The aim of the present research work was to formulate and characterize gastroretentive mucoadhesive tablets of lacidipine (LCDP) intended for the treatment of gastroparesis. Polymers such as sodium alginate, HPMC K4M, carbopol 974P, and chitosan were utilized in LCDP formulation to ensure gastric retention up to 8 h. Direct compression method was adopted in preparation of mucoadhesive tablets. Prior to compression, powder blends were evaluated in order to check their flow and compression properties. Fourier transform infrared spectroscopy and differential scanning calorimetry measurements were performed to assess the compatibility of LCDP with polymers. Tablets were characterized with respect to the uniformity of weight, hardness, friability, drug content, swelling index, surface pH and in-vitro drug release. All formulations exhibited acceptable physicochemical properties. Formulation F4 exhibiting in-vitro drug release of 95.510% was selected as the optimized formulation and was further characterized by scanning electron microscopy. In vitro dissolution data was fitted to various kinetic models, and formulation F4 was found to display non-Fickian mechanism of drug release. No major change was observed in drug release and drug content upon storage of optimized formulation under accelerated aging conditions. The obtained results revealed that carbopol 974P and chitosan can be used in combination to formulate gastroretentive mucoadhesive LCDP tablets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1. INTRODUCTION
Gastroparesis is considered to be a highly complicated and severe gastric motility disorder. Generally, strong smooth muscular contractions are responsible for propelling food throughout the gastrointestinal tract. However, the process of gastric emptying is delayed in gastroparesis because muscles present in the stomach walls fail to contract well enough [1]. Symptoms of this non-spontaneous opening of the pylorus are early satiety, nausea, bloating, indigestion and vomiting. Very limited choice of drugs is available for the treatment of gastroparesis, including metoclopramide, domperidone and erythromycin, which are often associated with a number of undesirable side effects [2].
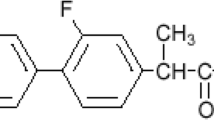
Lacidipine (LCDP), chemically diethyl(E)-4{2-[(tertbutoxycarbonyl) vinyl]phenyl}-1,4-dihydro-2,6 dimethylpyridine-3,5-dicarboxylate is a dihydropyridine calcium channel blocker (CCB) possessing antihypertensive activity. CCBs are known to have the ability to open closed pylorus by bringing about dilation of the smooth muscles thus enhancing gastric emptying in patients suffering from gastroparesis [3]. Reduction of the antihypertensive activity of LCDP was possible on designing its formulation as a controlled drug delivery system. The role of CCBs has been studied in the past for their effects on gastroparesis. Gastroretentive mucoadhesive microspheres of LCDP have been reported for the treatment of gastroparesis, in which the LCDP dose selected in the formulation of microspheres was 2 mg [4]. Research has also been carried out on nifedipine as a pylorospasm inhibitor [5].
Thus, there arose a need to develop gastroretentive mucoadhesive tablets for the treatment of gastroparesis. This dosage form is capable of being retained for a prolonged interval of time in the stomach thus providing drug release in a controlled manner. This prolonged retention also helps in improving the solubility of poorly soluble drug and thus its bioavailability by continuously supplying it at the absorption site [6].
Several high density mucoadhesive polymers were selected to be used in combination with LCDP, including sodium alginate, hydroxypropyl methyl cellulose K4M (HPMC K4M), carbopol 974P, and chitosan. All these polymers are capable of binding the gastric mucosa, thus prolonging the drug release along with localization at a specific site. Prepared LCDP tablets were characterized by organoleptic properties, thickness and diameter, uniformity of weight, friability, hardness, and the surface pH, swelling index, and in vitro drug release. The optimized formulation was also examined by scanning electron microscopy.
2. EXPERIMENTAL PART
2.1. Chemicals
Lacidipine was obtained as a gift sample from Unichem laboratories Ltd., Goa. Sodium alginate was obtained as a gift sample from Signet Chemical Corporation Pvt. Ltd., Mumbai. Hydroxy propyl methyl cellulose (HPMC K4M) was obtained as gift sample from Colorcon Asia Ltd., Goa. Carbopol 974P was obtained as a gift sample from Lubrizol advanced materials, Europe. Chitosan was purchased from Everest Biotech, Bangalore. Magnesium stearate and mannitol were purchased from SD Fine Chemicals Ltd., Mumbai.
2.2. Standard Drug Solutions
LCDP identification and standard calibration curve in 0.1 N HCl. Pure LCDP identification was performed by measuring its absorption maxima in 0.1 N HCl. By dissolving 10 mg of LCDP in 100 mL methanol, a 100 _g/mL stock solution was prepared. Then, serial dilutions of this stock solution were made to get a series of standard solutions ranging in concentration from 2 to 12 μg/mL. Standard solution (10 μg/mL) was scanned in UV range between 200 and 400 nm to determine λmax of LCDP in 0.1 N HCl. Further, absorbance of the standard solutions was measured at 240 nm against 0.1 N HCl which served as blank using UV-Visible spectrophotometer (Labindia UV 3092, Japan) [7, 8].
2.3. Drug – Excipient Compatibility Studies
Fourier transform infrared (FTIR) spectroscopy. FTIR spectroscopy with attenuated total reflectance (ATR) (Schimadzu corporation, affinity 1F, Japan) was used to evaluate the presence of any interactions between LCDP and polymers used in the formulations. A small quantity of powder sample was placed on the ATR cell and the arm of the ATR assembly was rotated so that a compact mass of the sample was formed on the cell. FTIR spectra of LCDP and physical mixtures of LCDP with polymers used in formulations were recorded in the 4000 – 400 cm-1 range. The spectra were analyzed and compared to the reference spectrum of pure LCDP to check for any alterations in appearance of the characteristic peaks [9].
Differential scanning calorimetry (DSC). DSC was used as a screening method for checking the compatibility of pure drug with polymers used in formulations. Samples were analyzed using DSC-60 (Shimadzu, Japan). Powder samples were carefully placed in aluminium pans and crimped. An empty sealed pan served as the reference. Samples were heated from 30 to 250°C at a scanning rate of 10°C/min. Thermal analysis of the samples was performed by measuring the heat flow as a function of temperature. DSC thermograms were recorded and analyzed as described in [10].
Pre-compression parameters. Prior to tablet compression, flow properties of individual powder blends for all formulations need to be investigated. Certain characteristic parameters of the powder blends were evaluated, including bulk density, tapped density, Hausner’s ratio, angle of repose, and compressibility index [11,12,13].
Preparation of gastroretentive mucoadhesive tablets. All ingredients were sifted through 60-mesh sieve. Accurately weighed quantities of LCDP along with polymers present in the formulation, mannitol, and magnesium stearate
(Table 1) were mixed together for approximately 10 min in a mortar adopting geometric progression method. Subsequently, each powder blend was compressed into tablets in a 10 Station tablet compression machine (Lab Press, Shakti Pharmatech Pvt. Ltd., Gujarat). Tablet compression was carried out using a punch of 8 mm diameter to obtain a tablet weight of 150 mg [14].
2.4. Evaluation of Gastroretentive Mucoadhesive Tablets
General appearance. Mucoadhesive tablets prepared were characterized by color, presence or absence of odor, and surface texture.
Uniformity of weight. Twenty tablets of each of the formulations were randomly taken. weighed individually, and the average tablet weight was calculated [15, 16].
Thickness and diameter. Vernier caliper (Mitutoyo, Japan) was used for the measurement of tablet thickness and diameter in a set of randomly taken tablets [16].
Hardness. LCDP mucoadhesive tablet hardness was evaluated using an automatic hardness tester (Labindia, TH 1050S, Mumbai). For each formulation, crushing strength of 3 tablets was determined and average hardness was calculated [16].
Friability. Twenty preweighed tablets were rotated in the Roche friabilator (VFT-DV Veego, Mumbai) for 4 min at 25 rpm. This was followed by dusting and re-weighing of tablets, after which percentage friability was calculated [16, 17].
Drug content uniformity. Three tablets of each formulation were randomly taken, powdered and mixed uniformly in a mortar. Powder amount corresponding to 2 mg of LCDP was placed in a 100 mL volumetric flask and dissolved in 10 mL methanol, followed by making up the volume with 0.1 N HCl. The solution was shaken for 15 min and filtered using Whatmann filter paper. Then, 1 mL of filtrate was diluted with 0.1 N HCl in a 25 mL volumetric flask and analyzed spectrophotometrically at 240 nm [16, 18, 19].
Surface pH. Surface pH of LCDP tablets for all formulations was determined using a pH meter (Esico 1010, Himachal Pradesh) with combined glass electrode. Tablets were placed into petri dishes containing 0.1 N HCl and allowed to swell for 2 h. Excess HCl present on the surface of tablet was removed using a tissue paper. Tablet surface was brought in contact with the electrode and permitted to equilibrate for 1 min, after which the and surface pH was determined [20].
Swelling index. For determining the swelling index, each tablet was weighed and placed on a preweighed 40 mesh size wire. The wire mesh holding the tablet was then submerged into 20 mL 0.1 N HCl previously placed in a petri dish. Tablets were removed after 1, 2, 4, 6 and 8 h and rendered free of any excess solvent present on the surface using tissue paper [21]. Then, tablets were again weighed post swelling, and the swelling index (S.I.) was estimated by formula:
Scanning electron microscopy (SEM). SEM studies of dry intact mucoadhesive tablet and tablet post swelling in 0.1 N HCl for 8 h were performed for optimized formulation F4 using Zeiss Evo-18 scanning electron microscope. Since the image formation by SEM requires high vacuum, the samples post swelling in 0.1 N HCl, must be totally dried and desiccated before being placed in the vacuum chamber. As prepared tablets were fixed on a sample holder and SEM images were taken by operating at an accelerating voltage of 15 kV [22].
In vitrodissolution studies.In vitro drug release study of LCDP mucoadhesive tablets was performed using USP dissolution apparatus II (Labindia, DS/8000, Mumbai) using 500 mL 0.1 N HCl as dissolution medium maintained at 37 ± 0.5°C with paddle speed set at 50 rpm. Aliquots were collected at 1, 2, 4, 6 and 8 h intervals and analysed spectrophotometrically at 240nm [23]. In- vitro dissolution data was fitted into various kinetic models to understand the mechanism of drug release [24,25,26].
Short-term stability study. Few tablets of optimized formulation F4 were packed in amber colored USP Type I glass vials, sealed hermetically with bromobutyl rubber stopper and crimped with aluminium cap. Samples were kept at accelerated aging conditions of 40 ± 2°C/75 ± 5 %RH for 90 days in photostability chamber (Osworld Scientific Equipment Co. Pvt. Ltd., Mumbai). Samples were withdrawn after 30, 60 and 90 days exposure and analyzed for drug content and in-vitro dissolution rate [27, 28].
3. RESULTS AND DISCUSSION
3.1. Preformulation Studies
Drug identification and standard calibration curve in 0.1 N HCl. Absorption maximum of LCDP was found to be 240 nm in 0.1 N HCl on scanning over 200 – 400 nm interval (Fig. 1). The calibration curve displayed linearity over a concentration range of 2 – 12 μg/mL with correlation coefficient R2 = 0.9996 (Fig. 2).
3.2. Drug – Excipient Compatibility
Fourier transform infrared spectroscopy. FTIR spectra of LCDP showed characteristic bands at 3344.57 cm–1 (N-H stretch), 1701.22 cm–1 (C=O stretch), 1672.28 cm–1 (C=C stretch) and 1188.15 cm–1 (C-O stretch). On comparison of the spectrum of pure drug with the spectra of physical drug mixtures with polymers, it was found that characteristic IR absorption peaks in the drug spectrum were close to those in the spectra of physical mixtures (Fig. 3). This result indicated the absence of any interaction between the parent drug and formulation excipients (Table 2).
Differential scanning calorimetry. DSC curve of LCDP exhibited a distinct endothermic peak at 181°C which corresponded to the melting transition temperature. DSC thermograms of 1:1 physical mixtures of drug with polymers (sodium alginate, HPMC K4M, carbopol 974, chitosan) are presented in Fig. 4, where the endothermic peaks of melting are observed at 178.76°C, 178.07°C, 181.02°C and 177.63°C, respectively. Thus, it can be concluded that no incompatibility exists between LCDP and polymers present in the formulations. DSC curve of the drug – polymer blend of optimized formulation also displayed no significant changes in peak positions.
Pre-compression parameters. Bulk density of formulated batches was in the range of 0.255 – 0.621 g/cm3 and the tapped density was within 0.306 – 0.722 g/cm3. All formulations showed the angle of repose values ranging within 25.375 – 27.813°. The Hausner’s ratio for the powder blends of formulations F1 – F8 was computed from the obtained bulk and tapped density values and were found to be ranging within 1.082 – 1.244. The percentage compressibility of all formulation powder blends were in the range of 7.664 – 19.763%. Collectively, results indicated that the powder mixtures possessed sufficient compression and flow properties needed for direct compression into tablets (Table 3).
Preparation of gastroretentive mucoadhesive tablets. Eight prototype tablet formulations F1 – F8 were developed containing 2 mg of drug LCDP and mucoadhesive polymers in various combinations. The weight of tablets was maintained constant at 150 mg for all formulations. Polymers sodium alginate, HPMC K4M, carbopol 974P, and chitosan were selected for use in various ratios in these formulations for their appropriate mucoadhesive characteristics. Mannitol was used as a tablet diluent to maintain the constant weight of the tablet and facilitate an increase in the flowability and compressibility of the ingredients. Magnesium stearate employed in the formulation served a lubricant. Gastroretentive mucoadhesive tablets were successfully prepared by direct compression method.
3.3. Evaluation of gastroretentive mucoadhesive tablets
General appearance. All tablets were round in shape, bisected on one side with a flat surface and had a smooth texture. The tablets were white to off-white in color. Tablets containing chitosan displayed some degree of off whiteness. The tablets did not exhibit any odor and no physical flaws were visible (Fig. 5).
Uniformity of weight. The prepared tablets passed the test for uniformity of weight as none of the formulations exceeded the limit (Table 4).
Thickness and diameter. The thickness of tablets prepared from all formulations ranged within 2.706 – 2.768 mm and their diameters ranged within 8.117 – 8.135 mm as reported in Table 4. Thickness and diameter were thus found to be uniform in all formulations.
Hardness. Formulated tablets were evaluated for crushing strength which was observed to be within the range of 4.951 to 6.010 kg/cm2. From the values given in Table 4, it is evident that hardness of tablets increased directly with increase in polymer concentration in the formulation. Hardness of the tablets was therefore satisfactory signifying good mechanical strength and ability to endure any stressful circumstances that arise while handling.
Friability. The total weight loss of the tablets post rotation in the friabilator for 100 revolutions was in the range of 0.004 – 0.638% (Table 4). The results obtained indicate that all the tablet formulations show friability less than 1%, thus ensuring good mechanical resistance.
Drug content uniformity. LCDP tablets must contain not less than 95% and not more than 105% of the stated amount of LCDP. The drug content in formulations F1 – F8 was found to be within the range of 97.012 – 103.94% as reported in Table 4. All formulations fall within the acceptable limit and therefore exhibit uniformity in drug content.
Surface pH. After swelling for 2 h, the tablets were found to display surface pH values very close to that of stomach pH. The pH of the formulations ranged within 1.246 – 1.536 as displayed in Table 4. Hence, it is clear that the tablet formulations will not bring about any irritation at the surface of the gastric mucosa when present in stomach after administration.
Swelling index. A direct relationship was found to exist between the concentration of polymer and swelling index. Formulations F7 and F8 showed swelling index of 4.128 and 4.35, respectively, at the end of 6 h exposure. This high degree of swelling could be attributed to the high amount of polymer present in these formulations along with absence of diluents. Formulation F4 containing chitosan and carbopol 974P showed a swelling index of 4.075 confirming the good swelling properties of these polymers. The swelling index varied in the order of F7 > F8 > F4 > F3 > F6 > F2 > F5 > F1 as seen in Fig. 6.
Scanning electron microscopy. Surface of dry intact F4 tablet was found to be fairly smooth without presence of any pores. The SEM image of the tablet after swelling for 8 h shows presence of pores (Fig. 7). This is likely to occur due to diffusion and erosion mechanism of LCDP release from the formulation.
In vitrodissolution studies. At the end of 8 h exposure, tablets of formulations F1 – F8 displayed percentage cumulative drug release within the range of 84.072 – 98.276%.
The ratio of polymer concentrations used in all formulations successfully controlled release of drug up to 8 h. From the results, it was found that formulations F3 and F4 containing polymers carbopol 974P and chitosan show maximum drug release of 98.276 and 95.510%, respectively. An electrostatic interaction is likely to exist between the two polymers as a result of their opposite charges. Thus, a strong attraction is present between components of these formulations. This further enhances the swelling characteristics and helps in maintaining the integrity of tablet. A decrease in in-vitro release of LCDP was also evident with increase in polymer concentration. This may be the result of an increase in diffusional path length which needs to be travelled by drug molecules.
On subjecting drug release data to Korsmeyer- Peppas model, good linearity was seen in R2 values (Fig. 8). By using Korsmeyer- Peppas equation, diffusion exponent n values of all formulations were determined (n = 0.557 – 1.071) as presented in Table 5. Formulations F1, F2, F5 and F6 display n values characteristic of super case-II transport mode. Whereas n values of formulations F3, F4, F7 and F8 suggest anomalous kinetics (non-Fickian diffusion) as the mechanism of LCDP release. Thus the drug release from formulations can be explained by more than one mechanism – i.e., diffusion and erosion. Super case-II transport indicates that the drug release occurs by swelling, relaxation, and erosion of mucoadhesive polymer with zero-order release kinetics. Non-Fickian or anomalous transport is known to be a conjunction of both diffusion and erosion based controlled release of drug. Kinetic constant k values must increase with increase in solubility of the matrix, which is manifested in k values of Peppa’s plot listed in Table 5.
Short-term stability study. On testing the stability of optimum formulation F4, results of which are displayed in Table. 6, it was found that no major change in drug content and in vitro drug release took place upon storage for 3 months. Thus, the formulation can be considered to possess good storage stability.
4. CONCLUSION
The proposed gastroretentive mucoadhesive drug delivery system has the ability to ensure sustained delivery of LCDP for a prolonged interval of time. The dose of LCDP selected for the formulation was 2 mg. FTIR spectra and DSC thermograms of drug and polymer mixtures revealed that there was no incompatibility between drug and excipients. The precompressed powder blends exhibited good flow properties. Gastroretentive mucoadhesive tablets were formulated using the polymers sodium alginate, HPMC K4M, carbopol 974P and chitosan in various combinations and concentrations by direct compression method. The drug release data revealed that the type and concentration of polymer directly influenced tablet physico-chemical properties and release of LCDP. Formulation F4 containing polymers chitosan and carbopol 974P was found to be the optimum formulation showing good physico-chemical and swelling properties and providing optimum release of LCDP for a period of 8 h. On fitting the drug release data of formulation F4 to Korsmeyer model, the mechanism of drug release was established to be non-Fickian diffusion. Controlled release of the drug occurred via both diffusion and erosion mechanisms. Thus, we conclude that the proposed gastroretentive mucoadhesive formulation of LCDP can be successfully utilized in the treatment of gastroparesis.
References
M. Camilleri and J. R. Malagelada, Eur. J. Clin. Invest., 14, 420 – 427 (1984).
H. P. Parkman,W. L. Hasler and R. S. Fisher, Gastroenterology., 127, 1592 – 622 (2004).
S. Sultana, S. Talegaonkar, D. Singh, et al., Saudi Pharm. J., 21, 293 – 304 (2013).
S. Sultana, Bhavna, Z. Iqbal, et al., J. Microencapsul., 26, 385 – 393 (2009).
S. Sultana, S. Talegaonkar, B. Ray, et al., Drug Dev. Ind. Pharm., 44, 1171 – 1184 (2018).
C. M. Lopes, C. Bettencourt, A. Rossi, et al., Int. J. Pharm., 510, 144 – 158 (2016).
S. Yogesh, V. Vitthal, D. J. Chaudhari Pravin, Pharm. Res., 3, 157 – 161 (2014).
P. T. Nagaraju, K. P. Channabasavaraj, Shantha Kumar, Int. J. PharmTech. Res., 3, 18 – 23 (2011).
R. V. Trivedi, J. H. Borkar, J. B. Taksande, et al., Int. J. Res. Pharm. Sci., 8, 608 – 615 (2017).
S. Patil and G. S. Talele, Drug Deliv., 22, 312 – 319 (2015).
L. Lachman, H. A. Lieberman and J. L. Kaling, in: The Theory and Practice of Industrial Pharmacy, K. E. Avis (Ed.), 3rd Edition, Varghese Publishing House: Bombay (1991), pp. 67 – 74.
United States Pharmacopeia 36, National Formulary 31, US Pharmacopeial Convention: Rockville (2013), The Standard of Quality, Powder Flow, Volume 2 (1174): 891 – 893.
H. H. Gangurde, M. A. Chordiya, S. Tamizharasi, et al., Int. J. Pharm. Investig., 1, 148 – 153 (2014).
A. ASyed and A. R. Syed, Int. J. Pharmacol. Res., 6, 176 – 182 (2016).
British Pharmacopoeia (BP 2013), Appendix XIIC, British Pharmacopoeia Commission: London, The Stationery Office Vol. V:A 353 – 354.
L. Lachman, H. A. Lieberman and J. L. Kaling, The Theory and Practice of Industrial Pharmacy, K. E. Avis (Ed.), 3rd Edition, Varghese Publishing House: Bombay (1991), pp. 296 – 302.
G. Arora, K. Malik, I. Singh, et al., J. Adv. Pharm. Tech. Res., 2, 163 – 169 (2011).
British Pharmacopoeia (BP 2013), Lacidipine Tablets, British Pharmacopoeia Commission: London: The Stationery Office Vol III: 3063 – 3064.
A. A. Shaikh, Y. D. Pawar and S. T. Kumbhar, Int. J. Pharm. Sci. Res., 3, 1411 – 1416 (2012).
G. D. Sankar, G. Sandeep, G. Deepak, et al., J. Pharm. Biomed. Sci., 12, 1 – 3 (2011).
R. Gunda, V. N. Karri, M. G. Mahendran Baskaran, and G. Ganesh, Int. J. Pharm. Pharm. Sci., 6, 422 – 427 (2014).
N. G. Sonani, S. P. Hiremath, F. S. Dasankoppa, et al., Pharm. Devel. Technol., 15, 178 – 83 (2010).
British Pharmacopeia (BP 2013), Laboratory Standard for UK Medicinal Products and Pharmaceutical Substances, BP Pharmacopoeia Commission: London, Vol. V: 332 – 334.
S. Dash, P. N. Murthy, L. Nath and P. Chowdhury, Acta. Pol. Pharm., 67, 217 – 223 (2010).
P. Costa and J. M. Lobo, Eur. J. Pharm. Sci., 13, 123 – 133 (2001).
K. H. Ramteke, P. A. Dighe, A. R. Kharat, and S. V. Patil, Sch. Acad. J. Pharm., 3, 388 – 396 (2014).
ICH Guidelines, Q1A (R2), International Conference on Harmonization, IFPMA: Geneva (2005).
S. K. Singh, S. B. Bothara, S. Singh, et al., Int. J. Pharm. Sci. Nanotechnol., 3, 152 – 158 (2010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Godinho, S., Harmalkar, D., Kumar, L. et al. Formulation and Characterization of Gastroretentive Mucoadhesive Tablets for Treatment of Gastroparesis. Pharm Chem J 53, 230–238 (2019). https://doi.org/10.1007/s11094-019-01985-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-01985-2