Abstract
Introduction
Targeting drug treatment to fungal infections that reside within or below the nail plate is problematic due to the highly restrictive barrier of the human nail. To optimise topical formulations for ungual drug delivery, inclusion of an effective penetration enhancer (PE) is imperative. At present, in vitro nail permeation studies can take weeks or months in order to obtain any meaningful data because the lack of a simple in vitro model to identify and develop nail PEs makes the selection and optimisation of novel PEs an empirical and inefficient process. The aim of this study was to compare three methods for pre-formulation screening of putative ungual PEs and then to select the most suitable technique for screening candidates that may enhance the permeation of therapeutic agents through the human nail.
Methods
Three screening techniques were evaluated; nail swelling (weight increase of human nail clippings), horse hoof swelling (weight increase of horse hoof clippings) and nail penetration of a radiolabelled permeability probe. Four test PEs were evaluated using each screening method and nail swelling was identified as a simple, rapid, economic, relevant and reliable technique. This screen was then used to evaluate 20 potential PEs. Thioglycolic acid (TA), hydrogen peroxide (H2O2) and urea H2O2 produced the greatest nail weight increases; 71.0 ± 4.6%, 69.2 ± 6.6%, and 69.0 ± 9.9 respectively. To confirm the relationship between human nail swelling and altered ungual barrier function, a permeation study was performed in human nails using caffeine as a model penetrant.
Results and Discussion
Human nails pre-treated with TA in vitro had a 3.8-fold increase in caffeine flux compared to the control (TA-free solution). This study illustrated the potential to use human nail clipping swelling as a surrogate marker of PE activity for topical ungual drug delivery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Onychomycosis, a fungal infection, is the most prevalent disease of the nail. The highly restrictive barrier properties of the human nail make this infection extremely difficult to treat (1,2). The condition is especially problematic in elderly and immunocomprised populations in whom the physical consequences of the infection include discomfort and pain. When toenails are severely affected, prolonged standing and walking become difficult. The psychological effects of onychomycosis include embarrassment, lowered self-esteem and social isolation (1,3,4).
Although ungual infections can be treated using oral medication, systemic antifungal therapy suffers from a high incidence and severity of adverse effects and propensity for drug interaction. For example, the hepatotoxic potential of oral itraconazole means that patients are required to take periodic liver function tests if they receive continuous treatment for more than one month and similar problems are associated with oral terbinafine (5,6). Thus, topical therapy appears more attractive, but in practice the barrier properties of the nail make it a challenge to accumulate therapeutically adequate drug concentrations in the ventral region of the nail and the nail bed. As a result, the cure rates using topical antifungal formulations are generally low and relapse rates are high. Whilst there are topical formulations available on the market in both Europe and the US, these have cure rates as low as 18% (1).
The structure of human nail has been likened to a hydrophilic gel membrane (7–9). It is considered to consist of three layers; the dorsal, intermediate and ventral. Of these the intermediate layer is believed to be the softest and thickest. The ventral layer connects the nail and the nail bed (10,11). The lipid content of the nail is reported to be low at between 0.1–1% and the nail is much more susceptible to water loss than the skin which contains more lipid (12). Despite the reported hydrophilic properties of the nail, hydrophobic compounds have been shown to diffuse into and through this barrier. For example Walters and co-workers reported that long chain alcohols permeate through the nail via a lipidic pathway (9,13). Subsequent work failed to find any evidence of a distinctive pathway for the transport of hydrophobic chemicals (8). These conflicting reports highlight the fundamental lack of understanding of human nail permeability determinants, a factor that confounds the rational design of PEs for the nail barrier.
As access to intact human nails is often limited, animal material has been used as a human nail substitute when studying nail permeability (14–16). However, significant differences in drug permeability have been reported between human nail and animal material such as hooves. In comparative studies, bovine hooves have been shown to be more permeable than human nails, possibly as a result of a distinct hoof keratin structure that is able to retain a higher percentage of water (17,18). Differences such as these suggest that while animal hooves can be used to model human nail permeability, caution is required in extrapolating such data to predict ungual penetration in humans in vivo.
Poor understanding of the barrier properties of human nail and the absence of a standard in vitro PE-screening model hinders the preformulation optimisation of ungual drug delivery systems. Current methods used for the investigation of nail permeation tend to be time consuming or difficult to perform and the generation of purposeful data can take weeks or months. This only slows down the process of identifying ways of improving nail permeation. Hence, the preliminary aim of the present study was to compare three screening methods for the identification of PE activity in the nail. Human nail swelling (measured as weight increase) has been used to test penetration-enhancing capabilities of chemicals such as N-acetyl-l-cysteine and urea (19). In this study, a nail swelling method was established as the first screen. For the second method, the swelling method was adapted to use horse hoof instead of human nail. The third screening technique monitored the permeation of [C-14]-mannitol through human nail. Mannitol was selected on the basis that it is a small (MW = 184), hydrophilic molecule and would, therefore, be expected to permeate the nail over a relatively short amount of time. Each of the screening methods was performed using the same four reported test PEs which were selected due to their potential as ungual penetration enhancers; resorcinol (20), TA, H2O2 (21) and cysteine (22).
The aim of this study was to compare the capability of three pre-formulation screening methods to assess the interaction between potential PEs and the nail. It was anticipated that the most appropriate model would be used to screen a whole range of compounds for PE activity and the best performing PE tested using a nail permeability study.
MATERIALS
Glycolic acid, hydrogen peroxide solution, L-cysteine hydrochloride hydrate, thioglycolic acid (TA), urea hydrogen peroxide addition compound (urea H2O2), thiouracil, 1,8 cineole, SIN-1, resorcinol and caffeine were purchased from Sigma–Aldrich (Dorset, UK). Ammonium thioglycolate, 1,4-dithio-DL-threitol (DTT), propyl gallate, p-coumaric acid, dithizone, benzoyl peroxide and oxalic acid were supplied by Fluka (Dorset, UK). Ethanol (EtOH) was from BDH (Dorset, UK). Catechin and curcumin were obtained from Extrasynthese (Genay, France) and Acros Organics (Leicestershire, UK), respectively. Acetone was provided by Fisher Scientific (Leicestershire, UK) and the 24-well cluster plates used were from Costar® (Dorset, UK). Human nail clippings were donated from healthy volunteers following approval by the King’s College Research Ethics Committee (Study Ref no. 04/05-126). Horse hoof shavings were obtained from Farriers Equipment Ltd (Surrey, UK). Validated ChubTur® permeation cells were kindly donated by MedPharm Ltd. [C-14]-mannitol was purchased from Amersham Biosciences (Bucks, UK) and scintillation fluid (hionic fluor) was supplied by Perkin Elmer (Bucks, UK). Ringers solution and PBS was from Oxoid (Hampshire, UK).
METHODS
Preparation of Penetration Enhancer Solutions
Unless otherwise specified, all candidate PEs were prepared in a mixture of water and ethanol (80:20 v/v). A saturated concentration of the enhancers was used but if this exceeded 5% w/w, this was accepted as the final working concentration, with the exception of H2O2 (used as a neat solution at 35% by weight) and urea H2O2 (used at the maximum concentration of 17.5% by weight). The PEs used in the three preliminary screening methods were prepared; cysteine (5% w/w), thioglycolic acid (5% w/w), H2O2 (used as a 35% solution by weight) and resorcinol (5% w/w). Additional candidate PEs were also prepared for use in the final human nail swelling screen for novel PE identification; ammonium thioglycolate (5% w/w), DTT (5% w/w), glycolic acid (5% w/w), sodium thioglycolate (5% w/w), urea H2O2 (17.5% H2O2 content w/v in water), propyl gallate (5% w/w in 70% EtOH), p-coumaric acid*, catechin*, curcumin*, thiouracil* (in 70% EtOH), 1,8 cineole*, SIN-1* (in water), dithizone* (in acetone), benzoyl peroxide* (in EtOH), oxalic acid (5% w/w) and potassium persulphate (5% w/w in water). Asterisk indicates saturated solutions.
Human Nail Swelling Screen
Human nail clippings were obtained from 15 donors (n = 10) aged between 18–65 years, were infection-free by visual inspection and of the weight range 9–12 mg. The nail clippings were washed with 70% EtOH (v/v) three times followed by rinsing in water. They were allowed to dry overnight in an open Petri dish at room temperature after which they were either used immediately or stored in a clean glass container at 4°C. Clean dry nails were weighed and then placed into individual wells of a 24-well plate. PE or control solution (1 ml) was added to each well to ensure that the nail was always submerged. The plates were covered using lids and incubated at room temperature for 20 h, after which the nails were then removed from solution, excess moisture was wiped away and the nails reweighed. Control solutions were prepared as the respective solvent solutions in absence of the PE.
Horse Hoof Swelling Screen
Hoof shavings were cleaned with water to remove any dirt and residue and were placed in a container with 1/4 strength Ringers solution and soaked for 3 h to soften them. A cable cutter (KNIPEX-Werk, Wuppertal, Germany) was used to cut the hooves into smaller sections and scissors were then used to trim the hoof sections to a similar size and weight as the nail clippings. The hooves were washed and dried as described for the human nails, then weighed into 24-well plates. Following 20 h incubation in either PE or control solutions, excess moisture was wiped away and the hoof clippings were reweighed.
Mannitol Permeability Screen
Clean, dry nail clippings were cut to size and mounted onto validated, pre-calibrated ChubTur® permeation cells. These permeation cells were similar in principle to Franz cells and the nail section (approximate size: 3 mm × 3 mm) was clamped and held in place between receiver and donor compartments. The dorsal nail surface area exposed to the applied formulation was approximately 0.05 cm2. The PEs used for pre-treatment were freshly prepared and 0.5 ml applied to the donor compartments of each cell. After 20 h at room temperature, the solution in the donor compartment was removed and the dorsal surface of the nail was washed three times with water. The receiver compartments were carefully filled with water and sampled (1 ml removed and replaced with fresh water) for assessment of background radioactivity. Radiolabelled mannitol solution (50 μl) containing 22.2 Bq/ml of activity was applied directly to the exposed nail surface. The diffusion cells were then sealed with parafilm to prevent evaporation and incubated in a water bath maintained at 32°C for 120 h, after which the receiver fluid was sampled. Samples (1 ml) were transferred into scintillation vials and after addition of 4 ml scintillation fluid, the vials were mixed thoroughly and analysed using a LS 6500 Multipurpose scintillation counter (Beckman Coulter™, Bucks, UK).
Enhancement of Caffeine Permeability in Human Nails
Clean, dry nail clippings were cut to size and mounted onto validated, pre-calibrated ChubTur® permeation cells, as described above. TA (5% w/w in 20% EtOH; 0.5 ml) was applied to the donor compartment of each diffusion cell so that the dorsal surface of the nail was submerged. For control samples, a 20% EtOH:water solution (v/v) was used to hydrate the nails over 20 h. After this time, solutions were removed from donor compartments and the nail surface was washed three times with water. The receiver compartment of each diffusion cell was filled with PBS. Saturated caffeine in PBS (ca. 21.6 mg/ml; prepared by incubating an excess of solid caffeine in PBS solution at room temperature) was applied (0.5 ml) to the dorsal surface of the nail. The diffusion cells were then sealed with parafilm to prevent evaporation and incubated in a water bath maintained at 32°C. Receiver fluid was sampled (1 ml) at predetermined time points for a total of 165 h and samples were analysed immediately by HPLC.
HPLC Analysis
The method used for analytical determination of caffeine was an adaptation of the method described by Akomeah and co-workers (2004). A Perkin–Elmer series 200 LC Pump with autosampler and vacuum degasser connected to a UV/Vis detector 785A was used for analytical determination of caffeine. This system was connected, via a PE Nelson network chromatography interface (NCI) 900 and PE Nelson 600 Series LINK to a PC with Turbochrome Navigation software which was used for data collection and interpretation. The sample injection volume was 50 μl and the mobile phase consisted of phosphate buffer (0.05M KH2PO4 containing 1% v/v triethylamine and adjusted to pH 3.5 using orthophosphoric acid):acetonitrile (92:8, v/v) set at a flow rate of 1 ml/min. A Phenomenex® Luna™ C18 column (150 × 4.6 mm, 5 μm particle size) was used and UV detection at 275 nm was employed.
RESULTS AND DISCUSSION
Penetration Enhancer Screening Method Evaluation
Without a clear understanding of the fundamental properties of the nail barrier, the development of a rapid screening procedure for the discovery of novel ungual penetration enhancers is an empirical exercise. Methods commonly used to monitor ungual drug penetration in vitro range in complexity from simple partition experiments (19) through to the construction of thermoregulated diffusion cells that house whole or parts of human nails (24). Most of these methods can be laborious and/or time consuming. By far, the simplest method reported for assessing the effects of ungual penetration enhancers is measurement of the ‘swelling’ of a human nail clipping under the influence of a specific PE in solution. This method is based on the principle that the rate and extent of drug penetration into the nail can be modelled by the ungual uptake (measured as weight increase) of small solvent molecules such as water. When immersed in water, the human nail has been shown to increase in weight as a result of uptake and retention of solution and previous work has reported that increased water uptake can be used as a good marker for enhanced nail permeability (19).
In the nail swelling experiments the four test PEs ranked similarly in terms of weight gain when using both the human nail and horse hoof; TA = urea H2O2 > resorcinol = cysteine (Fig. 1). TA and H2O2 produced human nail weight increases of 71 ± 5% and 69 ± 7%, respectively. Greater weight increases were observed in the hoof swelling assay; TA produced 82 ± 16% and H2O2 produced 93 ± 13%. The other test PEs, cysteine and resorcinol, induced no significant weight gain with either human nail or horse hoof compared to control (p > 0.05, ANOVA).
Increase in weight of human nail and horse hoof clippings following 20 h pre-treatment with selected chemical agent/s. The agents used for pre-treatment were resorcinol, thioglycolic acid (TA), H2O2 and cysteine. All agents were dissolved in 20% EtOH/H2O apart from H2O2 which was used as pre-prepared in H2O. The controls for the study were human nails or horse hooves immersed in H2O and 20% EtOH/H2O but these were not significantly different (p > 0.05, ANOVA) from each other and so only one data set is shown for each (n = 8–10, mean ± SD).
Animal hoof has been used as a human nail substitute in previous studies. For example, porcine hoof has been used to simulate the diffusion of compounds across the human nail matrix (14) and to investigate the effect of pressure sensitive adhesives on ciclopirox penetration (16). Bovine hooves have been used to investigate the permeation of compounds from an experimental nail lacquer (15). However, in the present study, the differences in the swelling of human nails and horses hooves as a result of solvent uptake in the presence of PE systems illustrated significant differences in their response. In addition, the increase in weight by the hooves was 40 ± 9% compared to a 27 ± 3% weight increase by human nails (Fig. 1) when immersed in control solutions. This provides further evidence that the mammalian hoof is capable of taking up and retaining more water than human nail (8). This species-dependant difference was also observed for H2O2 (69.2% for nails; 93.3% for hoof) and cysteine (38 ± 6% for nails; 74 ± 30% for hoof).
The hydration of the diffusion barrier has been shown to be pivotal in topical drug delivery. The higher hydration capacity of horse hoof compared to human nail may indicate that horses hooves do not provide a representative model in which diffusion through the nail can be evaluated (25). The higher hydration capacity may be due to differences in the quantity and type of keratin. Although the dominant keratin sub-type in human nail has not been elucidated the amino acid composition has been studied; for example, the amount of cysteine in the nail has been reported to be 10.6% whilst in the hoof, this value is believed to be almost half the amount at 5.7% (17,18). This suggests that less disulphide bonds, which are know to provide keratin with rigidity, may be present in the hooves.
In addition to the higher hydration capacity compared to nails, horse hooves displayed higher intra-experiment variability than human nails in the swelling screen (15.3%CV for human nail compared to 23.2%CV for hoof swelling). This may result, in part, from random orientation when sectioning the hooves. Like human nails, hooves consist of definite layers which may (although not studied) have different physical and permeability characteristics. Intact hoof shavings are hard and robust in nature, thus sectioning proved to be difficult, especially as the material required matching in terms of weight and surface area to the human nail. The only advantage of using hooves compared to human nail was the ready supply of material available.
In the mannitol permeability screen, the highest permeability was observed following pre-treatment with TA and H2O2 (2.8 and 3.2-fold increase above control, respectively; Fig. 2). This increase in permeability was significantly greater than the control (p < 0.05, ANOVA). No significant increase in the permeability of mannitol compared to control was produced after pre-treatment with resorcinol and cysteine (p > 0.05, ANOVA). Hence, using mannitol permeation as a marker ranked the effectiveness of the four test PEs in a similar manner to the swelling experiments. However, this model had several limitations. The intra experiment variability was high, not least in the control (83% CV). The in vitro ChubTur® model required nails with a dorsal surface area of at least 9 mm2 (large distal nail clipping) compared to nail swelling which required clippings of 10 mg rather than a specific size or shape. In addition, the health and safety limitations associated with using radioactivity also made this method less suitable as a simple screen compared to the human nail swelling assay. Furthermore, it is possible that mannitol permeation is not representative of the permeability of compounds with different physicochemical properties.
Permeation of [C-14]-mannitol through pre-treated human nail, 120 h after application. Pre-treatment time was 20 h. The agents used for pre-treatment were resorcinol, thioglycolic acid (TA), H2O2 and cysteine. All agents were dissolved in 20% EtOH/H2O apart from H2O2 which was used as pre-prepared in H2O. Only one control is shown as they only differed by 5.3% (n = 4, mean ± SD).
Novel Penetration Enhancer Identification
As nail swelling was simple, rapid and required only small amounts of nail material, this assay was used to screen 20 candidate compounds for penetration enhancing activity (Table I). In brief, the agents selected for the final screen included compounds from the literature (2,22) that represented groups of chemicals with the ability to act as reducing or oxidizing agents, those that were sulphur-containing, or were potential keratolytic agents which theoretically may be able to disrupt ungual barrier function.
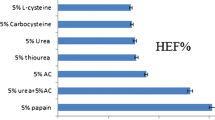
Several compounds that increased nail weights significantly compared to the control were identified. Nail weight was increased most by TA followed by H2O2, and urea H2O2, which produced equivalent increases. Glycolic acid was the only other chemical to cause a mean weight increase greater than 60%. Four test chemicals induced no significant weight increases in nails; dithizone, benzoyl peroxide, thiouracil, and p-coumaric acid.
TA, a strong reducing agent, is thought to reduce the disulphide bonds of nail keratin causing a disruption of the tightly structured disulphide bonding leading to solvent uptake and nail swelling. The thioglycolates have previously been shown to induce structural changes in hair keratin and are for this reason used in various depilatory products (26). It is possible that nail keratin exposed to TA solution undergoes structural expansion as well as bond disruption, leading to the formation of pores which facilitate uptake of the TA. Disulphide bond breakage is used not only in depilatory products but also by hairdressers for permanent hair waving (27,28).
H2O2-containing compounds were also effective in increasing nail swelling. The ability of H2O2 to influence the structural properties of human nails has previously been observed by Alkiewicz and Pfister (21), who suggested that nails could be softened using 10% thioglycolate or 20–30% H2O2. During the permanent waving process, H2O2 is applied to reform disulphide bridges in hair keratin so that the new style is ‘locked’ into place (29). Urea H2O2 is reported to be a more stable oxidising agent compared to H2O2 alone as this adducted compound is reported to release H2O2 in a controlled manner (30).
In the present study, compounds were screened using hydrated nail samples. The nail is known to be hydrated in vivo (containing between 7–25% water under normal physiological conditions) and for this reason most ungual permeability studies hydrate the nail samples prior to performing permeation experiments (2,24,31,32). Although it is accepted that some formulations applied to ungual surface may dehydrate the nail, the constant diffusion gradient of water out from the nail (in vivo) and the inherent ability of lacquers to dry quickly means that any dehydration should be localized to the upper layers of the nail.
Some of the agents that were not successful at increasing nail weight were poorly water soluble and were dissolved in lipophilic solvents (100% acetone and 100% ethanol). It was noted that only acetone did not allow the nail to swell at all and the use of 100% ethanol resulted in a very small amount of nail swelling (1.1 ± 0.8%). However, the inclusion of even a small proportion of water in the organic/water co–solvent mixtures caused significant (p<0.05, ANOVA) nail swelling. Therefore, as the agents that caused greatest swelling were dissolved in aqueous solutions, it is possible that there is a link between the solvent and swelling extent. The inability of pure acetone and ethanol to swell the nail may have reduced the ability of the model to determine the efficacy of the PEs in these solvents but, as only 2 PEs were dissolved in pure organic solvents this should have not impacted on the results presented in this study. These results suggest that the swelling model requires the inclusion of some water and this effect should be considered in the design of future studies.
Penetration Enhancement in Human Nails
The most effective penetration enhancer identified from the swelling pre-screen, TA, was applied to human nail sections and the permeation of a model drug, caffeine, was quantified over 165-h period. Caffeine permeability has previously been used to demonstrate the effectiveness of PEs in skin permeation studies (23,33). The steady state flux of caffeine through nails pre-treated with TA (14.10 ± 3.42 μg cm−2 h−1) was 3.8-fold higher compared to control nails which were treated with a TA-free solvent (3.64 ± 1.54 cm−2 h−1; Fig. 3).
TA was applied for 20 h and removed prior to the application of the saturated caffeine solution. This type of pre-treatment regimen was used to avoid issues of chemical incompatibility of TA and caffeine. One confounding issue brought about by applying the PE as a pre-treatment prior to the quantification of caffeine permeation is that if the effects of TA are reversible the barrier properties of the nail may start to recover during the time course of the permeation experiment. However, the permeation profiles recorded for both TA and the control nails were linear between 46–165 h (r 2 = 0.99 and 0.97) respectively, data not shown). This implies that any modification to the barrier properties of the nail is not reversible in this time course for TA. Further work is required to study if this is a compound specific phenomenon.
Although the similarity in the results from the human nail diffusion and swelling methods does not validate water uptake as a ubiquitous model for PEs, it provides evidence that such a screening method can be used to detect ungual PE activity. Further work is required to assess the ability of TA and the other compounds discovered by the screening method to enhance the permeability of a more diverse (in terms of physicochemical properties) set of therapeutic molecules.
CONCLUSION
This study compared three methods which were proposed as preformulation screens for ungual PEs. The simple nail swelling method was found to be the most suitable technique and it was used to screen 20 putative nail PEs. Pre-treatment of nails with TA was effective at inducing nail swelling. Penetration enhancement in the nail using TA was demonstrated by the increased permeation of caffeine in TA pre-treated nails. The mechanism and effectiveness of PE identified in the present study requires further verification along with an evaluation of their ability to enhance the permeation of drugs with differing physicochemical properties.
References
B. E. Elewski. Onychomycosis: Pathogenesis, diagnosis, and management. Clin. Microbiol. Rev. 11:415–429 (1998).
S. Murdan. Drug delivery to the nail following topical application. Int. J. Pharm. 236:1–26 (2002).
L. A. Drake, R. K. Scher, E. B. Smith, G. A. Faich, S. L. Smith, J. J. Hong, and M. J. Stiller. Effect of onychomycosis on quality of life. J. Am. Acad. Dermatol. 38:702–704 (1998).
R. K. Scher. Onychomycosis: A significant medical disorder. J. Am. Acad. Dermatol. 35:S2–S5 (1996).
C. Ajit, A. Suvannasankha, N. Zaeri, and S. J. Munoz. Terbinafine-associated hepatotoxicity. Am. J. Med. Sci. 325:292–295 (2003).
W. M. Chambers, A. Millar, S. Jain, and A. K. Burroughs. Terbinafine-induced hepatic dysfunction. Eur. J. Gastroenterol. Hepatol. 13:1115–1118 (2001).
Y. Kobayashi, M. Miyamoto, K. Sugibayashi, and Y. Morimoto. Drug permeation through the three layers of the human nail plate. J. Pharm. Pharmacol. 51:271–278 (1999).
D. Mertin and B. C. Lippold. In vitro permeability of the human nail and of a keratin membrane from bovine hooves: Prediction of the penetration rate of antimycotics through the nail plate and their efficiency. J. Pharm. Pharmacol. 49:866–872 (1997).
K. A. Walters, G. L. Flynn, and J. R. Marvel. Physicochemical characterization of the human nail—permeation pattern for water and the homologous alcohols and differences with respect to the stratum-corneum. J. Pharm. Pharmacol. 35:28–33 (1983).
R. P. R. Dawber. The ultrastructure and growth of human nails. Arch. Dermatol. Res. 269:197–204 (1980).
J. C. Garson, F. Baltenneck, F. Leroy, C. Riekel, and M. Muller. Histological structure of human nail as studied by synchrotron X-ray microdiffraction. Cell. Mol. Biol. 46:1025–1034 (2000).
G. B. E. Jemec, T. Agner, and J. Serup. Transonychial water-loss—relation to sex, age and nail-plate thickness. Br. J. Dermatol. 121:443–446 (1989).
K. A. Walters, G. L. Flynn, and J. R. Marvel. Physicochemical characterization of the human nail—solvent effects on the permeation of homologous alcohols. J. Pharm. Pharmacol. 37:771–775 (1985).
J. H. Kim, C. H. Lee, and H. K. Choi. A method to measure the amount of drug penetrated across the nail plate. Pharm. Res. 18:1468–1471 (2001).
D. Monti, L. Saccomani, P. Chetoni, S. Burgalassi, M. F. Saettone, and F. Mailland. In vitro transungual permeation of ciclopirox from a hydroxypropyl chitosan-based, water-soluble nail lacquer. Drug Dev. Ind. Pharm. 31:11–17 (2005).
Y. Myoung and H. K. Choi. Permeation of ciclopirox across porcine hoof membrane: effect of pressure sensitive adhesives and vehicles. Eur. J. Pharm. Sci. 20:319–325 (2003).
H. P. Baden, L. A. Goldsmit, and B. Fleming. Comparative study of physicochemical properties of human keratinized tissues. Biochim. Biophys. Acta 322:269–278 (1973).
R. C. Marshall and J. M. Gillespie. Keratin proteins of wool, horn and hoof from sheep. Aust. J. Biol. Sci. 30:389–400 (1977).
Johnson and Johnson patent. Method for increasing the permeability of horny human tissue. International Publication Number WO 99/4985 (1999).
I. Ghersetich, P. Teofoll, M. Gantcheva, M. Ribuffo, and P. Puddu. Chemical peeling: How, when, why?. J. Eur. Acad. Dermatol. Venereol. 8:1–11 (1997).
J. Alkiewicz, and R. Pfister. Atlas der Nagelkrankheiten. Schattauer–Verlag, Stuttgart, 1976, p. 8.
Y. Sun, J. C. Liu, J. C. T. Wang, and P. De Doncker. Nail penetration. Focus on topical delivery of antifungal drugs for onychomycosis treatment. In R. L. Bronaugh, and H. I. Maibach R. L. Bronaugh H. I. Maibach (eds.), Percutaneous Absorption. Drugs–Cosmetics–Mechanisms–Methodology, 3rd, Marcel Dekker Inc, New York, 1999, pp. 759–787.
F. Akomeah, T. Nazir, G. P. Martin, and M. B. Brown. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur. J. Pharm. Sci. 21:337–345 (2004).
Y. Kobayashi, T. Komatsu, M. Sumi, S. Numajiri, M. Miyamoto, D. Kobayashi, K. Sugibayashi, and Y. Morimoto. In vitro permeation of several drugs through the human nail plate: relationship between physicochemical properties and nail permeability of drugs. Eur. J. Pharm. Sci. 21:471–477 (2004).
C. R. Behl, G. L. Flynn, T. Kurihara, N. Harper, W. Smith, W. I. Higuchi, N. F. H. Ho, and C. L. Pierson. Hydration and percutaneous absorption 1. influence of hydration on alkanol permeation through hairless mouse skin. J. Invest. Dermatol. 75:346–352 (1980).
E. A. Olsen. Methods of hair removal. J. Am. Acad. Dermatol. 40:143–155 (1999).
S. Harrison and R. Sinclair. Hair colouring, permanent styling and hair structure. J. Cosmet. Dermatol. 2:180–185 (2004).
N. Nishikawa, Y. Tanizawa, S. Tanaka, Y. Horiguchi, and T. Asakura. Structural change of keratin protein in human hair by permanent waving treatment. Polymers 39:3835–3840 (1998).
C. Bolduc and J. Shapiro. Hair care products: waving, straightening, conditioning, and coloring. Clin. Dermatol. 19:431–436 (2001).
A. M. D. R. Gonsalves, R. A. W. Johnstone, M. M. Pereira, and J. Shaw. Dissociation of hydrogen–peroxide adducts in solution—the use of such adducts for epoxidation of alkenes. J. Chem. Res S8:208–209 (1991).
M. Egawa, Y. Ozaki, and M. Takahashi. In vivo measurement of water content of the fingernail and its seasonal change. Skin Res. Technol. 12:126–132 (2006).
X. Y. Hui, R. C. Wester, S. Barbadillo, C. Lee, B. Patel, M. Wortzmman, E. H. Gans, and H. I. Maibach. Ciclopirox delivery into the human nail plate. J. Pharm. Sci. 93:2545–2548 (2004).
D. A. Godwin and B. B. Michniak. Influence of drug lipophilicity on terpenes as transdermal penetration enhancers. Drug Devel. Ind. Pharm. 25:905–915 (1999).
Acknowledgements
Thank you to MedPharm Ltd for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khengar, R.H., Jones, S.A., Turner, R.B. et al. Nail Swelling as a Pre-formulation Screen for the Selection and Optimisation of Ungual Penetration Enhancers. Pharm Res 24, 2207–2212 (2007). https://doi.org/10.1007/s11095-007-9368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9368-3