The antianemic effect of an iron-containing composition that included iron-potassium-sodium hexacyanoferrate, iron sulfate, potassium sulfate, and pharmacopoeial micro-cellulose was studied in maleWistar rats. Iron deficiency anemia was induced by subcutaneous injection of deferoxamine (Desferal). The blood iron content, myelogram, erythrocyte count, and hematocrit were used as markers of anemia. The composition was administered perorally to rats with simulated anemia at a daily dose of 10 mg/kg for 3 and 12 days. The composition had three modifications differing in the percent content of iron and potassium salts. All modifications normalized hemoglobin, erythrocytes, iron in plasma, and hematocrit already on day 3 of the daily peroral administration. These parameters continued to increase by day 12 of the experiment. Modifications II and III showed more significant changes in the myelogram indicators as identifiable mitoses and a greater number of erythroid cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anemia is a pathological condition characterized by a reduced blood level of erythrocytes and/or hemoglobin that leads to poor oxygen transfer into tissues and the development of hypoxia, i.e., oxygen starvation of tissues [1].
According to the WHO, anemia in 1993 – 2005 afflicted 24.8% of the world population [2] and the incidence in both developing [3, 4] and developed countries [4] did not tend to decrease. Anemia in Russia was recorded for 6 – 40% of children according to different reports [5, 6].
The causes of anemia include iron deficiency due to blood loss, insufficient consumption of dietary iron, increased demand (in children and adolescents, during pregnancy and breast feeding), and problems with iron absorption in the intestines [1, 6, 7]. Iron deficiency anemia (IDA) comprises 90% of all anemias in children and up to 80% of all anemias in adults [1, 8]. Disturbed metabolism of compounds with IDA diminishes physical stamina, education effectiveness, and attention span [6, 9]. An iron deficit in IDA cases was demonstrated to lead to reduced immunological reactivity and increased susceptibility to infectious diseases at any age [9, 10]. Developmental abnormalities in children with IDA included delayed maturation of bone tissue and hard dental tissues [4]. Young children, women of reproductive age, pregnant women, and patients with chronic diseases are at risk of developing anemia [1,2,3,4, 6]. Regardless of the cause of IDA, the main treatment method is elimination of the iron deficit by prescribing peroral or parenteral (mainly intravenous and more rarely intramuscular) iron drugs [11, 12].
An iron-containing composition including iron-potassium-sodium hexacyanoferrate, iron sulfate, potassium sulfate, and pharmacopoeial micro-cellulose (hereinafter, the Composition) was formulated as a pharmacological composition for treating IDA and was designed for high medicinal effectiveness, good tolerability, and low toxicity as compared with existing preparations [13].
The previously developed form Bifege®, which was successfully used for a long time for veterinary purposes as a sorbent for heavy metals and cesium, can be considered the prototype of the Composition with analogous functionality [14,15,16]. All components included in it were allowed for use as drugs.
The goal of the present work was to study the possibility of improving the parameters of peripheral blood and hematopoietic marrow cell lines with experimental IDA by administering the Composition in three modifications differing in the percent contents of iron and potassium salts.
Experimental Chemical Part
The Composition containing KFe[Fe(CN)6] + FeSO4 chemically bonded to micro-cellulose was prepared by the original technology [13]. The Composition consisted of mixed iron-potassium-sodium hexacyanoferrate (A), FeSO4(B), K2SO4 (C), and pharmacopoeial micro-cellulose (D). Three modifications of the following formulations (A, B, C, D, wt%) were tested: Composition I, 64, 2, 4, 30; Composition II, 60, 3, 7, 30; Composition III, 61, 4, 5, 30. The formulations were prepared at SPE Eksorb Ltd. using chemically pure reagents.
Experimental Biological Part
The studies used 50 male Wistar rats (220 – 270 g) according to recommendations of international ethics committees (Committee Directive EC2010/63/EU). The studies were approved by the ethics committee of IIP, UrB, RAS (protocol No. 8 of Oct. 5, 2018).
The experimental technique was elaborated using 5 of 50 rats. The remaining animals were divided into groups of five each: 1, intact; 2 and 3, control with induced IDA for 3 and 12 d, respectively; 4, 6, and 8, treatment of anemia for 3 d by modifications I, II, and III, respectively; 5, 7, and 9, treatment of anemia for 12 d by modifications I, II, and III, respectively.
The method developed by Simanina, et al. in 2015 [17] in a local modification was used to model IDA. It consisted of dividing the total dose of deferoxamine not into two parts of 500 mg/kg each but into four parts of 250 mg/kg each to increase the duration of action of the deferoxamine. The modification of the Composition was administered daily per os calculated for 10 mg/kg of rat mass. The dose of the Composition was selected based on a test using an earlier analog, i.e., the veterinary drug Bifege, which is also manufactured by SPE Eksorb Ltd. According to the literature [14,15,16], the effective dose for small animals and birds is 10 – 15 mg/kg of body mass.
Reference groups in which the animals receive another drug known to treat anemia will be necessary in further studies on the advantages, specifics of the usage, and refinement of the dose of the new drug. However, results of preliminary studies of the drugs before their introduction into the clinic without a reference group with a known drug of analogous action [18,19,20] were published because this lengthened the experimental time and significantly increased the volume of analyzed results.
A weighed portion of the Composition was thoroughly mixed with distilled water to avoid settling. A volume of the suspension in proportion to the rat weight was administered.
The suspension was injected using an insulin syringe with a special fitting that avoided losing liquid during the injection. Blood samples were taken from animals of all groups under ether anesthesia from a tail vein for a complete blood count (CBC) on a Biocide Hycel Celly 70 automated hematological analyzer designed to analyze animal blood in experiments and veterinary medicine. The following CBC parameters were evaluated: RBC, red blood cell count (106/μL); Hb, hemoglobin content (g/dL); Hct, hematocrit (%); MCV, mean corpuscular (erythrocyte) volume (fL); MCH, mean corpuscular (erythrocyte) hemoglobin (pg); MCHC, mean corpuscular (erythrocyte) hemoglobin concentration (g/dL); and RDW, red-cell distribution width (%).
Animals were withdrawn from the experiment by an overdose of ether anesthesia.
Blood was centrifuged to determine the plasma iron content. Vital Diagnostics standard reagent kits (St. Petersburg, Russia) were used to determine iron. Optical density was measured on a Beckman DU-800 spectrophotometer (USA). Marrow was extracted from the femur to count myelograms. Marrow smears were fixed according to May–Gruenwald and stained according to Romanovskii–Giemsa. The myelogram was calculated after counting the total number of myelokaryocytes in marrow smears. Microscope studies used a Leica DM2500 microscope.
Nine series of experiments were conducted. Tables 1 and 2 summarize the results. The results were statistically analyzed using the Statistica 6.0 program (StatSoft Inc.) and were given as mean ± error. Nonparametric statistics were used because of the small set size. The statistical significance of differences obtained in compared independent sets was evaluated using the Mann–Whitney U-criterion; for dependent sets, the Wilcoxon W-criterion. The Kruskal–Wallis H-criterion (nonparametric analog of dispersion analysis) was used to estimate the effect of Composition modification and treatment time factors. Statistical hypotheses were checked at significance level 5% (p < 0.05).
Results and Discussion
Table 1 presents the red-blood parameters of the control animals (IDA, control, immediately after four injections of Desferal, 3 and 12 d after the start of IDA modeling) and animals with IDA that received modifications of the Composition.
The contents of erythrocytes and hemoglobin, hematocrit, and iron were significantly reduced for rats of control groups 2 and 3 (3 and 12 d) as compared to the corresponding parameters of intact rats (Mann–Whitney test, p < 0.05). These parameters were not normalized by the 12th day. Parameters of erythrocytes such as MCV, MCH, MCHC, and RDW did not deviate from the norms. Statistically significant differences between intact animals and animals of groups 2 and 3 were not observed (Mann–Whitney test, p > 0.05). However, the RDW index, which characterized the heterogeneity of the erythrocytes, was observed to increase on day 12 (Mann–Whitney test, U = 2.2, p = 0.02).
All red-blood parameters and the iron level for group 4 (IDA, administration of Composition I for 3 d) were at the level of the corresponding parameters of control groups (group 2, Mann–Whitney test, p > 0.05) before the start of treatment except for MCHC (Mann–Whitney criterion, U = 2.5, p = 0.01). A comparison of the parameters before and after treatment found significant increases of all parameters as compared to the initial level (Wilcoxon criterion, p < 0.05) except for RDW (Wilcoxon criterion, W = 0.67, p = 0.5). However, all red-blood parameters and the iron level in group 4 after treatment for 3 d corresponded to the normal values (intact animals, group 1, Mann–Whitney test, p > 0.05).
All red-blood parameters and the iron content in group 5 (IDA, administration of Composition I for 12 d) were at the level of the corresponding parameters of control groups (group 3, Mann–Whitney test, p > 0.05). A comparison of the parameters before and after treatment found statistically significant increases of contents of erythrocytes and hemoglobin, hematocrit, and iron as compared to the initial level (Wilcoxon test, p < 0.05) while MCV, MCH, MCHC, and RDW did not change (Wilcoxon criterion, p > 0.05). However, all red-blood parameters and the iron level in group 5 after treatment for 12 d corresponded to the normal values (intact animals, group 1, Mann–Whitney test, p > 0.05).
The duration of administration of Composition I did not affect the red-blood parameters and the iron level (Kruskal–Wallis test, p > 0.05).
All red-blood parameters and the iron level in group 6 (IDA, administration of Composition II for 3 d) before the start of treatment were at the level of the corresponding parameters of control groups (group 2, Mann–Whitney test, p > 0.05) except for MCV and MCHC, which differed significantly from the analogous value for the control group (Mann–Whitney criterion, p < 0.05). Acomparison of the parameters before and after treatment found significant increases of all parameters as compared to the initial level (Wilcoxon criterion, p < 0.05) except for MCH (Wilcoxon criterion, W = 1.48, p = 0.14) and RDW (Wilcoxon criterion, W = 0.13, p = 0.89). However, all red-blood parameters and the iron level in group 6 after treatment for 3 d corresponded to the normal values (intact group 1, Mann–Whitney test, p > 0.05).
All red-blood parameters and the iron level in group 7 (IDA, administration of Composition II for 12 d) before treatment were at the level of the corresponding parameters of the control group (group 3, Mann–Whitney test, p > 0.05). A comparison of the values before and after treatment found significant increases of all parameters as compared to the initial level (Wilcoxon criterion, p < 0.05) except for MCV, MCHC, and RDW (Wilcoxon criterion, p > 0.05). However, all red-blood parameters and the iron level in group 7 after treatment for 12 d corresponded to the normal values (intact animals, group 1, Mann–Whitney test, p > 0.05).
The duration of administration of Composition II affected the contents of erythrocytes and hemoglobin, hematocrit, and iron (increased) and MCV (reduced) and led to their changes with increased treatment time (Kruskal–Wallis test, p < 0.05). The other red-blood parameters did not depend on the treatment time for this modification of the Composition.
All red-blood parameters and the iron level in group 8 (IDA, administration of Composition III for 3 d) before the start of treatment were at the level of the corresponding parameters of the control group (group 2, Mann–Whitney test, p > 0.05) except for MCV and MCHC, which differed significantly from the analogous values of the control group (Mann–Whitney criterion, p < 0.05). Acomparison of the parameters before and after treatment found significant increases of all parameters as compared to the initial level (Wilcoxon criterion, p < 0.05) except for MCV (Wilcoxon criterion, W = 1.75, p = 0.08) and RDW (Wilcoxon criterion, W = 0.94, p = 0.35). Also, all red-blood parameters and the iron level in group 8 after treatment for 3 d corresponded to the normal values (intact animals, group 1, Mann–Whitney test, p > 0.05).
All red-blood parameters and the iron level in group 9 (IDA, administration of Composition III for 12 d) before treatment were at the level of the corresponding parameters of the control groups (groups 3, Mann–Whitney test, p > 0.05). A comparison of the values before and after treatment found significant increases of all parameters as compared to the initial level (Wilcoxon criterion, p < 0.05) except for MCV, MCHC, and RDW (Wilcoxon criterion, p < 0.05). However, all red-blood parameters and the iron level in group 9 after treatment for 12 d corresponded to the normal values (intact animals, group 1, Mann–Whitney test, p > 0.05).
The Composition modification for 3-day treatment affected only the MCH (Kruskal–Wallis criterion, H = 8.07, p = 0.02).
The type of modification of the Composition for 12-day treatment affected the contents of hemoglobin (Kruskal–Wallis criterion, H = 7.22, p = 0.03) and iron (Kruskal–Wallis criterion, H = 10.52, p = 0.0005). Modification I affected the hemoglobin content; modification III, the iron content.
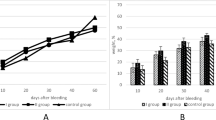
A complex analysis of the effect of the type of modification of the Composition over all obtained data, regardless of the treatment time, found a significant effect of the type of modification on the MCV (Kruskal–Wallis criterion, H = 6.37, p = 0.04) and the iron content (Kruskal–Wallis criterion, H = 6.66, p = 0.04) (Fig. 1).
Table 2 presents the myelograms of the experimental animals. The numbers of most erythrocyte precursors (pronormocytes, basophilic normocytes, polychromatophilic, oxyphilic) and the total number of erythroid cells decreased significant already on the 3rd day as compared to the corresponding parameters of intact rats. The numbers of basophilic and polychromatophilic cells in the control group with IDA remained below the norms on the 12th day. The numbers of erythroblasts and erythrocyte precursors decreased. The sum of all erythroid-type cells on the 12 day was significantly less than in intact rats, despite a certain natural reduction. Dividing cells were not observed in marrow of control animals. The number of mitoses was zero. The number of individual erythrocyte cell-precursors already on the 3rd day increased not only to the normal level but also rose above the norms as a result of peroral administration of the three modifications of the Composition to rats with IDA.
The numbers of all erythroid-type cells and individual fractions were still elevated on the 12th day in rats that received the three modifications of the Composition. The total number of erythroid cells was slightly greater in animals that received Compositions II and III on the 3rd and 12th days of IDA than in animals that received Composition I. Also, the number of mitoses sufficient to be detected increased in both studied periods for Compositions II and III, in contrast to I.
Thus, all three modifications of the Composition were effective in normalizing the iron level in blood plasma, hematocrit, and contents of hemoglobin and erythrocytes and increased erythroid cells in marrow already on the 3rd day of peroral administration at a daily dose of 10 mg/kg. Normalization of anemia was also found on day 12 of treatment with all three modifications of the Composition. However, signs of induced IDA after the fourth s.c. injection of Desferal by the modified method with a total dose of 1000 mg/kg in untreated animals persisted up to 12 d. Compositions II and III more than I changed the myelogram parameters. This was confirmed by the appearance of a rather large number of mitoses and large quantities of erythroid-type cells.
All modifications of the Composition in experiments with animals proved their efficacy for treating IDA and could be interesting for further development of a medicine.
References
A. I. Vorob’ev, Handbook of Hematology [in Russian], Vol. 3, Newdiamed, Moscow (2005).
B. de Benoist, E. McLean, I. Egli, et al., Worldwide Prevalence of Anaemia 1993 – 2005, WHO Global Database on Anaemia, WHO (2008).
S. R. Pasricha and H. Drakesmith, Hematol. Oncol. Clin. North Am., 30(2), 309 – 320 (2016).
V. Kumar, H. Haridas, P. Hunsigi, et al., J. Int. Soc. Prev. Community Dent., 6(5), 430 – 435 (2016); doi: 10.4103/2231–0762.192937.
M. Levi, M. Rosselli, M. Simonetti, et al., Eur. J. Haematol., 97(6), 583 – 593 (2016).
V. A. Rodionov and M. S. Agandeeva, Vestn. Chuvash. Univ., No. 3, 491 – 496 (2013).
A. G. Rumyantsev and Yu. N. Tokarev, Anemia in Children: Diagnosis, Differential Diagnosis, Treatment [in Russian], MAKS Press, Moscow (2004).
M. Nairz, I. Theurl, D. Wolf, et al., Wien. Med. Wochenschr., 166(13), 411 – 423 (2016); doi: https://doi.org/10.1007/s10354-016-0505-7.
I. S. Tarasova, Vopr. Sovrem. Pediatr., 10(2), 40 – 48 (2011).
Iron Deficiency Anaemia: Assessment, Prevention and Control. A Guide for Program Managers, World Health Organization, WHO / NHD / 01.3 (2001).
M. Auerbach and T. Deloughery, Hematology Am. Soc. Hematol. Educ. Program, No. 1, 57 – 66 (2016).
S. V. Moiseev, Klin. Farmakol. Ter., 21(2), 2 – 7 (2012).
V. P. Remez, RU Pat. 2,625,739, Jul. 18, 2016; PCT RU 2017/000563.
F. Akhmetzyanova and N. Mukhametgaliev, Molochn. Myasn. Skotovod., No. 1, 17 – 18 (2009).
P. N. Rubchenkov, L. L. Zakharova, G. A. Zhorov, and V. N. Obryvin, Vet., Zootekh. Biotekhnol., No. 4, 38 – 42 (2014).
A. N. Ratnikov, A. V. Vasiliev, R. M. Alexakhin, et al., Sci. Total Environ., 223(2–3), 167–176 (1998).
A. M. Dygai, E. V. Udut, et al., RU Pat. No. 2,553,334, Jun. 10, 2015.
J. E. Toblli, G. Cao, C. Rivas, et al., Int. J. Cardiol., 212, 84 – 91 (2016).
I. G. Danilova, T. S. Bulavintceva, I. F. Gette, et al., Biomed. Pharmacother., 201(95), 103 – 110 (2017).
H. Braxas, M. Rafraf, H. S. Karimi, et al., Can. J. Diabetes, 43(7), 490 – 497 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 55, No. 4, pp. 39 – 43, April, 2021.
Rights and permissions
About this article
Cite this article
Remez, V.P., Danilova, I.G., Gette, I.F. et al. Antianemic Effect of a Composition Containing Iron-Potassium-Sodium Hexacyanoferrate, Iron Sulfate, Potassium Sulfate, and Pharmacopoeial Microcellulose. Pharm Chem J 55, 350–354 (2021). https://doi.org/10.1007/s11094-021-02426-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-021-02426-9