Abstract
Purpose
Hydrogen/deuterium (H/D) exchange over a range of temperatures suggests a protein structural/mobility transition in the solid state below the system glass transition temperature (Tg). The purpose of this study was to determine whether solid-state protein stability correlates with the difference between storage temperature and apparent Td where an abrupt change in mobility occurs, or alternatively, the extent of H/D exchange at a single temperature correlates directly to protein stability in lyophilized solids.
Methods
Solid-state H/D exchange was monitored by FTIR spectroscopy to study the extent of exchange and the apparent transition temperature in both pure recombinant human serum albumin (rHSA) and rHSA formulated with sucrose or trehalose. H/D exchange of freeze-dried formulations at 11% RH and temperatures from 30 to 80°C was monitored. Protein stability against aggregation at 40°C/11% RH for 6 months was assessed by size exclusion chromatography (SEC).
Results
Both sucrose and trehalose showed equivalent protection of protein secondary structure by FTIR. The rHSA:sucrose formulation showed superior long-term stability at 40°C by SEC over the trehalose formulation, but the apparent Td determined from H/D exchange was much higher in the trehalose formulation. Instead, the extent of H/D exchange (X∞) was lower in the sucrose formulation at the temperature of the stability studies (40°C) than found for the trehalose formulation, which was consistent with better stability in the sucrose formulation.
Conclusions
While apparent Td did not correlate with protein stability for rHSA, the extent of H/D exchange, X∞, did.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freeze-drying is the drying process of choice to improve the stability of parenterally administered proteins, which undergo significant physical and/or chemical degradation in solution during storage. In order to achieve optimal long-term stability, it is recommended that freeze-dried proteins be stored below the glass transition temperature (Tg) of the solid (i.e., an assembly of protein molecules or protein molecules dispersed in a matrix) (1,2). The Tg of such a molecularly miscible system marks the onset temperature of viscous flow on a long time scale around 10–10−4 s. However, there is an increasing body of work demonstrating that physical and/or chemical degradations still occur during storage at temperatures, T, well below the measured system Tg of the solid (2,3,4). Moreover, there is no sensible correlation between protein stability and Tg-T at temperatures well below Tg (5).
Hydrogen deuterium (H/D) exchange has been employed extensively to better understand protein structural features and conformational dynamics (6,7,8,9,10,11). The rate and extent of H/D exchange can provide measures of protein structure and/or dynamics in the protein formulation, which are relevant to stability. A sudden increase in hydrogens accessible for exchange in pure solids consisting of bovine serum albumin (BSA) (12), insulin or human growth hormone (hGH) (13) suggested a transition to greater mobility in the protein molecule at temperatures below the reported Tg values of these proteins, typically around 130°C. The temperature of the sudden increase in exchangeable hydrogens was tentatively attributed to the protein dynamical temperature, Td, in the dry solid state. The low temperature motion within the protein molecule was suggested to be associated with local fluctuations of protein residues on a shorter time scale (i.e., 10−15-10−1 s) (2). According to this hypothesis, at temperatures exceeding the protein dynamical temperature, there is an increase in local internal motions, which eventually results in larger cooperative motions, and therefore may affect stability of the protein formulations. Evidence of loss in protein function below Td has been reported (14,15,16). The Td was modulated through the degree of hydration and addition of lyoprotectant (17,18,19,20,21,22) in solutions, but very little work has been reported in dry proteins (19,20,23).
Hill and co-workers suggested that the hydrogen bonding interactions between water and the amino acid residues facilitates the propagation of fast β-like motions into larger scale α-like motions, which are responsible for protein internal dynamics (17). Studies on the kinetics of ligand binding to carbon monoxymyoglobin in a dry trehalose matrix demonstrated that the glassy trehalose suppressed the inter-conversion of protein conformation sub-states, suggesting that protein internal motion is limited or locked in the relatively more rigid matrix (24). Therefore, effective coupling between protein internal dynamics and the dynamics of the matrices may play an important role in the stability of protein pharmaceuticals.
Both sucrose and trehalose are known to stabilize protein formulations during freeze drying (5). The “water substitute hypothesis” describes stabilization by the disaccharides through hydrogen bond formation with the protein molecule, whereas the “glass dynamics hypothesis” explains stabilization through reduced mobility in the rigid matrix (5). The addition of sucrose to a protein formulation results in a decrease in Tg of the formulation compared to protein alone (3,25,26) which is contrary to its stabilizing effect based on the simple interpretation of the “glass dynamics hypothesis”, which suggests that the system with the higher Tg will be more stable. It is possible that rather than the system Tg, the protein dynamical temperature (Td) may be a more appropriate measurement to determine stability, which can be modulated by the excipients.
The present study aimed to test the hypothesis that Td can be used to predict stability, where Td was determined from the extent of solid-state H/D exchange measured by FTIR as previously described (12,13). The extent of H/D exchange itself was also evaluated as a stability predictor. The model system was recombinant human serum albumin (rHSA) using sucrose and trehalose as lyoprotectants.
Materials and Methods
Materials and Sample Preparation
Stock solution of rHSA (50 mg/mL, ultrapure #9803, Albumin Bioscience, Huntsville, AL) was dialyzed using a 10 K MWCO dialysis cassette against 5 mM potassium phosphate buffer (pH 7.0) at 4°C twice for 3 h and the third time overnight. The recovered solution was filtered through 0.22 μm polyvinylidene difluoride (PVDF) low protein binding filter. The concentration of rHSA after dialysis was determined by absorbance at 280 nm (Cary Bio100, Varian Inc., Palo Alto, CA) using extinction coefficient of 0.58 mL/mg-cm (27). Sucrose (ACS grade, Sigma Aldrich, St. Louis, MO) or trehalose (high purity, trehalose dihydrate, Ferro Pfanstiehl, Waukegen, IL) was added to the protein solution at 1:1 weight ratio of disaccharide to protein. The protein was formulated at 3 mg/mL (or 12 mg/mL) in rHSA alone, and 3 mg/mL in the formulations with the disaccharide lyoprotecatnat.
D2O (99.8% D), lithium chloride (LiCl) (>99%, American Chemical Society grade), and potassium bromide (KBr; 99%, infrared grade) from Acros Organics (Morris Plains, NJ) were used for the H/D exchange study.
Freeze-Drying Procedure
Aliquots of one mL of each protein solution were filled in 5 mL glass tubing vials and were partially stoppered (Daikyo Fluorotec, West Pharmaceutical Services, West Whiteland, PA). Vials containing the protein solutions were placed on the center of the freeze-dryer shelf surrounded by 5% (w/w) sucrose solutions to minimize radiation from the edge and front of the chamber and maintain comparable product temperatures. Product temperature was monitored from a thermocouple glued with heat sink silicone grease (Chemplex 1381, FUCHS lubricants, Harvey, IL) and Kapton tape (Cole-Parmer, Vernon Hills, IL) to the outside of selected vials near the vial bottom. The samples were freeze-dried in Lyostar 3 at a shelf temperature of −30°C and 60 mTorr until the Pirani gauge signal converged with the capacitance manometer; the shelf temperature was raised at 0.2°C/min to 40°C and 60 mTorr for 2 h to complete drying. Once stoppered, protein formulation in the vials was sealed and stored in the −20°C freezer for further analysis. All freeze-dried cakes showed no collapse or shrinkage with no differences in appearance.
Physical Stability of rHSA Formulations during Storage
Freeze-dried rHSA samples were equilibrated at 11% RH at 40°C overnight and stored at 40°C for 6 months. At 0, 1, 3, and 6 months, samples were reconstituted with 1 mL of water (18 MΩ, distilled and deionized, Barnstead™ GenPure™, Thermo Scientific, Waltham, MA). No precipitation or visible particle was observed after reconstitution. The entire volume of reconstituted solutions was filtered with 0.22 μm PVDF filters and 50 μl of each sample were injected onto a column (TSKgel™ G3000SWXL, 7.8 mm × 30 cm, 5 μm, Tosoh Bioscience, San Francisco, CA) maintained at 25°C. The mobile phase (100 mM sodium phosphate buffer with 0.2 M NaCl at pH 7.0) was delivered at 1.0 mL/min by an HPLC system (Model 1100, Agilent, Santa Clara, CA). The absorbance of the effluent was measured at 280 nm (variable wavelength detector, G1315A, Agilent). Absorbance data were acquired and analyzed (ChemStation, Agilent). Monomer, dimer, and higher order aggregates were identified. Percent soluble aggregate reported below represents the areas under the dimer and higher order aggregate peaks relative to total area of the identified peaks.
Protein aggregation followed square root of time kinetics (3). Therefore, the rate constant of aggregation was determined by fitting the equation below to the stability data.
%P, %P0, and k represent the percent soluble aggregate at storage time t, time 0 (immediately after freeze-drying), and the rate constant of aggregation (percentage aggregation/√months), respectively.
Secondary Structure of rHSA in the Lyophilized Formulations
Spectra of the protein samples were recorded with a FTIR spectrometer (Nicolet Magma 560, Thermo Scientific, Madison, WI) in single beam mode accumulating 128 scans at 4 cm−1 resolution over the range of 400–4000 cm−1. Samples were prepared as described in previous publication (13). Briefly, samples of 0.3–0.5 mg of freeze-dried rHSA were gently ground with 150 mg of dried potassium bromide (KBr) in a glove bag purged with dry air. The resulting mixture was compressed at 10,000 psi for 2 min using a laboratory press (Carver Inc., Wabash, IN). The compressed pellet was transferred to a sample holder in the FTIR spectrometer. The CO level and water vapor in the spectrometer chamber were controlled by a purging system with moisture and CO traps (Puregas, LLC, Broomfield, CO). All spectra were processed using the software (Grams/AI 8.0, Thermo Electron, Madison, WI). Secondary structure of rHSA formulations was characterized from the amide I band in the region 1600–1700 cm−1 of the FTIR spectra. This region primarily consists of C=O stretch mode, which is known to be sensitive to protein secondary structure (28,29). The second derivative of the amide I region resolved the underlying peak components in this region.
Protein H/D Exchange in the Lyophilized Formulations
Solid-state H/D exchange of the freeze-dried rHSA formulations followed a previously described procedure (12,13). Briefly, the freeze-dried samples were incubated in a desiccator maintained at 11% RH using saturated LiCl in D2O. The desiccators were sealed under vacuum and stored at various temperatures from 30 to 80°C. The vacuum was sufficient to seal the desiccators firmly without boiling of D2O containing LiCl. It should be noted that saturated solution of LiCl gives a relatively constant humidity of 11%RH over the range of 20–80°C (30). The process proceeded for 4 days, with samples being removed at 1, 2, 3 and 4 days. At each sampling time, the H/D exchange reaction of samples was quenched by drying in the vacuum oven at room temperature overnight.
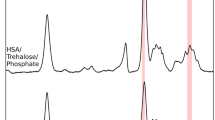
A portion (0.6 mg) of each quenched protein sample was mixed with 150 mg of KBr in a glove bag where humidity was controlled below 2% by purging dry air before analysis by FTIR. The extent of H/D exchange on the protein molecule was based on the intensity change of the amide II peak at 1535 cm−1, corresponding to an N-H stretch mode, as previously described (12,13). The absorption of sucrose and trehalose was primarily in the range of 1400 to 400 cm−1 (C-O stretch), and above 3200 cm−1 (O-H stretch); neither overlapped with the protein amide II peak. To further evaluate any interference of sucrose and trehalose with FTIR signal, the spectrum of freeze-dried amorphous sucrose was subtracted from the spectrum of rHSA:sucrose. The intensity of the amide I band was used for normalization. The analytical peak (amide II at 1535 cm−1) was unaffected by subtraction of the sucrose spectrum (Fig. 1). Similarly, the trehalose spectrum did not overlap with the amide II band (data not shown). Therefore, all analysis was performed using the spectral data without subtraction of the stabilizer spectra, following a previously established methodology (12,13).
FTIR absorption spectra of freeze-dried rHSA:sucrose formulation before (solid line) and after subtraction of the freeze-dried sucrose spectrum (dashed line). The intensity of amide I band (1655 cm−1) was normalized for comparison. The subtraction procedure did not affect the intensity of the amide II band at 1535 cm−1, which was further used to calculate the extent of H/D exchange.
Data Analysis of the H/D Exchange Process
The fraction of hydrogens remaining un-exchanged (denoted as X) at any time is the ratio of the intensity at 1535 cm−1 relative to its intensity before exchange, as previously described (13). The time course of the H/D exchange process was treated empirically as a first-order exponential decay. The extent of exchange at infinite time (1-X∞), was determined from the regression analysis using Eq. 5 of Mizuno and Pikal (12). Extrapolated to infinite time, the amide hydrogens remaining unexchanged (X∞) were not dynamically accessible by the D2O vapor on the time scale of the experiment (4 days), as they were presumably buried in the “rigid” interior of the protein-folded structure. For each formulation, a sigmoidal function was fitted to X∞ versus temperature to find the temperature midpoint of the transition (inflection point) and its associated error. As previously described (13), the transition temperature was interpreted as the Td, as discussed in the Results and Discussion Section.
Results and Discussion
The dynamical transition temperature where an abrupt change in mobility occurs, would affect stability due to any pathway that is dependent on mobility, including aggregation and chemical degradation. In previous reports, the extent of H/D exchange correlated well with aggregation and changes in acidity (presumably due to chemical degradation), but less with methionine oxidation (31). In the present study, only physical stability was assessed. Aggregate formation after freeze-drying is a well-known stability indicator for rHSA (26,32,33), and hence was used as the model protein to study the effect of protein dynamical transition temperature on physical stability in the solid state. The extent of H/D exchange, rather than the rate of H/D exchange, was monitored since the rate of H/D exchange could be complicated by the presence of the stabilizer. The time constant would likely represent a combination of mass transfer kinetics and H/D exchange kinetics. The stabilizer had the potential to alter the mass transfer of deuterium to the protein, potentially influencing the rate of H/D exchange, but not the final extent of H/D exchange. Freeze-dried rHSA samples with or without stabilizer were equilibrated with D2O of 11% RH over a range of temperature. Samples were taken for 4 consecutive days to determine the equilibrium extent of H/D exchange. The extent of exchange in freeze dried pure rHSA prepared using 3 mg/mL rHSA was highly variable. One possible source of such varibility was inter-vial variation in ice nucleation temperature that would lead to changes in protein structure as reported elsewhere (34). Such high variability was not evident when stabilizers were included in the formulation. To reduce the variation in extent of H/D exchange of freeze dried pure rHSA, a higher concentration of pure protein, (i.e., 25 mg/mL compared to 3 mg/mL in the presence of stabilizers) was freeze-dried such that all pure protein samples used for the H/D exchange were taken from the same vial that is well mixed to ensure uniformity and homogeneity.
The H/D exchange was monitored in the FTIR spectra as a function of temperature. Since H/D exchange can be a measure of both protein structure and dynamics, a greater extent of exchange is expected at a higher temperature when the interior of the protein molecule is exposed to the D2O vapor, and/or when there is greater fluctuation in protein molecular structure due to faster dynamics. At higher temperatures, the intensity of amide II band at 1535 cm−1 (N-H vibration) decreased, suggesting enhanced H/D exchange due to perturbation in protein structure or increase in mobility within the protein molecule (Fig. 2). As the N-H vibration decreased, the amide II band at 1445 cm−1 (N-D vibration) increased, but could not be used in the calculation of H/D exchange due to the contribution from H-O-D vibration mode at 1460 cm−1 (10).The extent of H/D exchange (X∞) shown in Fig. 3 provided a measure of structure or dynamics in the protein formulation.
Representative FTIR spectra of freeze-dried pure rHSA (left), freeze dried rHSA:sucrose (middle) and freeze dried rHSA:trehalose (right) recorded from 30 to 90°C after 4 days of the H/D exchange process. The amide I band was used to normalize the spectra. The decay in peak intensity of the amide II band (1535 cm−1) representing N-H vibrational modes was monitored to determine the extent of the H/D exchange reaction.
First order decay plot of the ratio of un-exchanged hydrogens (X) at various temperatures for freeze dried pure rHSA (left), freeze dried rHSA:sucrose (middle) and freeze dried rHSA:trehalose (right) equilibrated in D2O at 11% RH. The first order kinetics of the reaction was quantitatively analyzed by non-linear curve fitting using the following exponential model: (X = X∞ + (X0 − X∞) exp (−kt)), where t is time, k is an apparent rate constant for the observable exchange, X∞ and X0 are H/D un-exchanged fractions at the apparent plateau level (i.e., nominally infinite time) and t = 0 (X0 = 1.0), respectively.
The sucrose and trehalose formulations of rHSA showed no differences in key features of the native α-helix band such as FTIR peak position or intensity (Fig. 4). Similar observations were found in freeze-dried hGH:disaccharide 1:6 (w:w), where there was no difference in secondary structure by FTIR, although the aggregation rate constant at 40°C in the trehalose formulation was twice that in the sucrose formulation (13). In the present study, rHSA freeze-dried with no stabilizer was the least stable with an aggregation rate constant of 12.5% ∙ months−1/2. Both disaccharides stabilized rHSA reducing the rate constants to 3.6 and 1.2% ∙ months−1/2 for trehalose and sucrose, respectively (Fig. 5). Like the hGH study, sucrose was found to be the superior stabilizer, but this time by a factor of 3.
Second derivative of amide I region in the FTIR spectra of the three formulations after freeze-drying. This process allows the underlying components that overlap in the amide I region to be visually resolved. rHSA consists of predominantly α-helix structure with a peak at 1654 cm−1. The remaining structural elements are β-sheet and random turns. rHSA was formulated at 3 mg/mL in three formulations: freeze dried pure rHSA, freeze dried rHSA:sucrose 1:1 (w:w), and freeze dried rHSA:trehalose 1:1 (w:w).
As a first attempt to evaluate the correlation of Td with physical stability of the protein formulations, the samples were stored at 40°C to accelerate the aggregation within a reasonable experimental time frame. The extent of H/D exchange in the rHSA:sucrose formulation at 40°C was lower (p value = 0.002) than in rHSA:trehalose (Fig. 6), which agreed well with better stability in the sucrose formulation at a storage temperature of 40°C (Fig. 5). At and above 60°C, the trend was reversed with less H/D exchange in the trehalose formulation (p-values <0.05). Note that sucrose:rHSA (1:1) at 11% RH has a Tg of 58°C, but the corresponding trehalose formulation has a Tg of 70°C.
Better solid-state protein stability by sucrose compared to trehalose was also reported for IgG stored at 25 and 50°C, and for hGH stored at 40 and 50°C in sealed containers at low moisture (Table I). It was suggested that lower amplitude motions associated with “fast dynamics” in the hGH:sucrose formulation (on a nanosecond scale) as measured by neutron scattering correlated with the better stability in sucrose compared to trehalose (3). Micro-phase separation of trehalose formulations was another proposed mechanism of the lower stability in trehalose than in sucrose (17). In contrast, trehalose formulations provided superior stability of hGH at the same storage temperature, but with higher %RH, and for mAb at higher temperatures, 60°C. More specifically, the stabilization by sucrose relative to trehalose decreased with %RH and temperature in these two studies (3,31). This is consistent with the H/D exchange results that suggest greater mobility in the trehalose formulation (relative to sucrose) at lower temperatures, but lower mobility than the sucrose formulations at higher temperatures. Though stability was only studied at 40°C in current work, in future work, stability at 30°C and 50°C should be explored to further understand the interplay between mobility change as a function of temperature and physical stability.
Furthermore, the extent of H/D exchange seems to have sufficient sensitivity to correlate with modest changes in stability. Recently, Moorthy and colleagues demonstrated strong correlation between the deuterium incorporation at immediately after freeze-drying using H/D exchange-mass spectrometry (HDX-MS) and the extent of aggregation during storage of myoglobin (35) and monoclonal antibody (31) formulated with excipients with stabilization propensities very distinct from each other (i.e. sucrose, mannitol, NaCl). The present results are consistent with theirs, but with formulations expected to produce much smaller stability differences.
In previous studies, low-temperature endothermic events (40 ~ 60°C) well below the Tg of the protein were observed by differential scanning calorimetry (DSC) in solid pure BSA, insulin and hGH without excipients (12,13,36). It was proposed that these endotherms represented enthalpy recovery of a dynamical transition within the protein molecule. The temperature of the enthalpy recovery correlated well with a transition temperature found using H/D exchange monitored by FTIR, supporting the suggestion that the low temperature endotherms were associated with an abrupt change in mobility associated with a protein dynamical transition temperature, Td.
Based on extensive literature on the protein dynamical transition in hydrated systems (2), Hill, Shalaev and Zografi hypothesized that modulation of Td by lyoprotectants could alter protein molecule stability, providing better long-term stability of the formulation. Lyoprotectants are believed to form a hydrogen-bonding network with proteins which affects mobility and stiffness of the whole formulation. However, no experimental studies have tested this hypothesis in the solid state. Pre-Tg events were previously reported in hGH:disaccharide formulations (36); however, the events were attributed to the disaccharide itself, which has a pre-Tg signal in the same temperature range, due to a distribution of relaxation times within the non-equilibrium amorphous solid system. Therefore, a pre-Tg endothermic event in the protein molecule could not be uniquely identified from a DSC scan, at least when the protein formulation contained sucrose or trehalose. However, any change in the protein mobility/structure with temperature, which can be monitored by the extent of H/D exchange at the amide nitrogen, would be not complicated by the presence of a stabilizer such as sucrose or trehalose, at least in theory. To explore the impact of this transition associated with the protein dynamical temperature on protein stability in solid state, the H/D exchange data were analyzed for a transition temperature, which would presumably be the Td.
As in previous studies (12,13), a sigmoidal function was fitted to the temperature dependence of X∞. The mid-point of the transition (Tmid), nominally equivalent to the dynamical transition temperature (Td) of the protein molecule, was obtained from the fitted sigmoidal function (Fig. 7). According to the hypothesis of Hill, Shalaev and Zografi, the formulation that yields a higher Td of the protein would be effectively coupled to protein stability. The transition region for X∞ with temperature for pure rHSA was broad (compared to the previously reported transition in insulin and hGH (13) with a midpoint, or nominal Td of 56°C (±6.3°C) (Fig. 7). The transition temperature in rHSA:sucrose was 62.3°C (±3.3°C), which is not statistically different from that of pure unstabilized rHSA. In contrast, the X∞ versus temperature for rHSA:trehalose was not sigmoid in shape within the temperature range from 30 to 100°C. Perhaps the transition temperature of rHSA:trehalose is above this temperature range, beyond what was experimentally accessible due to the use of the 11% RH chamber. However, if the protein in trehalose formulation does have an inflection point associated with a Td or other relevant transition, it is clearly higher than that of protein alone or with sucrose.
Temperature dependence of the plateau level of H/D exchange (X∞) in pure rHSA (left), rHSA:sucrose (middle) and rHSA:trehalose (right), fitted to a sigmoidal function: X∞ = (X∞i − X∞f)/(1 + exp ((T − Tmid)/dT) + X∞f, where X∞i, X∞f, Tmid, dT are X∞ initial value, X∞ final value, temperature midpoint of the sigmoidal transition, and ¼ width of the transition, respectively. The mid-point of the sharp transition (Tmid) in pure rHSA was 56.2 ± 6.3°C. The mid-point of the transition (Tmid) in the rHSA:sucrose formulation was, 62.3 ± 3.3°C. No sigmoidal transition was found in the rHSA:trehalose formulation within the temperature range 30-100°C.
If the hypothesis that protein dynamical temperature (Td) can be used to predict protein stability were true, the trehalose formulation would be expected to be more stable than the sucrose formulation owing to the presumably higher Td of the protein in the trehalose formulation. However, clearly the rHSA:sucrose formulation was more stable at 40°C and 11%RH than the trehalose formulation with a degradation rate constant only 1/3 that of the trehalose formulation (Fig. 5). Thus, the stability data in the present study is not consistent with the hypothesis that trehalose should provide better stability due to a higher Td. Rather, the extent of H/D exchange over a short time period (i.e., 4 days in this case) may be a better predictor of long-term stability at the same temperature and humidity conditions.
Summary and Conclusions
An apparent protein dynamical temperature (Td) (2,17) in solid state proteins below the system glass transition temperature has been previously reported (12,13), corresponding to a cooperative transition in mobility within the protein molecule. According to the hypothesis of Hill, Shalaev, and Zografi, a higher Td in proteins lyophilized with a stabilizer was expected to correspond to a more stable protein formulation. However, rHSA:sucrose showed superior long-term stability at 40°C against aggregation than the trehalose formulation while exhibiting a lower apparent Td, which does not support the hypothesis.
Both sucrose and trehalose showed equivalent protection of protein secondary structure by FTIR, but often show differences in stabilization on long-term storage. The extent of H/D exchange (X∞) was lower for protein lyophilized in a sucrose matrix than in a trehalose matrix at 40°C and 11%RH, which corresponded to better stability in the sucrose formulation at least at these conditions. From literature reports, sucrose appears to be a better stabilizer at lower temperatures and humidity, while trehalose is superior at higher temperatures and humidity. The extent of H/D exchange extrapolated to infinite time, X∞, agrees with this general trend. Thus, X∞, rather than Td, may be a good short-term measurement predictive of long-term stability, indicating the rigidity of the structure and/or dynamics of the protein formulation.
Change history
23 February 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11095-021-03016-w
References
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48(1):27–42.
Hill JJ, Shalaev EY, Zografi G. The importance of individual protein molecule dynamics in developing and assessing solid state protein preparations. Journal of pharmaceutical sciences. 2014;103(9):2605–14.
Pikal MJ, Rigsbee D, Roy ML, Galreath D, Kovach KJ, Wang B, et al. Solid state chemistry of proteins: II. The correlation of storage stability of freeze-dried human growth hormone (hGH) with structure and dynamics in the glassy solid. J Pharm Sci. 2008;97(12):5106–21.
Wang B, Tchessalov S, Cicerone MT, Warne NW, Pikal MJ. Impact of sucrose level on storage stability of proteins in freeze-dried solids: II. Correlation of aggregation rate with protein structure and molecular mobility. J Pharm Sci. 2009;98(9):3145–66.
Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. J Pharm Sci. 2009;98(9):2886–908.
Hvidt A, Nielsen SO. Hydrogen exchange in proteins. Adv Protein Chem. 1966;21:287–386.
Englander SW, Kallenbach N. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1984;16:521–655.
Mayo SL, Baldwin RL. Guanidinium chloride induction of partial unfolding in amide proton exchange in RNase A. Science. 1993;262(5135):873–6.
Qian H, Mayo SL, Morton A. Protein hydrogen exchange in denaturant: quantitative analysis by a two-process model. Biochemistry. 1994;33(27):8167–71.
Dong A, Hyslop RM, Pringle DL. Differences in conformational dynamics of ribonucleases A and S as observed by infrared spectroscopy and hydrogen-deuterium exchange. Arch Biochem Biophys. 1996;333(1):275–81.
Sinha S, Li Y, Williams TD, Topp E. Protein conformation in amorphous solids by FTIR and by hydrogen/deuterium exchange with mass spectrometry. Biophys J. 2008;95(12):5951–61.
Mizuno M, Pikal MJ. Is the pre-Tg DSC endotherm observed with solid state proteins associated with the protein internal dynamics? Investigation of bovine serum albumin by solid state hydrogen/deuterium exchange. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2013;85(2):170–6.
Fang R, Grobelny PJ, Bogner RH, Pikal MJ. Protein internal dynamics associated with pre-system glass transition temperature endothermic events: investigation of insulin and human growth hormone by solid state hydrogen/deuterium exchange. J Pharm Sci. 2016;105(11):3290–5.
Parak F, Frolov EN, Kononenko AA, Mossbauer RL, Goldanskii VI, Rubin AB. Evidence for a correlation between the photoinduced electron transfer and dynamic properties of the chromatophore membranes from Rhodospirillum rubrum. FEBS Lett. 1980;117(1):368–72.
Ferrand M, Dianoux AJ, Petry W, Zaccai G. Thermal motions and function of bacteriorhodopsin in purple membranes: effects of temperature and hydration studied by neutron scattering. Proc Natl Acad Sci U S A. 1993;90(20):9668–72.
Rasmussen BF, Stock AM, Ringe D, Petsko GA. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature. 1992;357(6377):423–4.
Hill JJ, Shalaev EY, Zografi G. Thermodynamic and dynamic factors involved in the stability of native protein structure in amorphous solids in relation to levels of hydration. J Pharm Sci. 2005;94(8):1636–67.
Perez J, Zanotti JM, Durand D. Evolution of the internal dynamics of two globular proteins from dry powder to solution. Biophys J. 1999;77(1):454–69.
Cornicchi E, Marconi M, Onori G, Paciaroni A. Controlling the protein dynamical transition with sugar-based bioprotectant matrices: a neutron scattering study. Biophys J. 2006;91(1):289–97.
Zanotti JM, Bellissent-Funel MC, Parello J. Hydration-coupled dynamics in proteins studied by neutron scattering and NMR: the case of the typical EF-hand calcium-binding parvalbumin. Biophys J. 1999;76(5):2390–411.
Tsai AM, Neumann DA, Bell LN. Molecular dynamics of solid-state lysozyme as affected by glycerol and water: a neutron scattering study. Biophys J. 2000;79:2728–32.
Librizzi F, Viappiani C, Abbruzzetti S, Cordone L. Residual water modulates the dynamics of the protein and of the external matrix in “trehalose coated ” MbCO: an infrared and flash-photolysis study. J Chem Phys. 2002;116(3):1193–200.
Doster W, Cusack S, Petry W. Dynamic instability of liquidlike motions in a globular protein observed by inelastic neutron scattering. Phys Rev Lett. 1990;65(8):1080–3.
Hagen SJ, Hofrichter J, Eaton WA. Protein reaction kinetics in a room-temperature glass. Science. 1995;269:959–62.
Liao YH, Brown MB, Nazir T, Quader A, Martin GP. Effects of sucrose and trehalose on the preservation of the native structure of spray-dried lysozyme. Pharm Res. 2002;19(12):1847–53.
Chang L, Shepherd D, Sun J, Ouellette D, Grant KL, Tang XC, et al. Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J Pharm Sci. 2005;94(7):1427–44.
Bos OJ, Remijn JP, Fischer MJ, Wilting J, Janssen LH. Location and characterization of the warfarin binding site of human serum albumin: a comparative study of two large fragments. Biochem Pharmacol. 1988;37(20):3905–9.
Dong A, Prestrelski SJ, Allison SD, Carpenter JF. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995;84(4):415–24.
Li Y, Williams TD, Schowen RL, Topp EM. Characterizing protein structure in amorphous solids using hydrogen/deuterium exchange with mass spectrometry. Anal Biochem. 2007;366(1):18–28.
Greenspan L. Humidity fixed points of binary saturated aqueous solutions. J Res Bur Stand. 1977;81A:89–96.
Moorthy BS, Zarraga IE, Kumar L, Walters BT, Goldbach P, Topp EM, et al. Solid-state hydrogen–deuterium exchange mass spectrometry: correlation of deuterium uptake and long-term stability of lyophilized monoclonal antibody formulations. Mol Pharm 2017.
Forney-Stevens KM, Bogner RH, Pikal MJ. Addition of amino acids to further stabilize lyophilized sucrose-based protein formulations: I. screening of 15 amino acids in two model proteins. J Pharm Sci. 2016;105(2):697–704.
Fang R, Bogner RH, Nail SL, Pikal MJ. Stability of freeze-dried protein formulations: contributions of ice nucleation temperature and residence time in the freeze-concentrate. J Pharm Sci. 2020;109:1896–904.
Fang R, Tanaka K, Mudhivarthi V, Bogner RH, Pikal MJ. Effect of controlled ice nucleation on stability of lactate dehydrogenase during freeze-drying. J Pharm Sci. 2018;107(3):824–30.
Moorthy BS, Schultz SG, Kim SG, Topp EM. Predicting protein aggregation during storage in lyophilized solids using solid state amide hydrogen/deuterium exchange with mass spectrometric analysis (ssHDX-MS). Mol Pharm. 2014;11(6):1869–79.
Pikal MJ, Rigsbee DR, Roy ML. Solid state chemistry of proteins: I. glass transition behavior in freeze dried disaccharide formulations of human growth hormone (hGH). J Pharm Sci. 2007;96(10):2765–76.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to add the following affiliation as a second affiliation to Dr. Wasfy Obeidat. The second affiliation is Department of Pharmaceutical Technology, Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan.
In Loving Memory of Professor Michael J. Pikal
Rights and permissions
About this article
Cite this article
Fang, R., Obeidat, W., Pikal, M.J. et al. Evaluation of Predictors of Protein Relative Stability Obtained by Solid-State Hydrogen/Deuterium Exchange Monitored by FTIR. Pharm Res 37, 168 (2020). https://doi.org/10.1007/s11095-020-02897-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02897-7