Abstract
Objective Although Parkinson’s disease is a common disorder in the elderly, there have been very few studies of the role of the pharmaceutical care services in detecting and reducing problems associated with drug treatment in community settings. The aim of this study was therefore to investigate the type and frequency of drug-related problems identified in patients with Parkinson’s disease by community pharmacists over an 8-month period and to assess the pharmaceutical service interventions, the type and frequency of intervention outcomes and the clinical benefits for the patients. Setting Community pharmacies in Germany. Method Thirty-two community pharmacists recruited 113 outpatients with idiopathic Parkinson’s disease who were receiving anti-Parkinsonian medication. Main outcome measure Drug-related problems. Results A total of 331 drug-related problems were identified by the pharmacists. Patients not receiving a medication, despite the presence of an indication or symptom, accounted for the highest proportion of drug-related problems (26.3%). The pharmacists proposed a total of 474 interventions, the most common of which was giving the patient treatment advice (19.6%). Intervention outcomes were recorded for 215 of the 331 drug-related problems, for which there were 553 individual outcome results. Adjustments of the drug regimen accounted for the highest percentage of individual results (43.6%). Conclusion Structured pharmaceutical care processes by community pharmacists have the potential to make a valuable contribution to health care and enhance the health outcomes of patients with Parkinson’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact of the findings on practice
-
Structured pharmaceutical care processes provided by community pharmacists have a high potential to provide a valuable contribution to improving the health care of patients with Parkinson’s disease.
Introduction
In 1999, the Pharmaceutical Care Network Europe (PCNE) agreed upon the definition of drug-related problems (DRPs) as “events or circumstances involving drug therapy that actually or potentially interfere with desired health outcomes” [1]. Since then, much research effort has been put into processes to identify, evaluate and solve these DRPs.
The role of community pharmacists has become increasingly important over the years and has changed from drug formulation and dispensing to providing advice and recommendations on drug regimes. Pharmacists are able to detect DRPs during the dispensing process. This includes continuous monitoring of potential drug interactions through current versions of the summary of product characteristics, and by review of the patient’s drug regimen [2, 3]. In situations where the pharmacist is aware of a problem in a specific therapeutic area, they can actively approach patients who may be affected or at risk [4]. The aim of such a pharmacist intervention is to reduce inappropriate medication use, to improve medication management for patients and to cooperate directly with other health care professionals [3, 5]. This takes the form of direct discussions and a regular quarterly letter from the pharmacist detailing the patient’s drug regimen and the potential for interactions and adverse events.
The increasing awareness of DRPs and their detrimental effect on patients’ health [6, 7] has led to a number of studies of pharmaceutical care services [8–11]. However, although Parkinson’s disease is the second most common neurodegenerative disorder in the elderly population, very few such studies exist in this area [12]. Given that age, multi-morbidity and polypharmacy are known risk factors for DRPs, Parkinson’s disease outpatients are at particular risk of experiencing problems associated with long-term drug treatment [13, 14]. Not least, Parkinson’s disease patients in the more severe stages of their disease often take up to 10 or more tablets/day for Parkinson’s disease alone [15], thus confirming a patient population at risk for DRPs. Further confirmation for DRPs in this patient population is provided by a previous study in which we retrospectively searched for anti-Parkinsonian drug DRPs communicated in Parkinson’s disease online forums. Over a 1-year period, 160 outpatients experienced 238 DRPs, of which 153 were adverse drug reactions (ADRs) [16]. The number and range of DRPs clearly substantiates the requirement for the facilitation of professional recommendation from pharmacists in primary care.
Aim of the study
The primary aim was to investigate the type and frequency of DRPs identified in Parkinson’s disease patients by community pharmacists and to assess the clinical benefits of providing DRP-targeted pharmaceutical service interventions.
Method
Pharmacy service and data collection
Thirty-two German community pharmacists participated in this multicentre, longitudinal study. The pharmacists were identified following their participation in an advanced training course on pharmaceutical care services in local pharmacies in the month prior to the start of the study. All pharmacists fulfilled requirements for qualification as “local pharmacies” in accordance with the cooperation agreement between the national health insurance fund “Barmer Ersatzkasse” and the German Pharmacists’ Association [17]. This cooperation agreement covers a structured program based on current principles of pharmaceutical care [18]. The “local pharmacies” agreement comprises two categories: “drug service” with its requirements for data collection and advising patients, and “pharmaceutical management”. This includes closer examination of patients’ history and drug regimens and software-supported checks for DRPs, as well as interventions for management/prevention of DRPs [19].
Pharmacists were provided with a list of frequently occurring DRPs in Parkinson’s disease patients. This checklist was prepared using a stepwise approach based on analysis of on-line patient forums [16], an unpublished survey among neurologists and systematic literature research (Fig. 1).
The unpublished survey involved sending a standardized questionnaire to 32 neurologists (20 office-based, 12 clinicians; mean practical experience 8 years) between November 2004 and August 2005. The neurologists were asked about their preferred treatment approaches for Parkinson’s disease and commonly occurring therapeutic complications in everyday clinical practice. DRPs were included in the checklist if reported by at least three doctors.
The literature search was performed in August 2005 in Medline and Embase, with the language restricted to English, French or German. Keywords comprised drug-related problems, medication problems, medication-related problems, adverse drug events, adverse drug effects, adverse drug reactions, prescription errors, medication errors, inappropriate medication, inappropriate drug, inappropriate prescription, medication misadventures in combination with [idiopathic] Parkinson’s disease, anti-Parkinsonian agents, anti-Parkinsonian drugs, and community, ambulance, outpatient or primary care. The final checklist provided a list of common DRPs associated with anti-Parkinsonian agents, problem descriptions and recommendations for problem solutions within the scope of the structured pharmaceutical service programme.
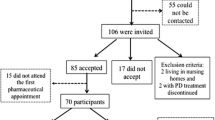
Between January 1st and March 1st 2006, the 32 pharmacists recruited outpatients who fulfilled the following inclusion criteria: diagnosis of idiopathic Parkinson’s disease according to UK Brain Bank Criteria[20] (determined by a neurology specialist prior to the study), age >35 years, receiving one or more anti-Parkinsonian medication(s), sufficient physical and cognitive ability to complete the questionnaires without assistance, no prior involvement in a “local pharmacies” service programme and German as a native language. Exclusion criteria were atypical Parkinsonism and dementia. All patients provided written informed consent. The pharmacists gave written confirmation that they would adhere to the general regulations for data protection. Ethical approval of the institutional review board was not required. The study was conducted within the scope of daily pharmaceutical routine and observed existing clinical and pharmaceutical practice in accordance with German pharmacy regulations.
Pharmacists had access to the information required in the study for providing pharmaceutical care service in daily pharmaceutical routine. As “local pharmacies”, they documented further information gathered from patients and physicians in accordance with the cooperation agreement between the “Barmer Ersatzkasse” and the German Pharmacists’ Association. There was no assignment to different groups, and the study used standard questionnaires with patient data remaining anonymous.
Following inclusion of the patients in the “local pharmacies” service programme, the pharmacists contacted the treating neurologist and provided information on the study and data communication among health care professionals in accordance with the service programme. The pharmacists received information on neurological status, date of Parkinson’s disease diagnosis, Hoehn and Yahr severity stage [21] (where 0 = no signs of the disease; and 5 = symptoms are very severe) and cardinal motor symptoms. Demographic data and medical history were collected and the patients completed questionnaires including a new 23-item Parkinson’s Scale (PS-23) (Sabrina Schröder, personal communication), Parkinson’s Disease Questionnaire-8 (PDQ-8) [22] and EuroQol (EQ-5D) [23] plus visual analogue scale (EQ-5D-VAS).
Symptom severity was determined by the PS-23 using a 5-point Likert-scale. The PS-23 has been shown to be a valid, reliable and responsive Parkinson’s disease-specific instrument (Sabrina Schröder, personal communication). The scale covers four dimensions of self-reported health status (bodily discomfort, mobility, cognitive functioning and emotional well-being), and includes one factor for total health status and one for non-motor symptoms worsened by ADRs. The PDQ-8 is the short form of the Parkinson’s Disease Questionnaire-39 (PDQ-39), the most widely used and validated Parkinson’s disease-specific measure for self-reported health status [16–19] with documented responsiveness [20]. The instrument covers eight dimensions of health (mobility, activity of daily living, emotional well-being, stigma, social support, cognition, communication and bodily discomfort). The generic EQ-5D covers five dimensions of self-reported health status (mobility, self-care, usual activities, pain/discomfort, anxiety/depression).
Within the scope of the pharmaceutical service routine, pharmacists recorded the services provided for the patients over an 8-month follow-up period. The number and duration of patient visits, patient details, medication details, description of DRPs, reasons for intervention, nature and description of intervention, and whether contact with the physician was established to discuss detected problems, were collected and anonymously documented.
Classification of drug-related problems
Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) system [24]. DRPs were coded according to the Problem Intervention Documentation (PI-Doc) system [25, 26]. The original PI-Doc system has two levels of classification (problem analysis and intervention recommendation)[1, 9, 25] whereby a code for a problem-solving intervention is allocated to each code for a DRP. The open hierarchical structure of the classification system made it possible to allocate DRPs to one of six predefined major problem categories: ADRs, drug interactions, inappropriate dosage, inappropriate drug use by the patient/compliance problems, inappropriate drug choice, and other problems. The modified version of the PI-Doc used in this study has a third level of classification that covers the intervention outcome: improving patient’s status of health, increasing patient’s safety and drug regimen adjustment [27].
The clinical significance of the DRPs was assessed by a multidisciplinary expert panel consisting of four physicians with clinical experience in internal medicine, geriatrics, neurology and psychiatry, and one experienced pharmacist. As described in a previous study [28], each expert independently rated the clinical significance of the DRPs as extremely important, or of major, moderate or minor importance (Table 1). The final evaluation of the clinical significance was categorized by consensus of the expert panel.
ADRs were coded according to the World Health Organisation Adverse Reaction Terminology (WHO-ART) and attributed to a specific System and Organ Class (SOC) [29]. The expert panel also rated the seriousness of ADRs according to the criteria of the Council for Informational Organizations of Medical Sciences (CIOMS), corroborated by the Working Group IV of the CIOMS and the European Medicines Agency (EMEA) in 1995 [30, 31]. The criteria for a serious ADR are: death, life threatening, hospitalisation or prolongation of hospitalisation, persistent or significant disability/incapacity, and congenital anomaly/birth defect. The panel also checked for unexpected ADRs defined as an adverse reaction, the nature or severity of which was not consistent with the product information as supplied to the patient in the form of the patient information leaflet or in the summary of product characteristics.
Statistical analyses
Simple descriptive statistical methods were used for the evaluation of DRPs. Frequency was calculated for each type of DRP according to PI-Doc (3 level) [27] and categories of clinical significance, as well as for the pharmacist service interventions and interventions’ outcomes. Categorical patient data were given as absolute frequency and percentages; continuous data were described using mean and standard deviation.
Results
Patient characteristics
Of the 148 eligible patients, 113 agreed to participate in the study. The baseline demographic and medical characteristics are shown in Table 2. Thirteen patients were lost to follow-up: five died, four had very severe disease, two were misdiagnosed with idiopathic Parkinson’s disease and two refused to complete follow-up.
The mean number of patients reviewed by each pharmacist at baseline was 3.5 (range 2–10); 3.1 patients per pharmacist (range 1–10) continued at follow-up. Over the 8-month follow-up period, the mean number of visits to the pharmacist was 4.8/patient (range 2–17).
Type and frequency of DRPs
A total of 331 DRPs (approximately 2.9/patient) were identified by the pharmacists over the 8-month period. Patients not receiving a medication despite the presence of an indication or symptom accounted for the highest proportion of DRPs (26.3%) (SP6) (Table 3). Other common categories included symptoms of an ADR (12.4%), unsuitable time of intake (10.0%), under-dosage (9.7%) and drug interactions (9.4%). Of the 331 DRPs, 159 (48.0%) were related to anti-Parkinsonian agents (33 to dopamine agonists, 92 to L-dopa and 34 to other agents).
Clinical significance of DRPs
The clinical significance of the DRPs was categorised as extremely important in 4.5% of cases, major in 27.2%, moderate in 29.0% and minor in 39.3%. The following three DRPs were rated as serious according to EMEA guidelines: bloody faeces (Stalevo®), falls (L-dopa) and insulin hypoglycaemia (insulin lispro). The first two required hospitalisation whilst the third was considered life-threatening. There were four unexpected ADRs according to EMEA guidelines: bloody faeces (Stalevo®), hair loss (L-dopa), hypertension (rasagiline) and angina pectoris (simvastatin).
DRP-targeted interventions
The pharmacists proposed a total of 474 interventions for the 331 DRPs. The most common intervention was giving the patient special treatment advice (19.6%) (Fig. 2), followed by interventions associated with compliance problems (15.6%) and ADRs (11.6%).
DRP-targeted intervention outcomes
Pharmacists recorded the observed outcomes an average of 5.4 months after the initial DRP/intervention documentation. Intervention outcomes were recorded for 215 of the 331 DRPs. For 89 DRPs (27%), no outcome was observed within the study period and there was no data available for 27 (8%) that occurred in the patients who withdrew prematurely. Evaluation of the 215 documented outcomes using the modified PI-Doc classification showed there were 553 individual outcome results, i.e. 2.57 per observed intervention outcome (Table 4). Adjustments of the drug regimen accounted for the highest percentage of individual results (241 cases), followed by improvement in the patient’s safety (223 cases) and improvement in health status (89 cases).
Amongst the 241 cases in which the drug regimen was adjusted, 140 (25.3%) involved the prescription being adjusted according to the patient information leaflet. The most common adjustment concerned the time of day at which the medication was taken (n = 24; 17.1%). This was followed by consideration of interactions (n = 19; 13.6%), increased dosage (n = 18; 12.9%), reduced dosage (n = 14; 10.0%) and consideration of ADRs (n = 14; 10.0%). Other adjustments involved changes in the interval between doses (n = 12; 8.6%), changes in formulation (n = 10; 7.1%), consideration of contraindications (n = 10; 7.1%), consideration of age (n = 9; 6.4%) and use of special application devices (n = 5; 3.6%).
Four patients were identified as being misdiagnosed with Parkinson’s disease and subsequently discontinued anti-Parkinsonian treatment. These misdiagnoses were corrected by the physicians into restless leg syndrome, hypertensive encephalopathy, essential tremor (in a patient who had been mistakenly treated for Parkinson’s disease for 25 years) and pathological cerebral contraction. There were a further two patients who received additional diagnoses due to the pharmacist’s closer collaboration with the patient’s physician. In one case, a diagnosis of cardiac insufficiency led to an adjustment in the medication regimen, whilst in the other, a diagnosis of Parkinson’s disease plus encephalitis resulting from borreliosis led to discontinuation from the study.
Discussion
The results from this study show that community pharmacists identified a wide array of DRPs within the primary care of Parkinsonian patients. Almost half of the recorded DRPs were related to anti-Parkinsonian medications (1.3 per patient over 8 months). This is almost as many as were identified in a previous study of Parkinson’s disease forum users (approximately 1.5/patient over 1 year) [16]. When comparing findings from the forum research with pharmacists’ records on DRPs, it is evident that inappropriate dosage and compliance problems are common in both cases. Comparison of data provided by forum users and pharmacy customers, however, might be limited due to differences in patient characteristics and the reporting methods by consumers versus health care professionals. Selection bias also cannot be excluded in either study.
Poor compliance can be partially explained by the nature of Parkinson’s disease. As the condition progresses, multiple symptoms result in a highly complex clinical picture and the drug regimens become more complicated than in other disease areas [15]. Inadequate timing of doses may be the consequence, and erratic drug-taking has been shown to be common in Parkinson’s disease [32].
In addition, anti-Parkinsonian agents are associated with side effects, especially psychosis, motor fluctuations, and dyskinesias [33, 34]. The pharmacists’ identification of ADRs (13.3% ADRs out of 331 DRPs) and ADR-related interventions (11.6%) was low when compared with forum research (64.2% of DRPs) and a previous non-indication specific study on community pharmacist services in which almost half of interventions were aimed at preventing ADRs [35]. It seems that prescribing physicians in our study were generally concerned about prevention of dosage-related dopaminergic ADRs [33, 34]. This was also reflected by a comparatively high percentage of under-dosage amongst the DRPs. It is not clear, however, whether these were unintentional or whether the physician chose to deviate from the recommended dosage. In addition, the physicians appeared to focus on amelioration of traditional cardinal motor symptoms. The highest percentage of DRPs was due to a lack of medication despite the presence of symptoms, especially non-motor symptoms. These results are in line with current literature showing that non-motor symptoms of Parkinson’s disease continue to remain underestimated in clinical practice [36].
Pharmacists’ collaboration with physicians and special clinical centres accounted for 16.3% of interventions, suggesting that the contribution of community pharmacists is particularly important in identifying and improving patients’ unmet medical needs. This is supported by another study showing that ‘need for additional drug’ was identified significantly more often through face-to-face interviews between patients and pharmacists than by routine examinations by the prescribing physician [28].
Although patient interviews have become part of established pharmacy practice [5], a high number of DRPs recorded in our study were proactively identified by pharmacists who had an increased awareness of problems due to using a checklist of common DRPs. These findings are in line with previous studies showing that proactive intervention by community pharmacists leads to modification of prescription errors [35, 37]. One practical recommendation that emerges from this study is that pharmacists who provide primary care should be given the opportunity to qualify in “pharmaceutical care service”. The use of the checklist of common DRPs significantly increased the awareness of problems in Parkinson’s disease and should be implemented in standardized pharmaceutical services for other chronic disorders.
Importantly, in our study, four patients were identified who had been misdiagnosed with Parkinson’s disease and subsequently received a different medication regime. In two other cases, additional diagnoses were made following close collaboration between pharmacists and physicians. These cases provide the first evidence that pharmaceutical care services in local pharmacies can make a valuable contribution to the health outcome of Parkinson’s disease patients.
One of the methodological limitations of our study was the selection of patients by the pharmacists, who may have recruited those patients who are more prone to DRPs (selection bias). In addition, although the patients were the primary source of information, the pharmacists assessed the outcome of their interventions themselves. Consequently, pharmacist-recorded outcomes of DRP-targeted interventions need to be seen in the light of interviewer and reporting bias, and can only reveal tendencies for the effectiveness of pharmaceutical care services rather than evidence. In addition, pharmacists were free to document more than one individual outcome result for each intervention, so conclusions about the quality of a single intervention cannot be drawn.
The clinical significance of DRPs assessed by an independent panel utilising explicit criteria might have been a more appropriate outcome measure. There is no officially recognised classification system for the significance of DRPs. The first quality-related classification of DRPs was developed by a team consisting of a specialist in pharmacotherapeutics as well as clinical pharmacists [28]. However, there are no strict guidelines for this classification and the assessment depends on the experience of the physician. Hence, evaluations should be conducted by at least three health care professionals, as in the current study. The expert panel assessed almost 40% of DRPs as being of minor clinical significance. This is in clear contrast to a previous study [28], which indicated that DRPs identified by hospital pharmacists might be of greater clinical significance (only 10% were considered of little clinical importance). It must be considered though that the type of DRP differs markedly between different patient groups, and that a valid comparison of the clinical value of services offered by community and hospital pharmacists cannot be made [38].
Future research on pharmaceutical management in local pharmacies should focus on the identification of DRPs and the effects of pharmaceutical interventions on patient outcomes among different patient groups or indications. In addition, the German model will need to be compared with that in other countries worldwide in order to develop strategies aimed at improving pharmaceutical services for patients with chronic diseases on an international basis.
Conclusion
Our study generates new information on Parkinson’s disease patients’ individual problems identified in a community setting. The community pharmacists identified a considerable number of DRPs that covered a wide spectrum of conditions, almost half of which were related to anti-Parkinsonian medication. The findings indicate that structured pharmaceutical care processes by community pharmacists have a high potential to provide a valuable contribution to the health care of these patients.
References
van Mil JWF. Proceedings of the international workings conference on outcomes measurement in pharmaceutical care 9. Pharmaceutical care network Europe (PCNE). Hilleroed, Denmark; 1999. p. 84. http://www.pcne.org/dokumenter/PCNE%20scheme%20V4.00.pdf.
Lipton HL, Bero LA, Bird JA, McPhee SJ. The impact of clinical pharmacists’ consultations on physicians’ geriatric drug prescribing. A randomized controlled trial. Med Care. 1992;30:646–58.
Goldstein R, Hulme H, Willits J. Reviewing repeat prescribing—general practitioners and community pharmacists working together. Int J Pharm Pract. 1998;6:60–6.
Munroe WP, Kunz K, Dalmady-Israel C, Potter L, Schonfeld WH. Economic evaluation of pharmacist involvement in disease management in a community pharmacy setting. Clin Ther. 1997;19:113–23.
Scottish Department of Health Clinical Resource and Audit Group. Clinical pharmacy practice in primary care. Edinburgh: Scottish Office; 1998.
Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash). 2001;41:192–9.
Gandhi TK, Seger DL, Bates DW. Identifying drug safety issues: from research to practice. Int J Qual Health Care. 2000;12:69–76.
Westerlund T, Almarsdottir AB, Melander A. Factors influencing the detection rate of drug-related problems in community pharmacy. Pharm World Sci. 1999;21:245–50.
van Mil JW, Dudok van Heel MC, Boersma M, Tromp TF. Interventions and documentation for drug-related problems in Dutch community pharmacies. Am J Health Syst Pharm. 2001;58:1428–31.
Paulino EI, Bouvy ML, Gastelurrutia MA, Guerreiro M, Buurma H. Drug related problems identified by European community pharmacists in patients discharged from hospital. Pharm World Sci. 2004;26:353–60.
Westerlund LT, Marklund BR, Handl WH, Thunberg ME, Allebeck P. Nonprescription drug-related problems and pharmacy interventions. Ann Pharmacother. 2001;35:1343–9.
Hubert MA, Mayer R, Müller CE. Parkinson-Therapeutika. Teil II: Wirkstoff-Dossiers, Therapiekonzepte und pharmazeutische Betreuung. Deutsche Apotheker Zeitung. 2002;39:62–71.
Jameson JP, Van Noord GR. Pharmacotherapy consultation on polypharmacy patients in ambulatory care. Ann Pharmacother. 2001;35:835–40.
Cunningham G, Dodd TR, Grant DJ, McMurdo ME, Richards RM. Drug-related problems in elderly patients admitted to Tayside hospitals, methods for prevention and subsequent reassessment. Age Ageing. 1997;26:375–82.
Leoni O, Martignoni E, Cosentino M, Michielotto D, Calandrella D, Zangaglia R, Riboldazzi G, Oria C, Lecchini S, Nappi G, Frigo G. Drug prescribing patterns in Parkinson’s disease: a pharmacoepidemiological survey in a cohort of ambulatory patients. Pharmacoepidemiol Drug Saf. 2002;11:149–57.
Schröder S, Zöllner YF, Schaefer M. Drug related problems with Antiparkinsonian agents: consumer Internet reports versus published data. Pharmacoepidemiol Drug Saf. 2007;16:1161–6.
Kooperationsvereinbarung zwischen der BEK und dem DAV. Vereinbarung zwischen der Barmer Ersatzkasse (BEK) und dem Deutschen Apothekerverband e.V. (DAV) zur Intensivierung der Kooperation bei der qualitätsorientierten Versorgung der Versicherten. 2003.
Schaefer M, Schulz M. Manuale zur Pharmazeutischen Betreuung. Band 1, Grundlagen der Pharmazeutischen Betreuung. Govi-Verlag; 2000. p 24.
Himstedt S, Kirchhoff G. Hausapotheke: Pharmazeutische Dienstleistung für die Patienten. Pharm Ztg. 2004. 19/44. http://www.pharmazeutische-zeitung.de/index.php?id=titel_19_2004; cited June 10 2005.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992;55:181–4.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–42.
Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The PDQ-8: development of a short form Parkinson’s disease questionnaire. Psychol Health. 1997;12:805–14.
The EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208.
ATC-Index. Anatomisch-therapeutisch-chemische-Klassifikation mit Tagesdosen. Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland im Jahr 2005. Status: 1 January 2005.
van Mil JWF, Westerlund T, Hersberger KE, Schaefer M. Drug-related problem classification systems. Ann Pharmacother. 2004;38:859–67.
Schaefer M. Discussing basic principles for a coding system of drug-related problems: the case of PI-Doc. Pharm World Sci. 2002;24:120–7.
Mattenklotz A. Vergleichende Validierung von zwei Klassifizierungssystemen für arzneimittelbezogene Probleme (PCNE und PiDoc). Master thesis, Charité—University Medicine Berlin; 2006.
Viktil KK, Blix SH, Moger TA, Reikvam A. Interview of patients by pharmacists contributes significantly to the identification of drug-related problems (DRPs). Pharmacoepidemiol Drug Saf. 2006;15:667–74.
WHO Adverse Drug Reaction Dictionary. The WHO Adverse Reaction Terminology WHO-ART. Terminology for coding clinical information in relation to drug therapy (December 2005). http://www.umc-products.com/graphics/3149.pdf.
CIOMS Working Group IV. Benefit-risk balance for marketing drugs: safety signals. Geneva: CIOMS; 1998.
EMEA. Note for guidance on clinical safety data management: definitions and standards for expedited reporting. June 1995—CPMP/ICH/377/95. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002749.pdf.
Grosset D, Antonini A, Canesi M, Pezzoli G, Lees A, Shaw K, Cubo E, Martinez-Martin P, Rascol O, Negre-Pages L, Senard A, Schwarz J, Strecker K, Reichmann H, Storch A, Löhle M, Stocchi F, Grosset K. Adherence to antiparkinson medication in a multicenter European study. Mov Disord. 2009;24:826–32.
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson’s disease therapy. Ann Neurol. 2003;53(Suppl 3):3–15.
Rascol O, Goetz C, Koller W, Poewe W, Sampaio C. Treatment interventions for Parkinson’s disease: an evidence based assessment. Lancet. 2002;359:1589–98.
Buurma H, De Smet PA, Leufkens HGM, Egberts AC. Evaluation of the clinical value of pharmacists’ modification of prescription errors. Br J Clin Pharmacol. 2004;58:503–11.
Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–74.
Rupp MT. Value of community pharmacists’ interventions to correct prescribing errors. Ann Pharmacother. 1992;26:1580–4.
Viktil KK, Blix HS, Reikvam A, Moger TA, Hjemaas BJ, Walseth EK, Vraalsen TF, Pretsch P, Jorgensen F. Comparison of drug related-problems in different patient groups. Ann Pharmacother. 2004;38:942–8.
Acknowledgments
The authors would also like to thank: the 32 study pharmacists for participation in the study; the 32 neurologists for participation in a survey on DRPs and unmet medical needs in Parkinson’s disease; and the members of the expert panel for the assessment of the clinical significance of DRPs and the seriousness and unexpectedness of adverse events; and Jane Irons and Mark Hickery for their assistance in editing the final manuscript.
Funding
The study was supported by an unrestricted grant from Solvay Pharmaceuticals GmbH, Hanover, Germany.
Conflicts of interests
None of the authors has any declarable conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schröder, S., Martus, P., Odin, P. et al. Drug-related problems in Parkinson’s disease: the role of community pharmacists in primary care. Int J Clin Pharm 33, 674–682 (2011). https://doi.org/10.1007/s11096-011-9526-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-011-9526-x