cabrera JL, Agnese AM, Ortega MG, Pierini AB (2013) Study of the interaction of Huperzia saururus Lycopodium alkaloids with the acetylcholinesterase enzyme. J Mol Graph Model 44:136–144. https://doi.org/10.1016/j.jmgm.2013.05.009
Qiu WD, Yang JY, Shen Tu YQ, Chen L, Zheng HG, Lou XK (2009) The effect of huperzine on cognitive dysfunction after aabdomenal surgery in elder patients. Pharm J Chin PLA 25(3):222–224
Rafii MS, Walsh S, Little JT, Behan K, Reynolds B, Ward C, Jin S, Thomas R, Aisen PS (2011) A phase II trial of huperzine A in mild to moderate Alzheimer disease. Neurology 76(16):1389–1394. https://doi.org/10.1212/WNL.0b013e318216eb7b
Rao LJM, Kumari GNK, Rao NSP (1983) Two further acylated flavone glucosides from anisomeles ovata. Phytochemistry 22(4):1058–1060. https://doi.org/10.1016/0031-9422(83)85067-5
Raves ML, Harel M, Pang YP, Silman I, Kozikowski AP, Sussman JL (1997) Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat Struct Biol 4(1):57–63. https://doi.org/10.1038/nsb0197-57
Rhee IK, Rijn RM, Verpoorte R (2003) Qualitative determination of false-positive effects in the acetylcholinesterase assay using thin layer chromatography. Phytochem Anal 14:127–131. https://doi.org/10.1002/pca.675
Rollinger JM, Ewelt J, Seger C, Sturm S, Ellmerer EP, Stuppner H (2005) New insights into the acetycholinesterase inhibitory activity of Lycopodium clavatum. Planta Med 71:1040–1043. https://doi.org/10.1055/s-2005-873130
Sacher JR, Weinreb SM (2012) Construction of the Azocane (Azacyclooctane) Moiety of the Lycopodium Alkaloid Lycopladine H via an Intramolecular hydroaminomethylation strategy. Org Lett 14(8):2172–2175. https://doi.org/10.1021/ol3007277
Sakornrat T, Prateep W, Nisachon K, Nitirat C, Apiwan J, Somsak R, Nopporn T (2014) Three new Lycopodium alkaloids from Huperzia carinata and Huperzia squarrosa. Tetrahedron 70(43):8017–8022. https://doi.org/10.1016/j.tet.2014.08.042
Sano T, Fujimoto T, Tsuda Y (1970) Clavatol: a novel triterpenoid of the bisnoronocerane type isolated from Lycopodium clavatum. J Chem Soc D Chem Commun 20:1274–1275. https://doi.org/10.1039/C29700001274
Sano T, Tsuda Y, Inubushi Y (1970) Serratenediol: a new skeletal triterpenoid containing a seven membered ring. Tetrahedron 26(12):2981–2986. https://doi.org/10.1016/S0040-4020(01)92878-9
Shang YZ, Ye JW, Wang H, Tang XC (1999) Improving effects of huperzine A on abnormal lipid peroxidation and superoxide dismutase in aged rats. Acta Pharmacol Sin 20(9):824–828
Sharma M, Prajapati S, Gupta P (2019) Effect of Lycopodium clavatum (Homeopathic drug) on developmental and behavioural defects in Denio rerio embryo. Paper presented at the 6th biennial international conference DDNPTM-2018 and a mini symposium on traditional Chinese medicine, NIPER, Mohali, India, 12 February 2019
Shen YC, Chen CH (1994) Alkaloids from Lycopodium casuarinoides. J Nat Prod 57(6):824–826. https://doi.org/10.1021/np50108a021
Shi LL, He YZ (2012) Serrantane triterpenoid from Lycopodium japonicum. Chin J Exp Med Formul 18(9):90–92. https://doi.org/10.13422/j.cnki.syfjx.2012.09.074
Shi H, Li ZY, Guo YW (2005) A new serratane-type triterpene from Lycopodium phlegmaria. Nat Prod Res 19(8):777–781. https://doi.org/10.1080/14786410500044906
Shi QH, Han R, Fu JF, Wei J, Ge D, Ran JH, Liu ZX (2013) Effects of huperzine A on acute hypobaric hypoxic-induced apoptosis of hippocampal neurons in rats. Med J Chin PLA 38(2):103–106
Shi YC, Li RS, Li JY, Fan FC, Sun HJ (2014) Effect of huperzine A on c-Fos and GFAP expression in the paraventricular nucleus and the brainstem of mice with acute inflammation. J Henan Normal Univ 42(1):105–110. https://doi.org/10.16366/j.cnki.1000-2367.2014.01.030
Shigeyama T, Katakawa K, Kogure N, Kitajima M, Takayama H (2007) Asymmetric total syntheses of two phlegmarine-type alkaloids, lycoposerramines-V and -W, newly isolated from Lycopodium serratum. Org Lett 9(20):4069–4072. https://doi.org/10.1021/ol701871v
Siengalewicz P, Mulzer J, Rinner U (2013) Lycopodium alkaloids-synthetic highlights and recent developments. Alkaloids Chem Biol 72:1–151. https://doi.org/10.1016/B978-0-12-407774-4.00001-7
Staerk D, Larsen J, Larsen LA, Olafsdottir ES, Witt M, Jaroszewski JW (2004) Selagoline, a new alkaloid from Huperzia selago. Nat Prod Res 18(3):197–203. https://doi.org/10.1080/14786410310001620600
Southon IW, Buckingham J (1989) Dictionary of alkaloids. Chapman & Hall, London
Sun Y, Yan J, Meng H, He CL, Yi P, Qiao Y, Qiu MH (2008) A new alkaloid from Lycopodium japonicum Thunb. Helv Chim Acta 91:2107–2109. https://doi.org/10.1002/hlca.200890225
Sun ZH, Li W, Tang GH, Yin S (2017) A new serratene triterpenoid from Lycopodium japonicum. J Asian Nat Prod Res 19(3):299–303. https://doi.org/10.1080/10286020.2016.1208182
Szarek WA, Adams KAH, Curcumelli-Rodostamo M, Maclean DB (1964) Lycopodium alkaloids xvi. annotine. Can J Chem Eng 42(11):2584–2594. https://doi.org/10.1139/v64-378
Takayama H, Katakawa K, Kitajima M, Seki H, Yamaguchi K, Aimi N (2001) A new type of Lycopodium alkaloid, lycoposerramine-A, from Lycopodium serratum Thunb. Org Lett 3(26):4165–4167. https://doi.org/10.1021/ol0167762
Takayama H, Katakawa K, Kitajima M, Yamaguchib K, Aimi N (2002) Seven new Lycopodium alkaloids, lycoposerramines-C, -D, -E, -P, -Q, -S, and -U, from Lycopodium serratum Thunb. Tetrahedron Lett 43(46):8307–8311. https://doi.org/10.1016/S0040-4039(02)02026-9
Takayama H, Katakawa K, Kitajima M, Yamagughi K, Aimi N (2003) Ten new Lycopodium alkaloids having the lycopodane skeleton isolated from Lycopodium serratum Thunb. Chem Pharm Bull 51(10):1163–1169. https://doi.org/10.1248/cpb.51.1163
Tam TW, Liu R, Arnason JA, Krantis A, Staines WA, Haddad PS, Foster BC (2011) Cree antidiabetic plant extracts display mechanism-based inactivation of CYP3A4. Can J Physiol Pharmacol 89:13–23. https://doi.org/10.1139/Y10-104
Tanaka R, Tsujimoto K, Matsunaga S (1999) Two serratane triterpenes from the stem bark of Picea jezoensis var. hondoensis. Phytochemistry 52(8):1581–1585. https://doi.org/10.1016/S0031-9422(99)00318-0
Tang XC, Han YF (1999) Pharmacological profile of huperzine A, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev 5(3):281–300. https://doi.org/10.1111/j.1527-3458.1999.tb00105.x
Tang XC, Zhu XD, Lu WH (1988) Studies on the nootropic effects of huperzine A and B: two selective AChE inhibitors. In: Giacobini E, Becker R (eds) current research in Alzheimer therapy: cholinesterase inhibitors. Taylor and Francis, New York, pp 289–293
Tang Y, Xiong J, Hu JF (2015) Lycopodium alkaloids from Diphasiastrum complanatum. Nat Prod Commun 10(12):2091–2094. https://doi.org/10.1177/1934578x1501001219
Tang Y, Xiong J, Zhang JJ, Wang W, Zhang HY, Hu JF (2016) Annotinolides A−C, three lycopodane-derived 8,5-Lactones with polycyclic skeletons from Lycopodium annotinum. Org Lett 18(17):4376–4379. https://doi.org/10.1021/acs.orglett.6b02132
Tao YM, Fang L, Yang YM, Jiang HL, Yang HY, Zhang HY, Zhou H (2013) Quantitative proteomic analysis reveals the neuroprotective effects of huperzine A for amyloid beta treated neuroblastoma N2a cells. Proteomics 13:1314–1324. https://doi.org/10.1002/pmic.201200437
Teng CC, He YZ, Feng JL, Ba XY, Li DY (2010) Studies on the chemical constituents of Lycopodii Herba. Chin Tradit Herb Drugs 41:1960–1963
Thorroad S, Worawittayanont P, Khunnawutmanotham N, Chimnoi N, Jumruksa A, Ruchirawat S, Thasana N (2014) Three new Lycopodium alkaloids from Huperzia carinata and Huperzia squarrosa. Tetrahedron 70:8017–8022. https://doi.org/10.1016/j.tet.2014.08.042
Thu DK, Vui DT, Tung BT (2019) Two Lycopodium alkaloids from the aerial parts of Huperzia phlegmaria. Pharmacogn Res 11(4):396–399. https://doi.org/10.4103/pr.pr_82_19
Tori M, Shimoji T, Shimura E, Takaoka S, Nakashima K, Sono M, Ayer WA (2000) Four alkaloids, lucidine B, oxolucidine A, lucidine A, and lucidulinone from Lycopodium lucidulum. Phytochemistry 53(4):503–509. https://doi.org/10.1016/S0031-9422(99)00592-0
Tori M, Mukai Y, Nakashima K, Sono M (2005) Three glucosides from Lycopodium clavatum. Heterocycles 65(1):107–115. https://doi.org/10.3987/com-04-10239
Trofimova NN, Gromova AS, Semenov AA (1996) Serratene triterpenoids from Lycopodium clavatum L. (Lycopodiaceae). Russ Chem B 45(4):961–963. https://doi.org/10.1007/BF01431333
Tsuda Y, Fujimo T (1970) The structure of lycoclavanin: triterpenoid of Lycopodium claoaturn. J Chem Soc D 5:260–261. https://doi.org/10.1039/C29700000260
Tsuda Y, Hatanaka M (1969) Triterpenoids of Lycopodium clavatum: The structure of 21-episerratriol. Chem Commun 18:1040–1042. https://doi.org/10.1039/C2969001040B
Tung BT, Hai NT, Thu DK (2017) Antioxidant and acetylcholinesterase inhibitory activities in vitro of different fraction of Huperzia squarrosa (Forst.) Trevis extract and attenuation of scopolamine-induced cognitive impairment in mice. J Ethnopharmacol 198:24–32. https://doi.org/10.1016/j.jep.2016.12.037
Vallejo MG, Ortega MG, Cabrera JL, Agnese AM (2013) N-demethyl-sauroxine, a novel Lycodine Group alkaloid from Huperzia saururus. Tetrahedron Lett 54(38):5197–5200. https://doi.org/10.1016/j.tetlet.2013.07.068
Vasudeva SM (1999) Economic importance of pteridophytes. Ind Fern J 16:130–152
Ved HS, Koenig ML, Dave JR, Doctor BP (1997) Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. NeuroReport 8(4):963–968. https://doi.org/10.1097/00001756-199703030-00029
Vickers A (2002) Botanical medicines for the treatment of cancer: rationale, overview of current data, and methodological considerations for phase I and II trials. Cancer Invest 20:1069–1079. https://doi.org/10.1081/CNV-120005926
Vogl S, Picker P, Bison JM, Fakhrudin N, Atanasov AG, Heiss EH, Wawrosch C, Reznicek G, Dirsch VM, Saukel J, Kopp B (2013) Ethnopharmacological in vitro studies on Austria’s folk medicine—an unexplored lore in vitro anti-inflammatory activities of 71 Austriantraditional herbal drugs. J Ethnopharmacol 149(3):750–771. https://doi.org/10.1016/j.jep.2013.06.007
Wang H, Tang XC (1998) Anticholinesterase effects of huperzine A, E2020, and tacrine in rats. Acta Pharmacol Sin 19(1):27–30
Wang YE, Yue DX, Tang XC (1986) Anti-cholinesterase activity of huperzine A. Acta Pharmacol Sin 7(2):110–113
Wang XD, Zhang JM, Yang HH, Hu GY (1999) Modulation of NMDA receptor by huperzine A in rat cerebral cortex. Acta Pharmacol Sin 20(1):31–35
Wang R, Xiao XQ, Tang XC (2001) Huperzine A attenuates hydrogen peroxideinduced apoptosis by regulating expression of apoptosis-related genes in rat PC12 cells. NeuroReport 12(12):2629–2634. https://doi.org/10.1097/00001756-200108280-00009
Wang G, Zhang SQ, Zhan H (2006) Effect of huperzine A on cerebral cholinesterase and acetylcholine in elderly patients during recovery from general anesthesia. J South Med Univ 26(11):1660–1662. https://doi.org/10.3321/j.issn:1673-4254.2006.11.034
Wang R, Yan H, Tang XC (2006) Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin 27(1):1–26. https://doi.org/10.1111/j.1745-7254.2006.00255x
Wang XJ, Li L, Si YK, Yu SS, Ma SG, Bao XQ, Zhang D, Qu J, Liu YB, Li Y (2009) Nine new lycopodine-type alkaloids from Lycopodium japonicum Thunb. Tetrahedron 69:6234–6240. https://doi.org/10.1016/j.tet.2013.05.028
Wang XJ, Zhang GJ, Zhuang PY, Zhang Y, Yu SS, Bao XQ, Zhang D, Yuan YH, Chen NH, Ma SG, Qu J, Li Y (2012) Lycojaponicumins A-C, Three Alkaloids with an Unprecedented Skeleton from Lycopodium japonicum. Org Lett 14(10):2614–2617. https://doi.org/10.1021/ol3009478
Wang TT, Zhao LJ, He S, Yang R, Hou YD, Yan XJ (2012) Chemical constituents of volatile oils from Huperzia serrata by GC/MS. J Ningbo Univ (Natural Science & Engineering Edition) 25(4):16–19. https://doi.org/10.3969/j.issn.1001-5132.2012.04.006
Wang XJ, Li L, Yu SS, Ma SG, Qu J, Liu YB, Li Y, Wang YD, Tang WB (2013) Five new fawcettimine-related alkaloids from Lycopodium japonicum Thunb. Fitoterapia 91:74–81. https://doi.org/10.1016/j.fitote.2013.08.007
Wang XJ, Yu DQ, Yu SS (2014) Two new triterpenoids from Lycopodium japonicum Thunb. Chin J Chem 32:1007–1010. https://doi.org/10.1002/cjoc.201400456
Wang ZC, Wu JC, Zhao ND, Yang YY, Chen YG (2015) Two new Lycopodium alkaloids from Phlegmariurus phlegmaria (L.) Holub. Nat Prod Res 30(2):241–245. https://doi.org/10.1080/14786419.2015.1046131
Wang P, Yang XL, Yang B, Li CL, Qiu HC, Huang P (2015) Protect effects of huperzine A on methylglyoxal induced injury in the cultured human brain microvascular endothelial cell in vitro experimental study. Chin J Mod Pharm 32(3):277–281. https://doi.org/10.13748/j.cnki.issn1007-7693.2015.03.005
Wang LJ, Xiong J, Wang W, Zhang HY, Yang GX, Hu JF (2016) Lycopodium alkaloids from Lycopodium obscurum L. f. strictum. Phytochem Lett 15:260–264. https://doi.org/10.1016/j.phytol.2016.02.001
Williams JP, Friedrich D, Pinard E, Roden BA, Paquette LA, Laurent DRS (1994) Total synthesis of the Lycopodium alkaloids magellanine and magellaninone by three-fold annulation of 2-cyclopentenone. J Am Chem SOC 116(11):4689–4696. https://doi.org/10.1021/ja00090a017
Wojciech S, Agnieszka P, Piotr S, Olga O, Mirosława F, Olga K (2005) Somatic embryogenesis and in vitro culture of Huperzia selago shoots as a potential source of huperzine A. Plant Sci 168(6):1443–1452. https://doi.org/10.1016/j.plantsci.2004.12.021
Wu BG, Bai DL (1997) The First Total Synthesis of (+-)-Huperzine B. J Org Chem 62(17):5978–5981. https://doi.org/10.1021/jo970248f
Wu JH, Hunag R (2013a) Application of lycojaponicumin C in the treatment of ileocecal bowel cancer. CN 103446146 A, 18 Dec 2013
Wu JH, Liu Y (2013b) Application of lycojaponicumin C in the treatment of gastric cancer. CN 103446143 A, 18 Dec 2013
Wu JH, Hunag R (2013c) Application of lycojaponicumin C in the treatment of returning liver cancer. CN 103446150 A, 18 Dec 2013
Wu XD, Li XN, Peng LY, Zhao QS (2020) Huperserratines A and B, two macrocyclic Lycopodium alkaloids with an unusual skeleton from Huperzia serrata. J Org Chem 85:6803–6807. https://doi.org/10.1021/acs.joc.0c00623
Xiao XQ, Wang R, Han YF, Tang XC (2000) Protective effects of huperzine A on beta-amyloid25–35 induced oxidative injury in rat pheochromocytoma cells. Neurosci Lett 286:155–158. https://doi.org/10.1016/S0304-3940(00)01088-0
Xiao XQ, Zhang HY, Tang XC (2002) Huperzine A attenuates amyloid β-Peptide fragment 25–35-induced apoptosis in rat cortical neurons via inhibiting reactive oxygen species formation and caspase-3 activation. J Neurosci Res 67(1):30–36. https://doi.org/10.1002/jnr.10075
Xiong J, Meng WJ, Zhang HY, Zou YK, Wang WX, Wang XY, Yang QL, Osman EEA, Hu JF (2019) Lycofargesiines A-F, further Lycopodium alkaloids from the club moss Huperzia fargesii. Phytochem 162:183–192. https://doi.org/10.1016/j.phytochem.2019.03.015
Yan J, Zhang XM, Li ZR, Zhou L, Chen JC, Sun LR, Qiu MH (2005) Three new triterpenoids from Lycopodium japonicum. Helv Chim Acta 88:240–244. https://doi.org/10.1002/hlca.200590004
Yan J, Zhang XM, Li ZR, Zhou L, Chen JC, Sun LR, Qiu MH (2005) Three new triterpenoids from Lycopodium japonicum Thunb. Helv Chim Acta 88:240–244. https://doi.org/10.1002/hlca.200590004
Yang YF, Qu SJ, Xiao K, Jiang SH, Tan JJ, Tan CH, Zhu DY (2010) Lycopodium alkaloids from Huperzia serrata. J Asian Nat Prod Res 12(11):1005–1009. https://doi.org/10.1080/10286020.2010.522180
Yang Y, Wang XP, Sun QW, Xu F (2013) Identification of Lycopodii herba by UPLC fingerprint. Chinese Journal of Experimental Traditional Medical Formulae 19(15):117–120. https://doi.org/10.11653/syfj2013150117
Yang GZ, Cui YY, Wang DB (2015) Screening on the anti-inflammatory activities of compounds from Lycopodium species. J South-Cent Univ National 34(2):52–56
Yang Q, Zhu YQ, Peng W, Zhan R, Chen YG (2016) New Lycopodine-type alkaloid from Lycopodium japonicum. Nat Prod Res 30(19):2220–2224. https://doi.org/10.1080/14786419.2016.1146885
Yang YY, Wang ZC, Wu JC, Chen YG (2016) Chemical constituents of plants from the genus Phlegmariurus. Chem Biodivers 13(3):269–274. https://doi.org/10.1002/cbdv.201500043
Yang Q, Zhu YQ, Zhan R, Chen YG (2018) A new fawettimine-related alkaloid from Lycopodium japonicum. Chem Nat Compd 54(4):729–731. https://doi.org/10.1007/s10600-018-2456-2
Yao LQ, Ren H, Ai QL, Chang LH (2006) Efficacy of rehabilitation practice together with Huperzine A on the cognitive impairment in patients with Parkinson’s disease. Foreign Med Sci Psychiatry 33(3):204–206. https://doi.org/10.3969/j.issn.1673-2642.2006.03.002
Yeap JSY, Lim KH, Yong KT, Lim SH, Kam TS, Low YY (2019) Lycopodium Alkaloids: Lycoplatyrine A, an unusual lycodine-piperidine adduct from Lycopodium platyrhizoma and the absolute configurations of Lycoplanine D and Lycogladine H. J Nat Prod 11(4):396–399. https://doi.org/10.1021/acs.jnatprod.8b00754
Yin S, Fan CQ, Wang XN, Yue JM (2006) Lycodine-type alkaloids from Lycopodium casuarinoides. Helv Chim Acta 89:138–143. https://doi.org/10.1002/hlca.200690006
You XM, Yan YJ, Zou GX, Jiang H, Hou Z (2012) Study on the quality standard of Lycopodium Japonicum Thunb. J Liaoning Univ TCM 14(12):15–17. https://doi.org/10.13194/j.jlunivtcm.2012.12.17.youxm.049
Young JCF, Maclean DB (1963) Lycopodium alkaloids: xiv. flabelline. Can J Chem Eng 41:2731–2736. https://doi.org/10.1139/v63-403
Yuan SQ, Zhao YM (2003) A novel phlegmariurine type alkaloid from Huperzia serrata Thunb. Acta Pharm Sinica 38(3):596–598
Zeng YE, Ye MR, Xu H (1999) Experimental studies on anti-inflammation and analgesia pharmacological action of the different extracts from Lycopodium japonicum L. Li Shi zhen Medicine and Materia Medica Research 10(9):641–642. https://doi.org/10.3969/j.issn.1008-0805.1999.09.001
Zhang ZJ (2007) Clinical observation of huperzine A and nimotop in the treatment of Alzheimer’s fisease. Chin Mod Doc 45(13):23–24. https://doi.org/10.3969/j.issn.1673-9701.2007.13.013
Zhang LB, Iwatsuki K (2013) Lycopodiaceae. In: Wu ZY, Raven PH, Hong DY (eds) Pteridophytes. Flora of China, vol 2–3. Science Press, Beijing
Zhang HY, Tang XC (2003) Huperzine A attenuates the neurotoxic effect of staurosporine in primary rat cortical neurons. Neurosci Lett 340:91–94. https://doi.org/10.1016/S0304-3940(03)00023-5
Zhang SG, Wu YX (2011) Effect of huperzine A on cognitive dysfunction after stroke. Inner Mongolia J Tradit Chin Med 30(19):56–73. https://doi.org/10.3969/j.issn.1006-0979.2011.19.069
Zhang RW, Tang XC, Han YY, Sang GW, Zhang YD, Ma YX, Zhang CL, Yang RM (1991) Drug evaluation of huperzine A in the treatment of senile memory disorders. Acta Pharmacol Sin 12(3):250–252. https://doi.org/10.3891/acta.chem.scand.45-0546
Zhang ZX, Wang XD, Chen QT, Shu L, Wang JZ, Shan GL (2002) Clinical efficacy and safety of huperzine A in trreatment of mild to moderate Alzheimer disease, a placebo-controlled, double-blind, randomized trial. Natl Med J China 82(14):941–944. https://doi.org/10.3760/j:issn:0376-2491.2002.14.003
Zhang ZZ, Elsohly HN, Jacob MR, Pasco DS, Walker LA, Clark AM (2002) Natural products inhibiting Candida albicans secreted aspartic proteases from Lycopodium cernuum. J Nat Prod 65:979–985. https://doi.org/10.1021/np0200616
Zhang W, Qin SD, Lv ZP, Wu JZ (2006) A clinical study of the effect of nimodipine on the cognition impairment of diabetes. Medical Recapitulate 14(16):2539–2541. https://doi.org/10.3969/j.issn.1006-2084.2008.16.052
Zhang HY, Chun YZ, Yan H, Wang ZF, Tang LL, Gao X, Tang XC (2008) Potential therapeutic targets of huperzine A for Alzheimer’s disease and vascular dementia. Chem Biol Interact 175(1):396–402. https://doi.org/10.1016/j.cbi.2008.04.049
Zhang XY, Dong LB, Liu F, Wu XD, He J, PengHR LYLQS, Zhao (2013) New Lycopodium alkaloids from Lycopodium obscurum. Nat Prod Bioprospect 3(2):52–55. https://doi.org/10.1007/s13659-013-0015-x
Zhang Y, Yi P, Chen Y, Mei ZN, Hu X, Yang GZ (2014) Lycojaponicuminol A-F: cytotoxic serratene triterpenoids from Lycopodium japonicum. Fitoterapia 96:95–102. https://doi.org/10.1016/j.fitote.2014.04.012
Zhang ZJ, Zhu QF, Su J, Wu XD, Zhao QS (2018) Lycoplanines B-D, Three Lycopodium alkaloids from Lycopodium Complanatum. Natur Prod Bioprosp 8:177–182. https://doi.org/10.1007/s13659-018-0161-2
Zhang ZJ, Qi YY, Wu XD, Su J, Zhao QS (2018) Lycogladines A-H, fawcettimine-type Lycopodium alkaloids from Lycopodium complanatum var. glaucum Ching. Tetrahedron 74(14):1692–1697. https://doi.org/10.1016/j.tet.2018.02.034
Zhang ZJ, Wang C, Wu XD, Huang Y, Zhou WX, Zhao QS (2019) Phlegmadine A: a Lycopodium alkaloid with a unique cyclobutane ring from Phlegmariurus phlegmaria. J Org Chem 84:11301–11305. https://doi.org/10.1021/acs.joc.9b01723
Zhang ZJ, Zhu QF, Wu XD, Zhao QS (2020) Phlegmadines B and C, two Lycopodium alkaloids with 6/5/5/5/7 pentacyclic skeleton from Phlegmariurus phlegmaria.Tetrahedron Lett 61(2):1–3. https://doi.org/10.1016/j.tetlet.2019.151381
Zhao M, Wang YX, Zhang YL (2007) Effect observation of donepezil in the treatment of vascular dementia. J Med Forum 28(10):57–58. https://doi.org/10.3969/j.issn.1672-3422.2007.10.031
Zhao YH, Deng TZ, Chen Y, Liu XM, Yang GZ (2010) Two new triterpenoids from Lycopodium obscurum L. J Asian Nat Prod Res 12(8):666–671. https://doi.org/10.1080/10286020.2010.493881
Zhou BN, Zhu DY, Huang MF, Lin LJ, Lin LZ, Han XY, Cordell GA (1992) NMR Assignments of huperzine A, serratinine and lucidioline. Phytochemistry 34(5):1425–1428. https://doi.org/10.1016/0031-9422(91)80042-Y
Zhu DY, Jiang SH, Huang MF, Lin LZ, Cordell GA (1994) Huperserratinine from Huperzia serrata. Phytochemistry 36(4):1069–1072. https://doi.org/10.1016/S0031-9422(00)90493-X
Zhu N, Lin JZ, Chen QZ, Wei MD, Wang Y (2013) Anti-inflammatory effect of huperzine A on protection of rat neural stem cells in vitro. Chin J Pathophysiol 29(7):1160–1164. https://doi.org/10.3969/j.issn.1000-4718.2013.07.002
Zhu Y, Dong LB, Zhang ZJ, Fan M, Zhu QF, Qi YY, Liu YC, Peng LY, Wu XD, Zhao QS (2018) Three new Lycopodium alkaloids from Lycopodium japonicum. J Asian Nat Prod Re 21(1):17–24. https://doi.org/10.1080/10286020.2018.1427075
Zhu XL, Wang LL, Shi ZH, Xia D, Zhou ZB, Pan K (2019) Lycocasuarines I-Q, new Lycopodium alkaloids isolated from Lycopodiastrum casuarinoides. Fitoterapia 134:474–480. https://doi.org/10.1016/j.fitote.2019.03.027
Zhu XL, Xia D, Zhou ZB, Xie SS, Shi ZH, Chen GM, Wang LL, Pan K (2020) Lycosquarrines A−R. J Nat Prod, Lycopodium alkaloids from Phlegmariurus squarrosus. https://doi.org/10.1021/acs.jnatprod.9b00815
Zou GX, You XM, Wang GH (2010) HPLC determination of the content of α-obscurine in Lycopodium japonicum. J Chin Med Mater 33(6):934–936. https://doi.org/10.13863/j.issn1001-4454.2010.06.035
Author information
Authors and Affiliations
Contributions
CG and BW wrote the manuscript, performed the literature search and data analysis, and QF provided analysis and critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Guan, C. & Fu, Q. The traditional uses, secondary metabolites, and pharmacology of Lycopodium species. Phytochem Rev 21, 1–79 (2022). https://doi.org/10.1007/s11101-021-09746-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-021-09746-4