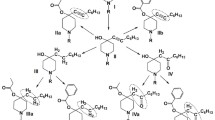
Catalytic processes involving Pd-containing catalysts for the synthesis of local anesthetics such as anesthesin (benzocaine), novocaine (procaine), lidocaine, mepivacaine, bupivacaine, trimecaine, and pyromecaine (bumecaine) are reviewed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Synthesis of Anesthesin
The classical industrial process for manufacturing the very important pharmaceutical drug anesthesin (ethyl p-aminobenzoate) incorporates a reduction step of ethyl p-nitrobenzoate (I) by iron filings in the presence of acetic acid.
The impurities are mainly products of incomplete reduction of the nitro group, i.e., ethyl p-nitrobenzoate (II), ethyl p-hydroxylaminobenzoate (III), diethyl azoxybenzene-(IV), azobenzene-(V), and hydroazobenzene-(VI) 4,4'-dicarboxylates. The amount of products from incomplete reduction of the nitro group and their rates of formation depend on the temperature, reductant, and solvent.
It was shown [5] that catalytic hydrogenation of I – VI under selected conditions occurs in the kinetic regime and is first-order in catalyst and hydrogen.
The dependence of the reaction rate on the substrate concentration is variable. For the instance where the substrate concentration becomes comparable with the amount of catalyst active centers, the reaction order in substrate changes from zero-to first-order.
The effective rate constant for hydrogenation of I as a function of Pd content in the catalyst shows a peak. The reaction rate with a metal content up to 4 mass % increases in proportion to the amount of Pd. Increasing the metal content further decreases the reaction rate. This is apparently due to lower catalytic activity because of the formation of Pd microcrystals. Thus, the optimum metal content in the catalysts was set at 4 mass %.
We studied the reduction of I on Pd-containing catalysts using the standard method [5] and found that they all produced anesthesin (VII) without forming detectable quantities of products from incomplete reduction of the nitro group. Tables 1 and 2 present the effective rate constants for the reaction calculated per kilogram of catalyst.
The hydrogenation was carried out over Pd/C catalysts and Pd-containing anion-exchangers AV-17-8 and AN-1 (AV-17-8-Pd and AN-1-Pd). The polytrimethylolmelamine AN-1 practically does not swell in organic solvents and is a weakly basic anion-exchanger whereas AV-17-8 [aminated chloromethylated copolymer of styrene (92%) and divinylbenzene (8%)] swells minimally in organic solvents and is a strongly basic anion-exchanger.
The temperature dependence of AV-17-8-Pd also shows a peak. The maximum rate is observed at 45°C. Increasing the temperature further causes a decrease in the reaction rate (Table 2). Apparently the appearance of the maximum is due, on one hand, to the increase in the partial pressure of the solvent vapor and, as a result, the lower H2 concentration in the reaction mixture and, on the other, to the polymeric nature of AN-1-Pd and AV-17-8-Pd [6].
The solvent has an important influence on the activity and selectivity for hydrogenation of I over all studied catalysts. Thus, the reaction rate in EtOH (Table 1) is greater than in toluene. However, VII undergoes additional alkylation in alcohol over Pd/C so that the yield of main product is lower.
The catalysts are placed in the following order of stability in the studied process: AV-17-8-Pd > AN-1-Pd > Pd/C. However, the initial rate over Pd/C is much greater than that for the Pd-containing polymers. The activity of Pd/C diminishes with repeated use because of leaching of Pd from the catalyst surface whereas this does not occur in the anion-exchangers because of the bond strength of the Pd to the polymer functional groups.
Thus, it can be concluded that I should be hydrogenated in EtOH at 45°C over AV-17-8-Pd and AN-1-Pd catalysts, which are superior to the heterogeneous catalyst Pd/C with respect to selectivity and stability and can produce high-quality VII without special purification [7].
Novocaine Synthesis
Novocaine (diethylaminoethyl p-aminobenzoate hydrochloride, VIII) was synthesized at the start of the 20th century [8 – 11]. Then, several industrial processes for synthesizing VIII were developed [12 – 14]. In all instances, the first step is esterification of p-nitrobenzoic acid to form I. Next, I is converted to VII, which is purified by recrystallization and trans-esterified to produce VIII, treating the corresponding base with alcoholic HCl.
The possibility of combining hydrogenation of I and trans-esterification of VII generated in situ was studied [15]. We demonstrated several times the ability in principle to perform simuntaneous hydrogenation of the nitro group and trans-esterification of the ester over Pd-containing polymers [7,16,17]. Hydrogenation of I and trans-esterification of the resulting VII was carried out over Pd/C, AV-17-8-Pd, and AN-1-Pd.
Preliminary results showed that hydrogenation of I and trans-esterification of VII under selected conditions occurs in the kinetic regime. Both processes are first-order in catalyst. The effective rate constant for trans-esterification of VII depends linearly on the amount of catalyst. The catalysts are placed in the following order with respect to this function: AV-17-8-Pd > AN-1-Pd > Pd/C. This correlates with their basicity. Apparently the basic properties of the metal-polymer catalysts enable them to be used effectively for combining the hydrogenation and trans-esterification. Obviously most of the anion-exchanger functional groups remain free upon loading the initial amount of Pd (4 mass %) [18] and act as active centers for the trans-esterification.
The lack of a dependence of the rate constant for formation of VIII on the initial concentration of I is consistent with a zero-order hydrogenation of I to VII. This agrees with prior investigations [5,16]. Therefore, the features of the hydrogenation of the nitro compound, including the high yield of product, persist under conditions where the hydrogenation and trans-esterification occur simultaneously.
However, the constant depends ambiguously on the initial concentration of diethylaminoethanol (IX). Thus, a first-order reaction is observed for the metal-polymer catalysts. The order is close to zero over the heterogeneous catalyst although the reaction rate increases slightly with increasing concentration of IX. Not only the rate of formation but also the yield of final product (VIII) increase with increasing initial concentration of IX (Table 3).
We studied the effect of acid and base on the formation rate constant of VIII from I during further improvement of the production of VIII because trans-esterification is known to be accelerated in the presence of acids or bases [19].
The results showed that preference should be given to the base-catalyzed reaction because high base concentrations were not required over the metal-polymer catalysts. As noted above, the metal-polymers contain free functional groups that also catalyze trans-esterification. Table 3 shows that the yield of VIII as a function of KOH concentration shows a peak. This is apparently related to the hydrolysis of VIII whereas the yield of VIII increases constantly as the concentration of IX is increased.
Thus, the results suggest that the catalytic synthesis of VIII from I via combination of hydrogenation and trans-esterification of the intermediate VII is a promising method for manufacturing the important pharmaceutical drug novocaine [20].
Dicaine Synthesis
Dicaine is β-dimethylaminoethyl p-butylaminobenzoate hydrochloride (X·HCl) and is a structural analog of VIII in which the amine in the benzene ring is substituted by butyl. Therefore, the synthetic scheme for X [21] has much in common with those for VII [12 – 14].
The industrial synthesis of X is carried out analogously to that of VIII from p-nitrobenzoic acid. The only difference is that alkylation of the amine formed by reduction of p-nitrobenzoic acid (XI)to p-aminobenzoic acid (XII)appears as an additional step.
The method proposed in the literature [22] for preparing X using catalytic hydrogenation enables many such drawbacks to be avoided partially or completely. Traditional Pd/C and Pd-containing anion-exchangers (AN-1-Pd and AV-17-8-Pd) were used as catalysts.
The starting material is I. It is hydrogenated in the presence of butanal (XIII) and dimethylaminoethanol (XIV)to form VII, which reacts with the aldehyde (XIII) in the reaction mixture to form an azomethine, ethyl p-butylideneaminobenzoic acid (XV). Then compound XV is reduced to ethyl p-butylaminobenzoic acid (XVI). It was found earlier that the catalyst has a substantial influence on condensation of aldehydes with aromatic amines [23 – 27]. The yield of XV in the presence of catalyst is much greater than condensation without a catalyst [25 – 27]. The role of the catalyst in shifting the equilibrium toward formation of XV is not fully understood. However, it was shown that Pd-containing polymers are far superior to Pd/C in this respect. Apparently this explains the relatively low yield of XI over Pd/C (Table 4).
Compound XVI generated in situ undergoes trans-esterification to form X. Amination of XIII and trans-esterification of XVI occur simultaneously but at different rates. This causes intermediates VII and XV to accumulate in the reaction mixture (Scheme 1).
It was shown earlier that hydroamination of aliphatic aldehydes by aromatic amines [23] and heterocyclic aldehydes by cyclohexylamine [24] and aromatic amines [25] over Pd/C and Pd-containing polymers [26,27] forms the target products, secondary amines, in 84 – 100% yields. Moreover, it seemed possible [5, 25 – 27] to perform simultaneous hydrogenation of the nitro group, hydroamination of the aldehydes, and trans-esterification of the ester over Pd catalysts. Thus, the overall scheme for producing X from I can be represented as shown in Scheme 2 according to the experimental results and literature data.
The produced base X does not require additional purification because the alcoholic HCl used for conversion of X to the hydrochloride salt contains water and EtOH. The developed method is suitable for catalytic synthesis of X base in one step from I in high yield (up to 94%) under mild conditions [22].
Lidocaine Synthesis
Lidocaine is 2-(diethylamino-N-(2,6-dimethylphenyl)acetamide (XVII) and is widely used in medicine as a local anesthetic. Its high therapeutic activity, rapid onset, and rather prolonged action make it suitable for practically any clinical application [12 – 14]. However, the high cost of the drug due to an imperfect industrial synthetic process prevents its wide use (like, for example, VIII). The starting material for industrial manufacture of XVII is 2,6-dimethylaniline [28].
Reductive acylation starts with 2,6-dimethylnitrobenzene (XVIII), which is reduced with simultaneous addition of the acyl group to the reduced N atom (Scheme 3).
Our experience with the synthesis of drugs containing an amine, hydrogenation [5,15], hydroamination [22], and reductive acylation [29] over Pd catalysts led to the production of the target products in high yields. The selectivity and activity of the process was determined by various process factors, mainly the nature of the reagents, catalyst, solvent, temperature, pH, etc. The new metal-polymer catalysts AV-17-8-Pd and AN-1-Pd enable the process to be performed under mild conditions. This increases significantly the selectivity of the synthesis (Table 5) by minimizing the destruction and polymerization of the substrates and target products. Their properties are comparable with classical Pd/C. Reductive acylation over all studied catalysts occurs without forming significant amounts of products from incomplete reduction of the nitro group. Tables 5 – 7 present the effective rate constants calculated per kilogram of catalyst.
As it turns out, the lower selectivity for producing XVII over Pd/C is due to a side reaction involving alkylation by solvent of the amine in XXIII. Increasing the temperature above 45°C favors this process (Table 6). The temperature dependence for AV-17-8-Pd shows a peak. The maximum effective rate is observed at 45°C. Increasing the temperature further reduces the reaction rate (Table 6). Apparently the presence of a maximum is due, on one hand, to the increase of the partial pressure of solvent vapor that results in a reduction of the H2 concentration in the reaction mixture and, on the other, to the polymeric nature of AN-1-Pd and AV-17-8-Pd.
The solvent has a significant effect on the activity and selectivity of reductive acylation of XVIII in the presence of all studied catalysts. Thus, the reaction rate in EtOH (Table 5) is much greater than in toluene. However, the reduction product of XVIII is alkylated by solvent molecules over Pd/C, i.e., reductive amination of the alcohols occurred.
The catalysts are placed in the following order according to stability and activity in this process (Tables 5 – 7): AV-17-8-Pd > AN-1-Pd > Pd/C. The diminished activity of Pd/C upon repeated use is due to leaching of Pd from the catalyst surface whereas this does not occur in the an ion-exchangers because of the bond strength of the Pd to the polymer functional groups.
However, not only the nature of the catalyst and substrate but also the solution pH turn out to have a substantial influence on the increased activity of the process. The acylation is known to be accelerated in the presence of acids or bases. Thus, the reaction rate increases, most over Pd/C, if HCl is added to the reaction mixture (Table 7). The effective reaction rate with [HCl] = 0.10M over the heterogeneous catalyst becomes comparable with that over AN-1-Pd but remains less than that over AV-17-8-Pd. The observed differences in the trends for the heterogeneous and metal-polymer catalysts suggests that they are involved not only in hydrogenation of XVIII but also in the most problematical step of the whole process, acylation of the reduction product of XVIII that is formed in situ.
The influence of base (Table 7) also confirms this hypothesis because increasing the KOH concentration has no effect on reactions involving the metal-polymers whereas KOH has an even greater influence than HCl on acylation over Pd/C. The zero-order in base is consistent with direct involvement of free highly basic and weakly basic functional groups of AV-17-8-Pd and AN-1-Pd in reductive acylation of XVIII.
Thus, the experimental results indicate that preference should be given to the base-catalyzed reaction because the use of large base concentrations is not required over the metal-polymer catalysts. Catalytic synthesis of XVII from XVIII by combining the hydrogenation and acylation reactions of the produced amine is a promising method for manufacturing the very important pharmaceutical drug lidocaine (XVII).
Synthesis of Mepivacaine, Bupivacaine, Trimecaine, and Pyromecaine
The possibility for catalytic synthesis of the newest pharmaceutical drugs, aminoamide local anesthetics bupivacaine [1-butyl-N-(2, 6-dimethylphenyl)-2-piperidinecarboxamide, XIX], mepivacaine [1-methyl-N-(2, 6-dimethylphenyl)-2 piperidinecarboxamide, XX], trimecaine (a-diethylamino2,4,6-trimethylacetanilide, XXI), and pyromecaine (1-butyl2,4,6-trimethyl-2-pyrrolidinecarboxamide, XXII) was studied [29].
Current catalytic and non-catalytic methods for preparing acetanilides of 2,6-dimethylaniline (XXIII) and 2,4,6-trimethylaniline require the use of a multi-component system and forcing conditions [30].
Two main methods were proposed for industrial manufacture of XX. One of them includes not only chemical steps but also catalytic ones.
-
a)
The first method involves synthesis using Grignard reagents and is performed by reacting ethyl 1-methylpiperidine-2-carboxylate with 2,6-dimethylanilinyl magnesium bromide, which was prepared in turn by treating XXIII with ethyl magnesium bromide [31 – 33].
-
b)
The second method [34] produces XX via reaction of XXII with pyridine-2-carboxylic acid chloride (XXIV). This formed initially the 2,6-xylylide of a-picolinic acid (XXV), the aromatic pyridine ring of which was reduced into piperidine by H2 over Pd/C. Compound XXV produced in this manner was N-methylated into XX by formaldehyde with simultaneous reduction by H2 over Pd/C (hydrogenating amination).
Compound XIX is chemically similar to XX and differs only in the substituent on the N atom of the piperidine ring (butyl instead of methyl). Therefore, the synthetic methods for these compounds have much in common. Two methods were proposed for preparing XIX.
-
a)
The first method produces XIX from XXV [32,35]. It is alkylated by butylbromide to produce the corresponding pyridinium salt. Then, the salt is hydrogenated into the piperidine derivative over platinum oxide to produce XIX.
-
b)
The second method uses XXIV as starting material [36 – 38]. It is reacted with amine XXIII. The amide produced in this manner is further alkylated by butylbromide into XIX.
It can be seen that the use of several steps and forcing conditions reduces in general the effectiveness and selectivity of the process. Therefore, a method was proposed [39] for production of XIX – XXII via reductive acylation of XVIII and 2,4,6-trimethylnitrobenzene by the appropriate carboxylic acid derivatives over Pd catalysts. This enables the aforementioned drawbacks to be avoided (Scheme 4).
The catalysts were Pd-containing anion-exchangers AV-17-8 and AN-1. They were demonstrated to be highly effective in such processes [5,15,22,29].
Reductive acylation of the nitrobenzene derivatives into the corresponding carboxylic acids (Scheme 4) occurs in the kinetic regime under the selected conditions. Performing the process under mild conditions over metal-polymers enables polymerization and destruction of the starting materials and reaction products to be avoided. This increases substantially the selectivity of the synthesis (Table 8).
As it turns out, hydrogenolysis of the reaction products is not observed over the metal-polymers. However, the maximum yield of the target product is less than 60 – 70% over Pd/C. Then, the concentration of the target product is observed to decrease and one of the starting compounds, in particular, the hydrogenation product of the nitro derivative, increases. This clearly indicates that the target products are being destroyed. It should be noted that the heterogeneous analog Pd/C has a characteristic tendency toward hydrogenolysis and rapid deactivation due to leaching of Pd from the catalyst surface or poisoning by polymerization products [5,15,22,29].
The high selectivity and activity of the metal-polymers is apparently related, on one hand, to their high selectivity in hydrogenation of nitro compounds [5,15,22,29] and, on the other, the involvement of free functional groups of the anion-exchangers as catalysts for acylation of the intermediate amine derivative formed in situ.
In general, the catalysts can placed in the following order relative to selectivity in the synthesis of local anesthetics by reductive acylation of nitrobenzene derivatives: AV-17-8-Pd > AN-1-Pd > Pd/C (Table 8).
References
M. V. Klyuev and M. G. Abdullaev, Catalytic Synthesis of Amines [in Russian], Izd. IvGU, Ivanovo (2004).
A. Limpricht, Ann., 303, 278 (1898).
Org. Synth., 8, 66 (1928). nd
Org. Synth. Coll., Vol. 1, 240 (2Ed. 1941).
M. G. Abdullaev, Khim.-farm. Zh., 35(1), 42 – 45 (2001).
M. V. Klyuev and E. F. Vainshtein, Metal-Containing Polymers, A Special Type of Catalyst [in Russian], Izd. Inst. Khim. R-ov RAS, Ivanovo (1999).
M. V. Klyuev and M. G. Abdullaev, Katal. Promsti., 6, 57–60 (2002).
Ger. Pat. No. 179,627 (1904).
Ger. Pat. No. 194,748 (1905).
USA Pat. No. 812,554 (1906).
A. Einhorn, Ann., 317, 125, 131, 142, 162 (1909).
USSR SP, Vol. 9, Meditsina, Moscow (1987), p. 467.
L. P. Senov, Pharmaceutical Chemistry [in Russian], Meditsina, Moscow (1971), pp. 361 – 367.
A. G. Melent’eva, Pharmaceutical Chemistry [in Russian], Meditsina, Moscow (1968), pp. 315 – 322.
M. G. Abdullaev, Khim.-farm. Zh., 35(10), 30 – 33 (2001).
M. V. Klyuev and M. G. Abdullaev, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 42(5),3–13 (1999).
M. V. Klyuev, T. G. Volkova, and M. G. Abdullaev, Neftekhimiya, 42(1), 32 – 35 (2002).
V. D. Kopylova, T. V. Pogodina, and M. V. Klyuev, Zh. Fiz. Khim., 64(3), 724 – 728 (1990).
R. T. Morrison and R. N. Boyd, Organic Chemistry,3Ed., Allyn and Bacon, Boston (1973).
M. G. Abdullaev and M. V. Klyuev, Katal. Promsti., 1, 25–30 (2003).
USA Pat. No. 1,889,645 (1932).
M. G. Abdullaev, Khim.-farm. Zh., 36(1), 28 – 33 (2002).
RF Pat. No. 2,039,599; Byull. Izobret., No. 20 (1995).
M. G. Abdullaev, M. V. Klyuev, and A. A. Nasibulin, Zh. Org. Khim., 31(3), 416 – 418 (1995).
M. G. Abdullaev, A. A. Nasibulin, and M. V. Klyuev, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 37(7–9), 55–58 (1994).
M. G. Abdullaev, A. A. Nasibulin, and M. V. Klyuev, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 37, No.7–9, 58–62 (1994).
M. V. Klyuev, A. A. Nasibulin, and M. G. Abdullaev, Neftekhimiya, 34(5), 413 – 420 (1994).
USA Pat. No. 2,441,498 (1948).
M. G. Abdullaev, Khim.-farm. Zh., 39(12), 32 – 34 (2005).
V. A. Zagorevskii, I. V. Chernyaeva, T. I. Ivanova, et al., Khim.-farm. Zh., 23(3), 292 – 296 (1989).
USA Pat. No. 2,799,679 (1957).
B. Ekenstram, Acta Chem. Scand., 11, 1183 (1957).
H. Rindernecht, Helv. Chim. Acta, 42, 1324 (1959).
USA Pat. No. 4,110,331 (1977).
G. Br. Pat. No. 869,978 (1959).
USA Pat. No. 2,792,399 (1957).
USA Pat. No. 2,955,111 (1960).
B. F. Tullar, J. Med. Chem., 14, 891 (1971).
M. G. Abdullaev, M. V. Klyuev, Z. Sh. Abdullaeva, et al., Khim.-farm. Zh., 42(6), 46 – 48 (2008).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 44, No. 8, pp. 31 – 37, August, 2010.
Rights and permissions
About this article
Cite this article
Klyuev, M.V., Abdullaev, M.G. & Abdullaeva, Z.S. Palladium catalysts in the synthesis of local anesthetics (review). Pharm Chem J 44, 446–451 (2010). https://doi.org/10.1007/s11094-010-0487-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-010-0487-5