ABSTRACT
Purpose
Liposomal ciprofloxacin nanoparticles were developed to overcome the rapid clearance of antibiotics from the lungs. The formulation was evaluated for its release profile using an air interface Calu-3 cell model and further characterised for aerosol performance and antimicrobial activity.
Methods
Liposomal and free ciprofloxacin formulations were nebulised directly onto Calu-3 bronchial epithelial cells placed in an in vitro twin-stage impinger (TSI) to assess the kinetics of release. The aerosol performance of both the liposomal and free ciprofloxacin formulation was characterised using the next generation impactor. Minimum inhibitory and bactericidal concentrations (MICs and MBCs) were determined and compared between formulations to evaluate the antibacterial activity.
Results
The liposomal formulation successfully controlled the release of ciprofloxacin in the cell model and showed enhanced antibacterial activity against Pseudomonas aeruginosa. In addition, the formulation displayed a respirable aerosol fraction of 70.5 ± 2.03% of the emitted dose.
Conclusion
Results indicate that the in vitro TSI air interface Calu-3 model is capable of evaluating the fate of nebulised liposomal nanoparticle formulations and support the potential for inhaled liposomal ciprofloxacin to provide a promising treatment for respiratory infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Respiratory tract infections (RTIs) cause significant morbidity and mortality in patients with chronic obstructive lung diseases such as cystic fibrosis (CF) and non-CF bronchiectasis (BE). Both diseases have similar characteristics in terms of accumulation of mucus in the lungs, causing recurrent pulmonary infection and inflammation (1,2). The cycle of infection and inflammation periodically develops into exacerbations where chronic colonisation of the airway by bacteria, especially Pseudomonas aeruginosa (P. aeruginosa) causes a more severe decline in lung function, increased hospitalization and high mortality rates (3,4).
CF patients generally receive extensive antibiotic treatments to maintain lung function and prevent exacerbations. Inhalation antibiotics have become a standard part of treatment for CF patients with P. aeruginosa lung infections as it directly targets the lung minimising systemic toxicity and reducing emergence of drug resistance. Current inhalation treatments for CF, approved by the Food and Drug Administration, are limited to tobramycin (TOBI®) and more recently aztreonam (Cayston®). Conversely, standardised antibiotic treatment for non-CF bronchiectasis are yet to be approved (5) as tolerability is an issue when patients require long-term treatment (6). Another major challenge faced by localised treatment of respiratory tract infections (RTIs) is the rapid absorption and clearance of antibiotics from the lungs. Multiple dosing each day and lengthy treatment time of those administrations is therefore required to maintain sufficiently high levels of antibiotics for antimicrobial efficacy.
To reduce the burden of treatment, a controlled release antibiotic formulation would be beneficial as it increases the residence time through gradual and predictable release of antibiotics in the lungs. Hence, liposomal ciprofloxacin for inhalation (CFI) was formulated with lipids and cholesterol designed to be delivered locally, targeting the site of infection directly. Currently, there is no regulatory approved aerosolised form of ciprofloxacin for human use that could directly target the area of primary lung infection. Ciprofloxacin is a suitable candidate as it is an established broad-spectrum fluoroquinolone antibiotic indicated for exacerbation treatment of RTI including P. aeruginosa in its oral and parenteral dosage forms. In comparison to the current ciprofloxacin formulation, aerosol liposomal ciprofloxacin formulations have been shown to improve pharmacokinetics and biodistribution, by providing higher drug concentration and sustained release at site of infection, decrease systemic toxicity and enhance activity against extracellular pathogens, in particular overcoming bacterial drug resistance (7–9).
Previous studies with aerosolised delivery of liposomal ciprofloxacin have been performed to evaluate the kinetic profiles and efficacy of liposomal ciprofloxacin inhalation on human and animal subjects (10–12). Although in vivo analyses are the preferred methods to evaluate drug deposition, distribution and absorption after pulmonary administration, in vitro cell models are appropriate systems to study absorption and metabolism, localization and retention time of drugs and carrier systems at the airway epithelium at a cellular level (13). In addition, cell culture models of the pulmonary epithelium could potentially be used as an alternative or complementary method to expensive and time consuming in vivo studies, especially to screen the potential of various preclinical formulations (14).
Recognising that there is a need for the development of an in vitro model that could potentially be used to reflect the transport characteristics in vivo, our previous study established an air-interface Calu-3 cell model coupled with a modified twin stage impinger for release profiling of controlled release dry powder respiratory formulations (15). That study found that the release kinetic profiles of controlled release ciprofloxacin formulation using polyvinyl alcohol as its release controller showed a higher transport rate compared to ciprofloxacin alone in the cell model. However, contrary results were observed using the diffusion cell suggesting that the cell model provides a more physiologically relevant model. The Calu-3 cell model is a sub-bronchial epithelial cell line that has similar in vivo characteristics in terms of morphology, electrical resistance and mucus production (16) to native pulmonary epithelium. Additionally, it has been well established and characterised model for drug delivery and toxicology (17–20). In the present study, this model is further evaluated for its release profiling capacity in terms of nebulised liposomal nanoparticle formulations. The formulation is nebulised directly onto the Calu-3 cells placed within an in vitro twin stage impinge (TSI). This experimental design may help clarify the interactions between the drug and its liposomal carrier system on the epithelial cells as it produces a more representative exposure than the alternative of direct application of the formulation onto the surface of the cells. Additionally, the use of the TSI helps simulate the impaction of respirable droplets onto the mucosa surfaces of the epithelial cells and by extension, similar proportion of the aerosols is assumed to reach the patient’s lung and have specific effects and activity retained.
Hence, the aim of the current study is to investigate the newly developed nebulised controlled release ciprofloxacin nanoparticle formulation, liposomal ciprofloxacin (Ciprofloxacin for Inhalation, CFI) and compare it to the free ciprofloxacin (FCI) formulation, in terms of release profiles, antimicrobial efficacy and aerosol performance using in vitro methodologies. The highlight of this work is the applicability of the air interface Calu-3 cell model together with a twin stage impinger (TSI) to simulate the deposition of aerosolised liposomal ciprofloxacin to the trachea bronchiolar regions of the lungs. Subsequently, this method was used to study the interaction between the CFI formulation and the epithelial cells with reference to drug localization, absorption and retention of drug within the model system. Furthermore, to fully characterise the physical properties of the CFI formulation, cryogenic transmission electron microscopy and dynamic light scattering measurements were performed, whereas the nebulised aerosol generated was analysed using laser diffraction and cascade impaction for its geometric diameter and aerosol performance, respectively.
MATERIALS AND METHODS
Materials
Non-essential amino acids solution, trypan blue solution (0.4% w/v) and CelLytic™ M Cell Lysis, protease inhibitor cocktail, were purchased from Sigma-Aldrich (Sydney, Australia). Other cell culture reagents including trypsin-EDTA solution (2.5 g/L trypsin, 0.5 g/L EDTA), Dulbecco’s Modified Eagle’s Medium (DMEM, without phenol red and L-glutamine, including sodium bicarbonate and 15 mM HEPES), Phosphate buffered saline (PBS), L-glutamine solution (200 mM), fetal bovine serum (FBS), Hank’s balanced salt solution (HBSS) were obtained from Invitrogen (Sydney, Australia). Transwell cell culture inserts (0.33 cm2 polyester, 0.4 μm pore size) were purchased from Corning Costar (Lowell, MA, USA) and all other sterile culture plastic wares were from Sarstedt (Adelaide, Australia). All solvents used were of analytical grade and were supplied by Biolab (Victoria, Australia).
Preparation of Liposomal and Solution Ciprofloxacin
Ciprofloxacin for Inhalation (CFI), 50 mg/mL, pH 6.0, is an aqueous liposome formulation comprised of lipids (hydrogenated soy phosphatidyl-choline, HSPC) (70.6 mg/mL) and cholesterol (29.4 mg/mL). CFI was prepared by an active loading method via a transmembrane pH gradient, generated in response to an ammonium sulphate gradient (11). Active loading is a simple and efficient process that has been used successfully for clinical development of several liposome-based drug delivery systems and the process is detailed in literature (21). In simple terms, the un-ionised form of ciprofloxacin is membrane permeable, and thus crosses into the aqueous lumen of the liposome. Upon entering the liposome, the ciprofloxacin gains a proton and becomes charged, therefore remaining trapped within the liposome, as it cannot diffuse back across the bilayer in the charged form. CFI is a unilamellar preparation by design, and of small enough size to allow for sterile filtration during production (22). Free Ciprofloxacin for Inhalation (FCI), 20 mg/mL, is an acetate-buffered aqueous formulation at pH 3.3. This pH was chosen because ciprofloxacin is poorly soluble at neutral pH (21). In order to minimize the nebulization volume, and thus the administration time, the ciprofloxacin concentrations in both the CFI and FCI formulations were set to the highest level that provided acceptable long-term stability (data not shown).
Transmission Electron Microscopy of the Liposomal Formulation
Cryogenic transmission electron microscopy (cryo-TEM) was performed using a JEOL 2100 instrument (Tokyo, Japan), operating at 200 kV. CFI samples were prepared in a climate chamber in which the temperature and humidity was controlled at 25°C and 100% RH, respectively. Three μL of sample solution was directly placed on glow-discharge Quantifoil carbon grids (Jena, Germany). Grids were blotted once with filter paper, at a blotting angle of 2 mm for 2 s. Using an automated vitrobot F.E.I. (Eindhoven, Netherlands), samples were subsequently vitrified by plunging into liquid ethane. Vitrified samples were kept in liquid nitrogen for protection against atmospheric conditions during both transfer and examination.
Dynamic Light Scattering Measurement of the Liposomal Formulation
Particle size distribution of CFI was measured using dynamic light scattering Malvern Zetasizer Nano ZS (Malvern, UK). The measurements were performed with a refractive index of 1.42 and absorption value of 0.1. Samples were measured in a quartz cuvette at 25°C and were analysed in triplicate.
Ciprofloxacin Analysis
Quantification of both ciprofloxacin formulations were performed using high-performance liquid chromatography (HPLC). CFI samples were first diluted with methanol to a concentration of 80:20 (% v/v) methanol:water to rupture the liposomes when required. Subsequently, the samples were centrifuged at 10,000 g for 5 min to allow sedimentation of the lipids. The supernatant was collected and further diluted 10 X with deionised water prior to HPLC assay.
The HPLC system Shimadzu Prominence UFLC system, Shimadzu Corporation (Kyoto, Japan) consisted of a SPD-20A UV–vis detector, LC-20AT liquid chromatography, RID 10A refractive index detector, SIL-20A HT Autosampler and an Optimal ODS-H column (3 μm, 150 X 4.6 mm) Capital HPLC Limited, (Broxburn, West Lothian, Scotland). The mobile phase was a mixture of methanol and 0.1 M sodium dihydrogen phosphate at a 30:70 (v/v) ratio, with pH adjusted to 3.35 with phosphoric acid. The flow rate was set to 0.8 mL/min and 100 μL of each sample was injected into the column. The UV detector was set to 275 nm. Linearity of ciprofloxacin was obtained between 0.01 and 20 μg/mL (R2 = 0.99) with a retention time of 15.38 min.
Characterisation of Nebulised Formulations
Nebulisation of the ciprofloxacin formulations were performed using the PARI LC Sprint® jet nebuliser powered by the Pari Turbo Boy S compressor (Starnberg, Germany). These are the same nebulizer systems that were used to deliver CFI and mixtures of CFI and FCI in vivo to subjects with CF and non-CF bronchiectasis (12,22). The reservoir of the nebuliser was filled with 2 mL of the respective formulations prior to nebulisation.
Laser Diffraction
The aerosol size distribution of nebulised formulation was determined by laser diffractometry using a Spraytec particle sizer, Malvern Instrument Ltd (Malvern, UK). Briefly, an open bench configuration was employed to reduce the distance between the nebuliser’s mouthpiece and laser measurement. The short distance between the mouthpiece of the nebuliser and the measuring zone implies that droplet evaporation is negligible and hence ambient conditions can be used. A 10 mm gap was maintained between the mouthpiece and measurement zone to comply with standards set by the European Committee for Standardisation of particle sizing with laser diffraction (23). Prevention of aerosol re-entry into measurement zones was ensured using air extraction by a vacuum pump. Measurements were made at room temperature (25°C) and an approximate relative humidity of 30%. Aerosol size measurements were initiated 5 s prior to nebulisation and ceased 10 s after no aerosol was detectable. The samples were analysed in triplicates. As for data analysis, detectors 1-6 were excluded to account for beam steering and an algorithm to correct multiple scatter within the Malvern software was activated. Light transmission was maintained above 70% for all measurements ensuring the absence of multiple scattering and vignetting (24). Median droplet size and geometric standard deviation (GSD) were determined by averaging all data points excluding the first and last 10 s of nebulisations.
Cascade Impaction
Further characterisation of nebulised aerosols for aerosol performance and correlation with laser diffractometry was performed using the next generation impactor (NGI). Cascade impaction was calibrated to a flow rate of 15 L/min in accordance to the standards of British Pharmacopeia (Apparatus E) to mimic the tidal breathing of an adult (25). To avoid post-aerosolisation evaporation, which could lead to undersizing of nebulised aerosols, the nebuliser and NGI were placed within a polycarbonate box with controlled temperature (20 ± 1°C) and relative humidity (>95%) (26). Activation of the sampling airflow for 2 min prior to nebuliser attachment allowed for environmental equilibration between the NGI and the surrounding experimental housing. The nebuliser was fitted to a rubber adapter to obtain a sealed attachment to the United States Pharmacopoeia (USP) throat that was connected to the NGI. Collection of aqueous aerosol does not require impactor plates to be coated. Samples were nebulised at a flow rate of 15 L/min for approximately 1 min.
For the mass recovery assay, each NGI stage cup and USP throat were oven dried at 70°C to evaporate the aqueous phase before cooling to ambient temperature. The components were then individually rinsed with either 5 mL of deionised water for the FCI formulation or 10 mL of 80:20 (% v/v) methanol:water for the CFI formulation. Quantification of the ciprofloxacin in these samples was achieved using the HPLC method described previously. Each formulation was tested in triplicate.
Deposition and Evaluation of Drug Transport Using an Air Interface Pulmonary Epithelial Model
Calu-3 cells were grown at the air-interface as described previously (15,19) to allow cell monolayer differentiation. Experiments were performed between day 11 and 14 from passages 35-42. Deposition of aerosolised formulation onto the Calu-3 cells was performed using a modified glass twin stage impinger (TSI; British Pharmacopoeia Apparatus A) Copley Scientific (Nottingham, UK). The set-up of the apparatus is described in detailed elsewhere (15,18). Briefly, the TSI was assembled according to the British Pharmacopoeia, aside from the absence of solution in the second lower chamber where a Transwell insert was firmly affixed to the connecting tube. A custom-made mouthpiece adapter connecting the PARI LC Sprint® nebulizer, powered by the PARI TurboBoy®S compressor, was fitted to the entrance of the TSI. Prior to the transport study, the CFI and FCI formulations were diluted to a final concentration of 2.5 mg/mL with HBSS to allow <20 μg of ciprofloxacin to be deposited onto the Calu-3 cells when formulations were nebulised. This was calculated before hand and confirmed from the subsequent drug transport study across the cells. Two mL of the diluted CFI or FCI was introduced into the reservoir of the nebulizer. The vacuum pump was switched on for 5 s at a flow rate of 15 L/min to allow for equilibration before samples were nebulised for an additional 5 s.
The Transwell insert was immediately removed after deposition and the outer surface wiped dry to remove any aerosol droplets adhering to the outer surface. Subsequently, the Transwell insert was placed into a well of a 24-well plate containing 600 μL of pre-warmed HBSS (receiver fluid). Samples were taken at pre-determined time points (up to 4 h) while maintaining sink conditions by transferring the Transwell to a new well with fresh HBSS. At the final time point, the cells were gently washed with HBSS buffer to collect the remaining drugs on the cell surface. Finally, to quantify intracellular drug, cells were trypsinised and subsequently lysed on ice in CellLyticTM M Cell Lysis reagent with 1% (v/v) protease inhibitor cocktail according to manufacturer’s recommendation. Lysates were centrifuged at 10,000 g for 10 min at 4°C and the supernatant was aspirated and diluted to an appropriate volume. Samples were analysed by HPLC. The estimated quantity of ciprofloxacin deposited on the cell is the sum of the drug that has been transported across the epithelial cells and the drug recovered from the surface of the cell and within the cells after the 4 h period.
Transepithelial electrical resistance (TEER) measurements were performed as previously described (15,16) to assess the integrity of the Calu-3 monolayers using an epithelial voltohmmeter (EVOM) World Precision Instruments (Florida, USA). TEER measurements were taken after the deposition experiments were complete and were compared to untreated cells.
Confocal Microscopy Live-Cell Imaging
Ciprofloxacin formulations were nebulised onto the air-interface Calu-3 cells using the modified TSI as described above. Immediately after deposition at the pre-determined time points, image acquisition was performed on the Transwell inserts in the 24-well plate using a confocal microscope LSM 510 Microscope Meta, Carl Zeiss (Goettingen, Germany) with 10X objective (15). The UV laser (405 nm) was used for excitation of ciprofloxacin and settings (aperture, gain and laser power) were maintained constant throughout the experiment. Image analyses were performed using Image J software.
In Vitro Antimicrobial Activity
Minimum inhibitory concentration (MIC) was determined using a standard twofold serial dilution method with cation-adjusted Mueller-Hinton broth (CAMHB) according to NCCLS guidelines (27). The drug concentration used ranged from 32 to 0.153 μg/mL. Cultures of P. aeruginosa (NCTC7244) and Staphylococcus aureus (Oxford NCTC6571) were selected from the microorganism culture bank and maintained on Trypticase soy agar. The final inoculum was prepared using bacteria from the stationary growth phase and diluted with CAMHB to obtain a viable count of approximately 5 X 106 CFU/mL. The final inoculum size was verified by counting visible colonies by spread plate technique. The lowest antibiotic concentration that after incubation for 18-24 h at 37°C inhibited visible growth, was defined as the MIC.
The minimum bactericidal concentration (MBC) was determined by sampling 100 μL from each MIC broth dilution that lacked visible growth, and inoculating on blood agar and incubating at 37°C for 24 h or 48 h for P. aeruginosa and S. aureus respectively. The bactericidal endpoint for MBC determination was defined as the drug concentration that demonstrated a reduction of ≥ 99.9% of the initial inoculum.
Statistical Analysis
All results are expressed as mean ± standard deviation of at least three separate determinants. One-way ANOVA or unpaired 2-tailed t-tests were performed to determine significance (which was quoted at the level of p < 0.05) between treatment groups and control.
RESULTS AND DISCUSSION
There are no approved or marketed products for inhalation containing liposomally encapsulated drugs or biologics, but much progress has been made recently with respect to the preclinical and clinical development of inhaled liposome formulations of antibiotics (28,29). In this study, two nebulized ciprofloxacin formulations intended for pulmonary delivery, liposomal ciprofloxacin (CFI) and free ciprofloxacin (FCI) were assessed in terms of their physical properties, aerosol performance, antimicrobial efficacy and release profiles using in vitro methodologies. The in vitro data supports the concept that inhaled liposomal ciprofloxacin may become an important new approach for the treatment of bacterial infections in CF and non-CF BE.
Transmission Electron Microscopy of the Liposomal Formulation
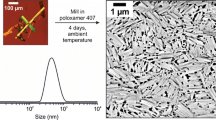
The cryo-transmission electron micrograph for the CFI formulation is shown in Fig. 1. It is observed that the liposomes are unilamellar, comprised of a lipid bilayer. The liposomes also display a spherical geometry with a diameter within the nano range of approximately 30-80 nm, which is in relatively good agreement with, if somewhat smaller than, the size distribution data determined by light scattering previously (22).
Dynamic Light Scattering Measurement of the Liposomal Formulation
The size distribution of CFI as measured by dynamic light scattering is shown in Fig. 2. The liposomes display a mean diameter of 89.27 ± 0.21 nm and polydispersity index of < 0.1 indicating that the liposomes are monodisperse with a narrow size distribution. This data is consistent with the light scattering size distribution reported previously (22) but somewhat larger than the sizes observed under cryo-TEM. This difference may be attributed to dynamic light scattering being an indirect method of size measurement; it measures the hydrodynamic diameter based on the calculation of mean size from a large sample population rather than an actual physical diameter. The technique of dynamic light scattering not only measures the size of the particle core but also includes surface structure concentration and ions in the medium leading to a bias towards a larger reported size than for cryo-TEM (30). Additional parameters such as sample temperature, concentration and particle sedimentation may also influence the reported measurement (30).
Evaluation of Nebulised Formulations Using Laser Diffraction and Cascade Impaction
Laser diffraction is an established alternative to cascade impaction for size characterisation of aqueous aerosols as nebulised droplets can be approximated by unit density spheres. Hence, high levels of correlation have been found between geometric and aerodynamic diameter of droplets from laser diffraction and cascade impaction that has been corrected for evaporation (31,32). However, laser diffraction has been subjected to varying data interpretation as manufacturers utilise different algorithms and orientation of detectors, leading to differences in results. In addition, laser diffraction suffers from beam stearing, multiple scatter and vignetting (26). Hence, cascade impaction was performed to validate results obtained via laser diffraction.
The characteristics of the aerosol generated from the nebuliser in terms of volumetric diameter and aerodynamic diameter using laser diffraction and cascade impaction respectively are shown in Fig. 3a and Table I. The median droplet sizes from both laser diffraction and cascade impaction were compared and found to be within the range for respiratory delivery and not significantly different (p > 0.05) for both formulations. Therefore, cascade impaction confirmed the recognised correlations with laser diffraction for nebuliser aerosol sizing. Similarly, there was also no significant difference found in the mean droplet sizes between the CFI and FCI formulations (p > 0.05) as would be expected given that the formulation properties (density and viscosity) are similar and the same aerosol delivery system was used for both formulations. Previous analysis of the aerosol characteristics of CFI using the PARI LC Sprint nebulizer powered by the PARI Proneb Ultra II® compressor (marketed in North America but has been phased out and is replaced by the PARI Vios® compressor) reported a slightly smaller aerosol with median size of 3.6 μm (GSD = 2.3) using laser diffraction (22). These differences could be attributed to the different compressors used, normal batch-to-batch variations in the performance of the jet nebulizers, or differences in the laser diffraction analysis assumptions or execution.
The deposition of ciprofloxacin on each stage of the NGI after nebulisation of CFI and FCI is shown in Fig. 3b. Data are represented as the percentage of total drug deposited in the throat and at each stage of the NGI over the emitted dose. The total emitted dose is defined as the total amount of drug recovered from the throat and the stages of the NGI. Furthermore, the fine particle fraction (FPF) (<5 μm), mass median aerodynamic diameter and geometric standard deviation (GSD) were calculated from regression of log-linear plots of stage-size verses cumulative stage-deposition. The total emitted dose recovered was found to be 2840.0 ± 480.9 μg and 6935.0 ± 646.5 μg for FCI and CFI formulation respectively due to the different concentration of drug within the formulations. On the other hand, the aerosol performance of the CFI formulation (FPF = 70.47 ± 2.03%) was not significantly different to that of FCI formulation (FPF = 64.20 ± 5.52%) (p = 0.1412). These findings were in good agreement with FPF predictions made from previous studies based on volumetric diameters of aerosols <4.95 μm (12,22), where the size distribution of the aerosols generated from the nebuliser was consistent with broad deposition in the lung. Findings from Bruinenberg et al. (12) predicted a lung dose of approximately 16.7% of the 150 mg CFI nebuliser loaded dose and 22.9% of the 300 mg CFI nebuliser loaded dose (unpublished data) which resulted in a sputum ciprofloxacin concentration of about 80-500 μg/mL, following administration in CF patients. The concentration is well above the MIC of many respiratory infection-causing bacteria including P. aeruginosa and S. aureus, which will be discussed further in later sections.
Critical features of any liposomal product include drug stability and retention of encapsulated drug during the manufacturing process and over the shelf life, and for inhaled applications must additionally ensure that the aerosolisation process does not alter the liposome size distribution or encapsulation of drug. This is particularly important for targeted local release of antibiotics to treat respiratory infection as liposomes must be delivered to the lung predominantly intact and must not rapidly release the drug after reaching the lungs (28). The CFI formulation was shown to meet these minimum requirements in a study by Cipolla et al. (22). Batches of CFI manufactured to good manufacturing practices (GMPs) possessed a mean diameter of ~91 nm (SD = 5, n = 12) at release and the mean liposome size did not change for at least 2 years when stored refrigerated. Typically greater than 99% of the ciprofloxacin is encapsulated within the vesicles after manufacture and this is also maintained for at least 2 years when stored under refrigeration (22). The liposomes retain their integrity and size distribution following nebulisation with no loss in drug encapsulation (22).
Deposition and Evaluation of Drug Transport Using an Air Interface Pulmonary Epithelial Model
Since the FPF are equivalent for both formulations as shown previously, cell deposition and transport studies can be performed confidently. The release profiles over time and the transport rate of the nebulised CFI and FCI formulations using the modified TSI Calu-3 cell model are shown in Figs. 4 and 5 respectively. Drug concentrations in Fig. 4 are expressed in terms of the percent total recovery throughout the experiments and data were plotted as mean cumulative percentage (± standard deviation) of drug transported across the Calu-3 cells over 4 h. The total amount of ciprofloxacin deposited onto the Calu-3 cells from both the formulations were equivalent, with ciprofloxacin recovery from the CFI formulation of 8.67 ± 2.08 μg and FCI formulation of 13.15 ± 4.11 μg deposited on the cells. The lower amount of ciprofloxacin deposited on the cells in comparison to the fine particle fraction is expected as a portion of this FPF could escape through the openings around the transwell inserts. CFI successfully controls the release of ciprofloxacin, with only 1.5% of the drug being transported across the epithelium over 4 h and so the vast majority of the drug is still present on the apical cell surface, remaining in physical proximity to the site of the bacterial infection in vivo. In comparison, FCI showed >33% of the drug transported over the same period.
These results are in qualitative agreement with animal and human pharmacokinetic data where the CFI absorption-limited plasma half-life was ~10.5 h (11,12). It is assumed that following the deposition of the nebulised CFI formulation onto the cell surface, ciprofloxacin is slowly released from the liposomes and subsequently diffuses into the surrounding epithelial lining fluid and finally passages across the epithelium. The transport rate of the CFI was found to be at least an order of a magnitude slower than that for FCI on the epithelial cells (Fig. 5). In addition, the distributions of ciprofloxacin in the cell model in Fig. 6 further indicated that the rate-limiting step in the CFI formulation is due to the release of ciprofloxacin from the liposomes. Drug concentration in each compartment from Fig. 6 is expressed in terms of the percentage of total recovery. It showed that after 4 h, >95% of the ciprofloxacin remained on the cells for the CFI formulation in comparison to the FCI formulation, where approximately 60% of the drug remained on the cell layer. Subsequently, due to the higher availability of free ciprofloxacin in the surrounding epithelial lining fluid from the FCI formulation, more drugs are allowed to diffuse into the cells giving a higher percentage of intracellular drugs and drug transported across the cell monolayer. Conversely, due to the retention of ciprofloxacin within the liposomes, smaller amounts of drugs were available for absorption and transport through the cells.
This slow release may be due to a combination of factors: the expected slow decomposition or modifications to the liposome membrane allowing leakage of drug from the vesicle, the slow dissipation of the transmembrane ion gradient resulting in a greater proportion of uncharged drug molecules able to migrate across the intact bilayer, or potential alteration in the physical form of ciprofloxacin within the liposomes interior that attenuates the release of drug molecules in solution. Release of drug is likely to be slower for liposomes prepared by this method compared to passively encapsulated liposomes (21) except for the case of large, polar passively encapsulated drugs like amikacin which appear to be released extremely slowly (28). Hence, CFI is not only stable during storage and nebulisation but is also able to mediate a sustained release of ciprofloxacin at the lung epithelium.
While the rapid absorption of FCI is in excellent agreement with animal and human studies of unencapsulated ciprofloxacin delivered to the lung (11,33) the absorption from CFI in the present in vitro model appears to be much slower compared to the observations in humans (12,22,29). There are therefore likely to be release and clearance mechanisms in the lungs of animals and humans that are absent from the in vitro model used in the currently reported studies, such as mucociliary clearance, uptake and breakdown by other cells (e.g., macrophages) and P. aeruginosa infection which releases virulence factors that could potentially trigger the release of antibiotics from the liposomes (28).
The deposition and drug transport of CFI on the Calu-3 cells were also analysed qualitatively using the confocal microscope at different time points following nebulisation (Fig. 7). The inherent fluorescent properties of ciprofloxacin allow visualisation using confocal microscope providing a better understanding of the interaction between the drug and mucus on the cells. It was observed that the nebulisation of CFI onto the cells resulted in an uneven distribution of ciprofloxacin over the cell surface. It also showed slow diffusional spreading of the deposited liquid droplets into the surrounding epithelial lining fluid creating a concentration gradient after 4 h, presumably due to the slower release of ciprofloxacin from liposomes. As such, most ciprofloxacin still remained on the cells after the experiment. In comparison, the FCI diffuses and spreads rapidly into the epithelial lining fluid giving a relatively even distribution of antibiotics within 30 min (Fig. 8). Given the slow and controlled release of ciprofloxacin from liposomes in the CFI formulation, high focal concentrations produced during aerosol deposition are maintained giving it a patchy appearance after 4 h (Fig. 7). Conversely, the faint ciprofloxacin fluorescence on the cell surface after 1.5 h into the experiment indicated that significantly more antibiotic has been transported across the cells (Fig. 8). These observations further confirm the quantitative analysis of the release profile on the Calu-3 cells and the distribution of ciprofloxacin in the different compartments of the cell model. Thus, the different transport kinetics, distribution and interaction with the lung epithelium of the ciprofloxacin formulations observed were only made possible by the modified TSI air-interfaced Calu-3 cell system (15). This system allows for more realistic assessment of dissolution and transport rate after drug deposition from nebulisers with respect to in vivo conditions. However, the system also lacks some important clearance mechanisms including mucociliary clearance and uptake by other cells in the airways (e.g. macrophages).
To assess the integrity of the epithelial cells, transepithelial electrical measurements were performed after the experiments and were compared to the untreated cells. There were no significant differences found between the control cells (TEER = 548.31 ± 3.63 Ω cm2) and the cells treated with either CFI (TEER = 549.32 ± 24.79 Ω cm2) or FCI (TEER = 547.61 ± 11.82 Ω cm2) formulations (p > 0.05). Hence, the deposition of the nebulised formulations and high local concentration at the deposition point did not have any detrimental effect on the epithelial cell integrity under the conditions and timescale studied. The TEER values are similar to previous findings indicating high integrity of the Calu-3 monolayer (15,19).
In Vitro Antimicrobial Activity
The in vitro antimicrobial activity of CFI was compared to the FCI by determining the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) on laboratory culture collection strains of S. aureus and P. aeruginosa. The results are shown in Table II. The antimicrobial effects of empty liposomes were also tested on each microorganism as a control. No statistically significant difference was found between the CFI and FCI formulations in the MIC values for both organisms. However, the MBC value for P. aeruginosa was found to be significantly lower in the CFI formulation compared to the FCI formulation whereas no significant difference in MBC was found for S. aureus. Previous studies have also demonstrated similar improvements in antimicrobial activity of antibiotics when encapsulated within liposomes against Gram-negative bacteria such as P. aeruginosa (34–36), Francisella tularensis (10) and Salmonella typhimurium (21). According to the literature (10,34–36), the enhanced antimicrobial activity against Gram-negative bacteria is due to the fusional interaction of the phospholipids of liposomes with the bacterial outer membrane. In addition, the formulation is especially beneficial towards the eradication of P. aeruginosa as it has high intrinsic resistance owing primarily to its poorer outer membrane permeability due to the presence of drug efflux pumps coupled with smaller water-filled channels/porins compared to other Gram-negative bacteria (37,38).
Conversely, the CFI did not show an improvement in bactericidal activity with S. aureus presumably due to the presence of the thick peptidoglycan cell wall in Gram-positive bacteria. This barrier may hamper direct contact between the liposomes and the cytoplasmic membrane resulting in the liposomal formulation (CFI) being comparable to administration of the free drug (FCI). Furneri et al. (35), found that the composition of liposomes is an important parameter which could influence the effectiveness of the liposomes. The presence of negatively charged phospholipids was found to be a fundamental requisite to ensure improvement in antibacterial effectiveness as it could form hydrogen bonds or/and ionic interactions with the peptidoglycan and various other components (39). In addition, it was suggested that the liposomal size and fluidity property of the liposome’s bilayer are also important determinants for the interaction with the external peptidoglycan layer (35,40). Nonetheless, in the in vivo environment, the antimicrobial efficacy would be further enhanced, regardless of the bacterial type, due to the increased lung retention of ciprofloxacin provided by the sustained release from liposomes in comparison to the free ciprofloxacin that will be cleared rapidly from the lungs.
CONCLUSIONS
CFI has the potential to provide a promising new treatment for pulmonary infections particularly for patients with cystic fibrosis and bronchiectasis. This study has demonstrated that the liposomal formulation mediated a sustained release at the respiratory epithelium and enhanced antimicrobial activity against P. aeruginosa. The liposomal formulation may ultimately lead to greater compliance through less frequent dosing, improved efficacy through targeted delivery to the lung, potentially reducing the risk of systemic side effects and resistant.
The air-interface Calu-3 cell together with the TSI allows for a more realistic assessment of drug release and transport rate after drug deposition from nebulisers with respect to in vivo conditions. This model also helps to elucidate the underlying mechanism by which the formulation interacts with the airway epithelium and mucus at the cellular level. Therefore, this in vitro system can be a developmental tool to rapidly evaluate the release profiles of novel nebulised liposomal formulations prior to entry into the clinic. Still, the model would require further refinements to more closely approximate in vivo transport rates. Future studies will focus on correlating the absorption rate of this in vitro model with the in vivo state and on how to further refine the model to better approximate the transport kinetics and accurately represent the barrier system of the tracheo-bronchiolar epithelium. In vivo data with pulmonary delivered CFI in animals and in humans (11,12,22,29) show that ciprofloxacin is released slowly from the liposomes but at a substantially faster rate than in the in vitro model reported here. There are therefore likely to be release mechanisms in the lungs of animals and humans that are absent from the in vitro model used in the currently reported studies.
REFERENCES
Murray TS, Egan M, Kazmierczak BI. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr. 2007;19:83.
Barker AF. Bronchiectasis. N Engl J Med. 2002;346:1383–93.
Geller DE. Aerosol antibiotics in cystic fibrosis. Respiratory Care. 2009;54:658–70.
Barker AF, Couch L, Fiel SB, Gotfried MH, Ilowite J, Meyer KC, O'Donnel A, Sahn SA, Smith LJ, Steward JO. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am J Respir Crit Care Med. 2000;162:481–5.
Scheinbergand P, Shore E. A pilot study of the safety and efficacy of tobramycin solution for inhalation in patients with severe bronchiectasis*. Chest. 2005;127:1420.
Rubin BK. Aerosolized antibiotics for non-cystic fibrosis bronchiectasis. J Aerosol Med Pulm Drug Delivery. 2008;21:71–6.
Gubernator J, Drulis-Kawa Z, Dorotkiewicz-Jach A, Doroszkiewicz W, Kozubek A. In vitro antimicrobial activity of liposomes containing ciprofloxacin, meropenem and gentamicin against gram-negative clinical bacterial strains. Lett Drug Des Discovery. 2007;4:297–304.
Drulis-Kawaand Z, Dorotkiewicz-Jach A. Liposomes as delivery systems for antibiotics. Int J Pharm. 2010;387:187–98.
Bakker-Woudenberg IAJM, ten Kate MT, Guo L, Working P, Mouton JW. Improved efficacy of ciprofloxacin administered in polyethylene glycol-coated liposomes for treatment of Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 2001;45:1487.
Wong JP, Yang H, Blasetti KL, Schnell G, Conley J, Schofield LN. Liposome delivery of ciprofloxacin against intracellular Francisella tularensis infection. J Controlled Release. 2003;92:265–73.
Yim D, Blanchard JD, Mudumba S, Eastman S, Manda K, Redelmeier T, Farr SJ. The development of inhaled liposome-encapsulated ciprofloxcin to treat cystic fibrosis. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery X, vol. 2. River Grove: Davis Healthcare Int'l Publishing LCC; 2006. p. 425–8.
Bruinenberg P, Blanchard JD, Cipolla DC, Dayton F, Mudumba S, Gonda I. Inhaled liposomal ciprofloxain: once a day management of respiratory infections. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, editors. Respiratory drug delivery, vol. 1. River Grove: Davis Healthcare International Publishing; 2010.
Ehrhardt C, Fiegel J, Fuchs S, Abu-Dahab R, Schaefer U, Hanes J, Lehr CM. Drug absorption by the respiratory mucosa: cell culture models and particulate drug carriers. J Aerosol Med. 2002;15:131–9.
Steimer A, Haltner E, Lehr CM. Cell culture models of the respiratory tract relevant to pulmonary drug delivery. J Aerosol Med. 2005;18:137–82.
Ong HX, Traini D, Bebawy M, Young PM. Epithelial profiling of antibiotic controlled release respiratory formulations. Pharm Res. 2011;28:2327–38.
Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23:1482–90.
Cavet ME, West M, Simmons NL. Transepithelial transport of the fluoroquinolone ciprofloxacin by human airway epithelial Calu-3 cells. Antimicrob Agents Chemother. 1997;41:2693.
Grainger C, Greenwell L, Martin G, Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur J Pharm Biopharm. 2009;71:318–24.
Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time-and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev Ind Pharm. 2010;36:1207–14.
Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm Pharmacol Ther. 2005;18:165.
Webb MS, Boman NL, Wiseman DJ, Saxon D, Sutton K, Wong KF, Logan P, Hope MJ. Antibacterial efficacy against an in vivo Salmonella typhimurium infection model and pharmacokinetics of a liposomal ciprofloxacin formulation. Antimicrob Agents Chemother. 1998;42:45.
Cipolla DC, Dayton F, Fulzale S, Gabatan E, Mudumba S, Yim D, Wu H, Zwilinski R. Inhaled liposomal ciprofloxacin: In vitro properties and aerosol performance. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery, vol. 2. River Grove: Davis Healthcare Int'l Publishing; 2010. p. 409–14.
Vecellio None L, Grimbert D, Becquemin M, Boissinot E, Le Pape A, LemariÈ E, Diot P. Validation of laser diffraction method as a substitute for cascade impaction in the European Project for a Nebulizer Standard. J Aerosol Med. 2001;14:107–14.
Dumouchel C, Yongyingsakthavorn P, Cousin J. Light multiple scattering correction of laser-diffraction spray drop-size distribution measurements. Int J Multiphase Flow. 2009;35:277–87.
Pharmacopoeia British. Consistency of Formulated Preparations, vol. 5. London: HMSO; 2011.
Chan JGY, Kwok PCL, Young PM, Chan H-K, Traini D. Mannitol delivery by vibrating mesh nebulisation for enhancing mucociliary clearance. J Pharm Sci. 2011;100:2693–702.
National Committee for Clinical Laboratory Standards. Tests to assess bactericidal activity: approved standard M2-A6. Wayne: NCCLS; 1996.
Meers P, Neville M, Malinin V, Scotto A, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins W. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61:859.
Cipolla DC, Redelmeier T, Eastman S, Bruinenberg P, Gonda I. Liposomes, niosomes and proniosomes - a critical update of their (commercial) development as inhaled products. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery Europe 2011. Rover Grove: Davis Healthcare Int'l Publishing; 2010. p. 41–54.
Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69:1–9.
Zieglerand J, Wachtel H. Comparison of cascade impaction and laser diffraction for particle size distribution measurements. J Aerosol Med. 2005;18:311–24.
Clark AR. The use of laser diffraction for the evaluation of the aerosol clouds generated by medical nebulizers. Int J Pharm. 1995;115:69–78.
Bruinenberg P, Serisier D, Cipolla DC, Blanchard JD. Safety, tolerability, pharmacokinetics and antimicrobial activity of inhaled liposomal ciprofloxacin formulations in humans. Pediatr Pulm. 2010;45:354, #377.
Mugabe C, Halwani M, Azghani AO, Lafrenie RM, Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:2016–22.
Furneri PM, Fresta M, Puglisi G, Tempera G. Ofloxacin-loaded liposomes: in vitro activity and drug accumulation in bacteria. Antimicrob Agents Chemother. 2000;44:2458.
Jia Y, Joly H, Omri A. Characterization of the interaction between liposomal formulations and Pseudomonas aeruginosa. J Liposome Res. 2010;20:134–46.
Hancock REW. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27:S93.
Woodruff W, Parr Jr T, Hancock R, Hanne L, Nicas T, Iglewski B. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986;167:473.
Lohnerand K, Prenner EJ. Differential scanning calorimetry and X-ray diffraction studies of the specificity of the interaction of antimicrobial peptides with membrane-mimetic systems. Biochimica et Biophysica Acta (BBA)-Biomembranes. 1999;1462:141–56.
Hopeand MJ, Wong KF. Liposomal formulation of ciprofloxacin. In: Shek PN, editor. Liposomes in biomedical applications. Germany: Harwood Academic Publishers; 1995. p. 121–34.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, H.X., Traini, D., Cipolla, D. et al. Liposomal Nanoparticles Control the Uptake of Ciprofloxacin Across Respiratory Epithelia. Pharm Res 29, 3335–3346 (2012). https://doi.org/10.1007/s11095-012-0827-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0827-0