Compound T1059 (1-cyclohexanecarbonyl-2-ethylisothiourea hydrobromide) was found to be water soluble, moderately toxic (i.p. LD16 and LD50 of 274 and 380 mg/kg), and capable of competitively inhibiting nitric-oxide synthase (NOS) activity with significant selectivity toward inducible and endothelial isoforms (IC50 for nNOS, iNOS, and eNOS of 60.3, 1.8, and 3.2 μM, respectively). T1059 was rapidly absorbed after a single i.p. injection (dose range 10 – 30 mg/kg) and distributed in tissues, causing pronounced suppression of endogenous NO production. T1059 at a dose of 10 mg/kg in normotensive anesthetized Wistar rats produced long-term vasoconstriction. The observed changes in vascular tone did not influence inotropic heart function but were accompanied by weak bradycardia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Vasopressors (α1-adrenomimetics and peptide vasoconstrictors) play important roles in treating acute and chronic hypotension [1, 2]. However, they frequently fail to meet clinical requirements, especially during the prehospital phase, because of pharmacokinetic features (short duration of action) and broad spectra of side effects [3, 4]. Also, the weakness of prehospital medicines for serious cases associated with blood loss, especially where circulating blood cannot be supplemented, is mainly responsible for negative outcomes of acute hypotension. For example, data from S. M. Kirov Military Medical Academy, Ministry of Defense, Russian Federation, indicate that up to 35% of irretrievable personnel losses in Afghanistan were due to ineffective prehospital aid for the wounded and the development of serious hemorrhagic shock [5]. Furthermore, treatment of vascular dysfunctions resistant to existing vasopressors (sepsis, endotoxemia, serious hemorrhages, etc.) remains an extremely complex problem. The development of vasoplegic syndrome in the late stages of shock of any etiology increases the hospital lethality of such conditions by 50 – 65% [1, 6, 7]. These circumstances necessitate the development of new original vasopressors capable of maintaining effectively a target arterial pressure (AP) and thereby helping to save lives.
Biologically active compounds that influence endogenous nitric oxide (NO) production by vascular endothelium are considered a possible platform for constructing innovative vasopressors. Endothelium-produced NO is now known to be a universal mediator (NO/cGMP-pathway) for manifestation of the physiological and pathological vascular effects of vasodilators such as acetylcholine, bradykinin, estrogens, insulin, tissue growth factors, inflammatory cytokines, and microbial pathogens and endotoxins [8]. Many experiments demonstrated convincingly that NO synthase (NOS) inhibitors could considerably increase vascular tone [9,10,11,12,13].
Moreover, experimental results indicating that NOS inhibitors could suppress circulatory disorders caused by sepsis, endotoxemia, and blood loss were not confirmed clinically. Thus, results of Phase III clinical trials of L-NMMA (NG-monomethyl-L-arginine, tilarginine) enlisting patients with serious septic shock were unsatisfactory. Although L-NMMA (5 – 20 mg/kg for 7 – 14 d) without other vasopressors maintained AP at 70 mm Hg and reduced lethality from multiorgan dysfunction, overall lethality increased by 10% as compared with a placebo group (because of an increase of cardiovascular deaths) in the second week of L-NMMA administration. As a result, the trial was halted [14, 15]. Possible causes of the failure were identified as nonselective inhibitory activity of L-NMMA for NOS isoforms and its ability to inactivate irreversibly NOS [15, 16]. L-NMMA is structurally very similar to L-arginine and competitively inhibits at practically the same level all NOS isoforms. Part of the L-NMMAis metabolized by iNOS and nNOS into the N-hydroxy derivative that reacts covalently with the heme active center of these enzymes and inactivates them [17].
Therefore, it seemed promising to design drugs based on reversible selective iNOS and eNOS inhibitors because the endothelial and inducible NOS isoforms are known to play roles in regulation of vascular tone under normal and pathological conditions [8, 17].
An expansive series of N-acyl-S-alkyl-substituted isothioureas (ITUs) that were NOS inhibitors were recently synthesized at the Radiation Pharmacology Laboratory, A. F. Tsyb Medical Radiological Research Center (MRRC) [10, 18, 19]. Screening of a series of ITU derivatives selected the lead compound T1059 (1-cyclohexanecarbonyl-2-ethyl-ITU hydrobromide). The goal of the present study was to study systematically the NOS-inhibitory and vasopressor activities of T1059.
Experimental Part
T1059 was synthesized using methods developed in the MRRC and patented in Russia [19]. The purity of T1059 was monitored using TLC on Silufol UV-254 plates (Czech Republic) and C6H6—EtOH—Et3N (9:1:0.1). PMR spectra were taken in DMSO-d6 with TMS standard on a DRX-500 spectrometer (Bruker, Germany) at 500 MHz. T1059 was water soluble and was used in all in vitro and in vivo experiments as aseptic aqueous solutions prepared ex tempore.
Tests in vivo used male white outbred mice (4 – 5 months, 28 – 32 g), male F1 (CBA×C57BL6j) mice (2 – 2.5 months, 19 – 22 g), and male Wistar rats (3 – 4 months, 230 – 320 g). Animals were obtained from the nursery at the SCBMT, FMBA of Russia, had veterinary certificates, and were quarantined in the vivarium at the MRRC. Animals were kept in T-3 and T-4 cages with natural lighting and 16-fold hourly ventilation at 18 – 20°C and relative humidity 40 – 70% on bedding of sterilized wood shavings. Animals had free access to food and water and were fed standard (GOST R 50258-92) PK-120-1 briquette feed (OOO Laboratorsnab, RF). Operations with laboratory animals were performed according to accepted standards for treatment of animals and were based on standard operating procedures adopted at the MRRC that corresponded to rules of European Convention ETS 123.
Acute toxicity of T1059 was studied in male white outbred mice using i.p. injection of T1059 at doses of 100 – 1,000 mg/kg in solution (0.1 – 0.4 mL). Test animals were observed for 15 d. Acute toxicity parameters were calculated using Litchfield—Wilcoxon probit analysis.
The NOS-inhibitory activity and selectivity of T1059 were quantitatively evaluated in vitro using a radiometric method with recombinant NOS isoforms (Enzo Life Sciences Inc., USA) and the reagents and measurement protocol of an NOS Activity Assay Kit (Cayman Chemical, USA). The catalytic activity of the NOS isoforms was studied in the presence of various concentrations of T1059 (10–1 – 103 μM) and was estimated as the accumulation rate of [3H]-L-citrulline [20]. Radiometric measurements were made in an LS-6000 scintillation counter (Beckman Coulter, USA). The parameter of the inhibitory activity was IC50, the T1059 concentration suppressing NOS isoform activity by 50%. Selectivity was judged from the ratios of IC50 values for the isoforms. This same method also estimated the competitive nature of the NOS-inhibitory activity of T1059 using the change of the effect with increasing excess of L-arginine.
The NOS-inhibitory activity of T1059 was studied in vivo using male F1 (CBA×C57BL6j) mice and EPR spectrometry with diethyldithiocarbamate trap (Sigma-Aldrich, USA) [21]. Test groups of 7 – 8 mice were used. The influence of T1059 on spontaneous NO synthesis and that induced by lipopolysaccharides (LPSs) from E. coli (0111:B4; Sigma-Aldrich, USA; 2 mg/kg i.p.) in liver and brain of test animals was studied using the published methods [10, 22]. EPR spectra were recorded at 77 K and 9.61 GHz using an EMX-8 spectrometer (Bruker, Germany). NO contents in tissues were estimated from the amplitude of the first component of the triplet in the spectrum.
The influence of T1059 on the NO2–/NO3– contents in tissues was studied using male Wistar rats and a photometric method based on Griess reagent (Promega, USA) [23]. Test groups of 7 – 8 male Wistar rats (3 – 3.5 months, 200 – 230 g) were used. The NO2–/NO3– contents in blood plasma, liver, and cerebellum of untreated rats and those receiving E. coli LPS (5 mg/kg, i.p.) were assayed 24 h after a single i.p. injection of T1059 (10 mg/kg). Blood plasma and tissue homogenates were deproteinized by ZnSO4. Nitrates were reduced by metallic Zn. Optical density was measured at 520 nm using a KFK-3-01 photometer (ZOMZ, RF).
Cardiovascular effects of T1059 were studied using eight male Wistar rats (3.5 – 4 months, 240 – 320 g). Anesthetized animals (thiopental sodium; 60 mg/kg, i.p.; OAO Sintez, RF) underwent tracheotomy and tracheostomy before catheterization of the left jugular vein to measure central venous pressure (CVP) and of the left carotid artery to measure AP. Heparin (100 U) was injected i.v. EKGs (standard leads), heart rates (HR), respiration rates (RR), systolic and diastolic arterial pressure (SAP and DAP), and CVP were recorded after the animals stabilized using an RM-6000 polygraph (Nihon Kohden, Japan). Instantaneous blood volume was measured using thermodilution with the thermistor placed in the esophagus [24]. The parameters were recorded for 120 min after a single injection of T1059 (i.p., 10 mg/kg) that was the optimal vasopressor dose for T1059 for such animals and such administration mode according to preliminary investigations. Hemodynamics were analyzed by calculating standard parameters such as ejection volume (EV), cardiac index (CI), and specific peripheral vessel resistance (SPVR).
Differences between groups were checked statistically using nonparametric criteria. The Mann—Whitney U-criterion was used to compare pairs; Kruskal—Wallis one-way analysis of variance by ranks and Dunnett’s and Dunn’s tests, for multiple comparisons [25]. In all instances, differences were considered statistically significant for p < 0.05.
Results and Discussion
Figure 1 shows the synthetic scheme for T1059. Thus, thiourea (TU) with R1 = cyclohexane was reacted with an equimolar amount or excess (up to 100%) of alkylating agent R2–X in which R2 was ethyl and X, a leaving group (halide, sulfate, alkane/arenesulfonate, triflate, alkylsulfate, alkyl/dialkylphosphate) in a polar inert solvent (DMSO, sulfolane, Me2CO, MeCN) at temperatures from ambient or reflux.
Example synthesis. A mixture of 1-cyclohexanecarbonylthiourea (3.7 g, 20 mmol), ethyl bromide (4.4 g, 40 mmol), and anhydrous MeCN (10 mL) was heated in a sealed ampul on a boiling-water bath for 2 h. The precipitate was filtered off and recrystallized twice from 4-methyl-2-pentanone. Yield 2.8 g (48.5%).
PMR spectrum (500 MHz, DMSO-d6, δ): 1.3 (m, 9H); 1.62 – 1.84 (m, 5H); 3.65 (m, 1H); 10.8 (br, 3H). C10H19BrN2OS. mp 123 – 125°C. Rf 0.35 (C6H6—EtOH—Et3N, 9:1:0.1).
The quantitative content of 1-cyclohexanecarbonyl-2-ethylisothiourea hydrobromide in the substance was ≥ 95%.
Acute toxicity tests of T1059 showed that it corresponded to hazard class 3 or moderately hazardous compounds [26]. The LD16, LD50 ± m, and LD84 values for a single i.p. injection to outbred mice were 274, (380 ± 32), and 523 mg/kg.
Animals became lethargic and stationary and the respiration rate increased moderately 2 – 3 min after i.p. injection of T1059 at doses of 100 – 1,000 mg/kg. Doses >250 mg/kg caused motor disturbances and tremors in some of the mice at 10 – 20 min post-injection. Animals that received high doses of T1059 developed adynamia accompanied by suppressed reaction to tactile, painful, and audible stimuli. The worsening condition manifested as reduced RR and development of convulsive seizures, at the peak of which the animals perished. The lethal action of T1059 appeared in the first 2 – 5 h at all used doses. No signs of intoxication and changes of behavior, locomotor activity, external appearance, and demand for food as compared with untreated animals were observed in surviving mice 1 d after the injection and for the subsequent 15 d.
T1059 exhibited dose-dependent inhibition of all NOS isoforms in in vitro tests (Table 1). The quantitative parameters of T1059 inhibitory activity indicated that the N-acyl radical in this compound reduced in general its affinity for NOS as compared with S-ethyl/isopropyl-ITU with IC50 values in the μM range [27, 28]. The N-substituent also had a positive effect, the 1-cyclohexanecarbonyl hindered interaction of T1059 primarily with nNOS. Whereas S-ethyl/isopropyl-ITU were practically nonselective NOS inhibitors, T1059 showed substantial (by 15 – 30 times) statistically significant (p < 0.01) selectivity for inhibition of iNOS and eNOS. The inhibitory activity of T1059 for these NOS isoforms was quantitatively comparable with those of well-known NOS inhibitors such as L-NMMA, L-NNA, and L-NAME [28,29,30], which are currently regarded abroad as new drug platforms.
A comparison of the effective IC values of T1059 and its lethal doses showed that it could have a significant (>50%) inhibitory influence on eNOS and iNOS at concentrations 2 – 3 orders of magnitude less than toxic ones. Therefore, T1059 was expected to be highly physiologically active at relatively safe doses.
Compound T1059, like S-ethyl/isopropyl-ITU, exhibited fully competitive inhibitory activity in these in vitro tests. Its influence on all NOS isoforms was completely neutralized already with an eight-fold excess of L-arginine (Table 2).
NO production in the liver and brain of test mice with LPS effects was reduced statistically significantly in the first hour by a single i.p. injection of T1059 (10 mg/kg) (Table 3). Spontaneous NO production in brain tissues that were unaffected by endotoxins in these timeframes was suppressed by T1059. It blocked both spontaneous and LPS-induced NO synthesis in the liver. Table 4 shows that T1059 (10 mg/kg) at 1 d after a single i.p. injection reduced statistically significantly the NO2–/NO3– contents in various tissues of test animals. The reductions correlated with reduced endogenous NO production.
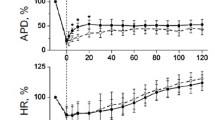
According to the results, T1059 (10 mg/kg ≈ 1/27 LD16) after a single i.p. injection had a long-term (80 – 90 min) vasoconstricting effect that manifested as a pronounced (by 35 – 50%) increase of total peripheral vessel resistance and increased DAP (Fig. 2).
Influence of T1059 (10 mg/kg) after a single i.p. injection on hemodynamics of anesthetized normotensive Wistar rats. Data were normalized to the initial value and expressed in %. Error bars correspond to M ± S. D. *Statistically significant differences (p < 0.05) by Dunnett’s test from the starting values.
Hemodynamic changes were biphasic in nature. The first 10 min gave a statistically significant hypertensive effect (DAP and SAP increased by 15 – 20% from the starting level) with slowing of the HR by 12 – 15%. Later, bradycardia starting at 10 min was accompanied by progressive reduction of SAP with elevated DAP and SPVR. At 90 min post-injection of T1059, peripheral vessel resistance began to subside so that the SPVR returned to the starting level by 120 min. Reduction of vessel tone was associated with normalization of the AP and HR.
The results indicated that T1059, like several other NOS inhibitors (aminoguanidine, L-NMMA, L-NNA, L-NAME, L-TC) that suppress effectively eNOS, possessed pronounced vasopressor activity [9, 11,12,13]. Furthermore, the results suggest that T1059 (10 mg/kg) is relatively safe because the cardiac ventricular ejection and instantaneous volumes practically did not change. Changes of peripheral hemodynamics of normotensive rats were moderate and transitory.
References
A. Belletti, M. L. Castro, S. Silvetti, et al., Br. J. Anaesth., 115(5), 656 – 675 (2015).
A. Havel, J. Arrich, H. Losert, et al., Cochrane Database Syst. Rev., 11(5), CD003709 (2011).
N. Ferguson-Myrthil, Cardiol. Rev., 20(3), 153 – 158 (2012).
J. G. Lampard and E. Lang, Ann. Emerg. Med., 61(3), 351 – 352 (2013).
G. G. Khubulaeva, A. A. Erofeev, and O. V. Maslyanyuk, in: Proceedings of the Scientific-Practical Conference “Promising Medical Technologies for Russian Federation Military Forces” [in Russian], VMedA, St. Petersburg (2013), pp. 142 – 145.
V. E. Volkov and S. V. Volkov, Shock. Sepsis. Multi-organ Dysfunction [in Russian], L. A. Naumov SP, Cheboksary (2009).
E. K. Stevenson, A. R. Rubenstein, G. T. Radin, et al., Crit. Care Med., 42(3), 625 – 631 (2014).
U. Forstermann and W. C. Sessa, Eur. Heart J., 33(7), 829 – 837 (2012).
R. M. Bateman, M. D. Sharpe, D. Goldman, et al., Crit. Care Med., 36(1), 225 – 231 (2008).
M. V. Filimonova, L. I. Shevchenko, V. I. Surinova, et al., Vopr. Biol., Med. Farm. Khim., No. 9, 35 – 39 (2011).
M. Lange, A. Hamahata, P. Enkhbaatar, et al., Shock, 35(6), 626 – 631 (2011).
M. V. Filimonova, T. P. Trofimova, G. S. Borisova, et al., Khim.-farm. Zh., 46(4), 14 – 16 (2012); Pharm. Chem. J., 46(4), 210 – 212 (2012).
K. Ishikawa, P. Calzavacca, R. Bellomo, et al., Crit. Care Med., 40(8), 2368 – 2375 (2012).
A. P. Lopez, J. A. Lorente, J. Steingrub, et al., Crit. Care Med., 32(1), 23 – 30 (2004).
A. Watson, R. Grover, A. Anzueto, et al., Crit. Care Med., 32(1), 13 – 21 (2004).
J. Viteceek, A. Lojek, G. Valacchi, et al., Mediators Inflammation, 2012 Art. ID: 318087 (2012).
W. K. Alderton, C. E. Cooper, and R. G. Knowles, Biochem. J., 357(3), 593 – 615 (2001).
M. V. Filimonova, L. I. Shevchenko, V. M. Makarchuk, et al., RU Pat. No. 2, 475, 479, Feb. 20, 2013; Byull. Izobret., No. 5 (2013).
M. V. Filimonova, L. I. Shevchenko, V. M. Makarchuk, et al., RU Pat. No. 2,552,529, Jun. 10, 2015; Byull. Izobret., No. 16 (2015).
H. M. van Eijk, Y. C. Luiking, and N. E. Deutz, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 851(1 – 2), 172 – 185 (2007).
A. F. Vanin and A. P. Poltorakov, Front. Biosci., 14, 4427 – 4435 (2009).
S. Ya. Proskuryakov, N. G. Kucherenko, M. V. Filimonova, et al., Byull. Eksp. Biol. Med., 134(10), 393 – 396 (2002).
D. Tsikas, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 851(1 – 2), 51 – 70 (2007).
M. V. Filimonova and A. A. Skvortsov, Byull. Eksp. Biol. Med., 127(5), 593 – 596 (1999).
S. Glantz, Primer of Biostatistics, 4th Ed., McGraw-Hill Inc., New York (1997), 473 pp.
GOST 12.1.007–76 Interstate Standard. Labor Safety Standard System. Hazardous Substances. Classification and General Safety Requirements, IPK Standart, Moscow (1999).
D. J. Wolff, D. S. Gauld, M. J. Neulander, et al., J. Pharmacol. Exp. Ther., 283(1), 265 – 273 (1997).
R. Boer, W. R. Ulrich, T. Klein, et al., Mol. Pharmacol., 58(5), 1026 – 1034 (2000).
L. E. Lambert, J. P. Whitten, B. M. Baron, et al., Life Sci., 48(1), 69 – 75 (1991).
W. S. Faraci, A. A. Nagel, K. A. Verdries, et al., Br. J. Pharmacol., 119(6), 1101 – 1108 (1996).
Acknowledgments
The work was sponsored in part by an RFBR grant and the Government of Kaluga Oblast (12-04-97524-p-tsentr-a).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 4, pp. 7 – 12, April, 2018.
Rights and permissions
About this article
Cite this article
Filimonova, M.V., Shevchenko, L.I., Makarchuk, V.M. et al. Vasopressor Properties of Nitric Oxide Synthase Inhibitor T1059. Part I: Synthesis, Toxicity, NOS-Inhibition Activity, and Hemodynamic Effects Under Normotensive Conditions. Pharm Chem J 52, 294–298 (2018). https://doi.org/10.1007/s11094-018-1809-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1809-2