Abstract
The effects of both CF4/O2 and Ar/O2 mixing ratios in three-component CF4 + O2 + Ar mixture on plasma parameters, densities and fluxes of active species determining the dry etching kinetics were analyzed. The investigation combined plasma diagnostics by Langmuir probes and zero-dimensional plasma modeling. It was found that the substitution of CF4 for O2 at constant fraction of Ar in a feed gas produces the non-monotonic change in F atom density, as it was repeatedly reported for the binary CF4/O2 gas mixtures. At the same time, the substitution of Ar for O2 at constant fraction of CF4 results in the monotonic increase in F atom density toward more oxygenated plasmas. The natures of these phenomena as well as theirs possible impacts on the etching/polymerization kinetics were discussed in details.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorocarbon (FC) gases with a general formula of CxFy are widely used in the microelectronic industry for dry patterning of silicon wafers and dielectric (SiO2, Si3N4) thin films [1, 2]. Among these, the CF4 is characterized by the highest F/C ratio and provides the domination of etching over the surface polymerization process under the typical reactive ion etching conditions [3,4,5]. Being used for the etching process, the CF4 is frequently combined with Ar or O2 in forms of binary CF4 + Ar or CF4 + O2 gas mixtures with the aims of accelerating the physical etching pathway, increasing the F atoms yield and suppressing polymerization on the surfaces which are in a contact with plasma [4, 5].
Recently, in order to satisfy the increasingly demanding requirements concerning device dimensions and performance, many dry etching processes require optimization through the appropriate choice of working gas and input process conditions. In this framework, an understanding of the plasma chemistry mechanisms involved in various gas systems is important for future progress in the dry etching technology. Until now, there were enough experimental and modeling studies focused on pure CF4, CF4 + Ar and CF4 + O2 low pressure (p < 50 mTorr) plasmas [6,7,8,9,10,11,12,13,14,15,16]. These works contain the results of plasma diagnostics by Langmuir probes [6, 7, 10,11,12,13], the measurements of the neutral species densities (absorption spectroscopy, optical emission actinometry, mass-spectrometry) [7,8,9, 13, 16] as well as provide the well-adjusted kinetic schemes determining the steady-state plasma parameters and composition [6, 8, 10, 12,13,14,15]. Thought the available data show some disagreements due to different input process conditions and types of plasma reactors, the key features of the CF4 + Ar and CF4 + O2 plasma chemistries can briefly be summarized as follows:
-
The dilution of CF4 by Ar at constant pressure and input power has no principal influence on the kinetics of neutral species (except the weak increase in the CF4 dissociation degree due to an increase in electron density [10,11,12]), so the densities of F atoms and CFx radicals decrease monotonically toward Ar-rich plasmas. Another important thing is a growth of positive ion density (due to a decrease in plasma electronegativity that lowers the decay rate of positive ions in ion–ion recombination process) as well as of their flux together with Ar fraction in a feed gas. This accelerates the physical etching pathway for both target material and FC polymer film.
-
The dilution of CF4 by O2 at constant pressure and input power results in the rapidly decreasing densities of CFx radicals as well as in the non-monotonic behavior of the F atom density which exhibits a maximum at 20–40% O2 [13,14,15]. Most authors reasonably attribute this effect to both stepwise dissociation of the CFx species due to their interaction with oxygen atoms and the consequent chemical reactions involving the CFxO products [14, 15].
When analyzing the existing works, one can conclude that modern plasma etching technology involves three- or more component gas mixture, for example CF4 + O2 + Ar. The ternary gas systems provide more pathways for the changes in gas mixing ratios in order to obtain the optimal process conditions. Particularly, one can fix the fraction of one component and change the mixing ratios for the remaining two ones. It is clear that, since the composition of the feed gas is different compared with the simple CF4 + O2 mixture, some principal differences in plasma parameters (through the electron energy distribution function and mean electron energy) and densities of plasma active species can take place. Earlier, Kimura and Hanaki [17] have reported the experimental study of CF4 + O2 + Ar plasma with variable CF4 fractions in a feed gas. They found that the behaviors of plasma parameters and F atoms density versus CF4/O2 gas mixing ratio at constant Ar fraction are quite similar to those obtained for the CF4 + O2 plasma. However, they did not provide analysis of plasma chemistry in order to understand the differences between the binary and ternary gas systems. In our previous works have dealt with CF4 + O2 + Ar plasma [18, 19], it was found that the variation in Ar/O2 mixing ratio at constant CF4 fraction causes the different changes of plasma parameters compared with Ref. [13,14,15, 17] as well as does not result in the non-monotonic behavior of F atoms density. Unfortunately, the data of Refs. [17,18,19] cannot be compared directly and/or analyzed in a comparative scale because of different input process conditions, reactor types and geometries. Another important thing is that all mentioned studies were mainly focused on bulk plasma characteristics and thus, did not provide enough information on the possible impacts of CF4/O2 and Ar/O2 mixing ratios on the dry etching kinetics. Such situation retards the optimization of the dry etching technologies with using the CF4 + O2 + Ar plasma.
The goal of this work was the study of CF4 + O2 + Ar inductively coupled plasma under the fixed set of operating conditions (gas pressure, input power and bias power), but in two different gas mixing regimes assumed the change in CF4/O2 or Ar/O2 mixing ratio. In other words, we would like to demonstrate the ability of CF4 + O2 + Ar gas system to adjust the plasma parameters and composition using the gas mixing ratios only. For this purpose, we applied the previously used research scheme based on plasma diagnostics by Langmuir probes and 0-dimensional plasma modeling [18]. The main attention was focused on the comparative analysis of the parameters which directly determine the dry etching mechanisms: ion energy flux, F atom flux, ion energy flux, F atom flux, polymerizing species (CF2 and CF) flux as well as various flux-to-flux ratios illustrating the changes in the etching/polymerization balance. We also performed the analysis of the formation-decay kinetics for neutral species in order to explain the obtained phenomena.
Experimental and Modeling Details
Experimental Setup and Plasma Diagnostics
The experiments were performed in planar inductively coupled plasma (ICP) reactor [20]. The reactor had a cylindrical (r = 15 cm) chamber made from the anodized aluminum. The 5-turns copper coil with a diameter of 29 cm was connected to the 13.56 MHz power supply and located above the 10 mm thick-horizontal quartz window on the top side of the chamber. A distance (l) between the window and the bottom electrode used as a substrate holder was 12.8 cm. The bottom electrode was connected to 12.56 MHz power supply to maintain a negative dc bias voltage (−U dc ). The temperature of the bottom electrode was stabilized at 17 °C using the water-flow cooling system.
All experimental series corresponded to the fixed total gas flow rate (q = 40 sccm), gas pressure (p = 6 mTorr) and input power (W = 900 W). The input power density \(W^{{\prime }} = W/\pi r^{2} l\) then became 0.9 W cm−3. In order to imitate actual etching conditions, the bottom electrode was biased by W dc = 200 W. The initial CF4 + O2 + Ar gas compositions were set by adjusting the partial flow rates. In one experimental series, the CF4 flow rate was fixed at 20 sccm while the flow rates of the O2 and Ar were variably set to a combined total of \(q_{{O_{2} }} + q_{Ar}\) = 20 sccm. Therefore the fraction of CF4 (\(y_{{CF_{4} }} = q_{{CF_{4} }} /q\)) in the feed gas was always 0.5, and the remaining half gas mixture was composed of various amounts of Ar and O2. Another experimental series assumed the constant flow rate for Ar (q Ar = 20 sccm) as well as the variable flow rates for CF4 and O2, so that \(q_{{O_{2} }} + q_{{CF_{4} }}\) = 20 sccm. In this case, the fraction of Ar (y Ar = q Ar /q) in the feed gas was always 0.5, and the remaining half gas mixture was composed of various amounts of CF4 and O2.
The plasma parameters were determined by a double Langmuir probe (LP), (DLP2000, Plasmart Inc.). The probe tip was installed through a hole in the sidewall of the chamber, 5.7 cm above the bottom electrode and centered in a radial direction. The output data were the electron temperature (T e ), ion current density (J +), floating potential (U f ), and total positive ion density (n +). The treatment of the I–V curves was based on Johnson and Malter’s double probe theory [21] and the Allen–Boyd–Reynolds (ABR) approximation for the ion saturation current density [22]. These assume \(J_{ + } \approx 0.61\,en_{ + } \upsilon\), where υ is the ion Bohm velocity. In our previous studies, it was shown that such an approach can be reasonably applied even for more electronegative plasmas than those used in this study [23, 24]. The effective ion mass needed to determine υ was evaluated simply through the mole fractions of the corresponding neutral species. In order to exclude the influence of the FC polymer film on the LP results, we conducted a set of preliminary experiments, where the \(I {-} V\) curves were recorded continuously at fixed-feed gas composition and operating parameters. Even for the non-oxygenated 50% CF4 + 50% Ar plasma, the differences between the results of such measurements did not exceed the standard experimental error for a period of at least 10 min after the plasma was turned on. Also, throughout the main experimental procedure, the probe tip was cleaned in 50% Ar + 50% O2 plasma before and after each measurement.
Plasma Modeling
In order to obtain the densities and fluxes of plasma active species, we used a simplified zero-dimensional kinetic model with the experimental data on T e and \(n_{ + }\) as input parameters [23, 24]. Similarly to our previous works [18, 19], the model was based on the well-adjusted reaction scheme (see Table 1) and used the following assumptions:
-
1.
The electron energy distribution function (EEDF) is quite close to Maxwellian one. The applicability of Maxwellian EEDFs for the description of the electron-impact kinetics for CF4-based low-pressure (p < 50 mTorr) ICPs has been confirmed in many works by reasonable agreements between the measured and model-predicted plasma parameters [12,13,14]. Also, Kimura et al. [13] reported that the experimentally determined EEDFs in CF4 plasma at p < 20 mTorr had the almost Maxwellian shapes. The postulation of Maxwellian EEDF allows one to obtain the rate coefficients for the electron-impact processes (R1–R17) as functions of T e in a form of \(k = AT_{e}^{B} { \exp }\left( { - C/T_{e} } \right)\) [13, 14].
-
2.
The half-diluted CF4 gas does not form a continuous FC polymer film on the chamber walls under plasma conditions [4, 25]. This allows one to describe the heterogeneous chemistry of atoms (F, C, O) and radicals (CF3, CF2, CF) in terms of the conventional first-order recombination kinetics. The rate coefficients for the heterogeneous loss of these species (R51–R56) can be evaluated as \(k_{S} \approx \gamma \upsilon_{T} /\Lambda\), where \(\Lambda = \left[ {\left( {2.405/r} \right)^{2} + \left( {\pi /l} \right)^{2} } \right]^{ - 1/2}\) is the diffusion length [5], υ T = (8k B T gas /πm)1/2, and γ is the recombination probability. The recombination probabilities for F (γ F ≈ 0.02), CF3 (\(\gamma_{{CF_{3} }}\) ≈ 0.05), CF2 (\(\gamma_{{CF_{2} }}\) ≈ 0.1) and CF (γ CF ≈ 0.1) species were taken from the modeling works [12, 13, 26] where these were adjusted in order to obtain the agreement between the measured and model-predicted densities of F atoms and CF2 radicals in CF4- and C4F8-based plasmas. All reaction pathways between the adsorbed (marked by the “s” index) and gaseous species inside R51–R56 were assumed to have equal probabilities of occurrence.
-
3.
The temperature of the neutral ground-state species (gas temperature, T gas ) is independent of the feed gas composition [18, 19]. Since experimental data on T gas were not available during this study, we took 600 K as the typical value for close ranges of p and W′ in ICP etching reactors with similar geometry [18, 19, 23, 24]. Accordingly, the rate coefficients for R18–R50 were taken from the NIST chemical kinetics database [27] for T gas = 600 K.
-
4.
For the given range of operating parameters, the formation of CxFy species with x > 1 may be neglected due to their low densities and negligible influence on the overall reaction balance [23].
The steady-state electron density (n e ) was calculated using the simultaneous solution of the chemical kinetic equation for negative ions and the quasi-neutrality condition \(n_{ + } = n_{e} + n_{ - }\). When neglecting the loss of negative ions on reactor walls [5], their kinetic equation for the given gas system may be written as
where k da is the dissociative attachment rate coefficients for corresponding species, n − is the density of negative ions, and k ii ≈ 1 × 10−7 cm3 s−1 is the rate coefficient for ion–ion recombination. Accordingly, the left-hand side of Eq. (1) represents the total formation rate for negative ions while the right-hand side is their total decay rate in bulk plasma. These allow one to obtain
where \(\nu_{da} \approx k_{{da,CF_{4} }} n_{{CF_{4} }} + k_{{da,F_{2} }} n_{{F_{2} }} + k_{{da,O_{2} }} n_{{O_{2} }}\) is the total frequency of dissociative attachment. The rate coefficients k da were taken from Refs. [13, 14].
The steady-state densities for neutral species were obtained from the system of chemical kinetics equations in the general form of \(R_{F} - R_{D} = \left( {k_{s} + 1/\tau_{R} } \right)n\), where R F and \(R_{D}\) are the volume-averaged formation and decay rates in bulk plasma for a given type of species, n is their density, τ R = (1/p 0)πr 2 lp/q is the residence time and p 0 = 101,325 Pa is the gas pressure corresponding to standard conditions. The fluxes of F atoms (\(\Gamma _{F}\)), O atoms (\(\Gamma _{O}\)) and polymerizing radicals (\(\Gamma _{pol} =\Gamma _{CF} +\Gamma _{{CF_{2} }}\)) to the etched surface were calculated in one and the same manner as \(\Gamma \approx 0.25n\sqrt {8k_{B} T_{gas} /\pi m}\). The total flux of positive ions was simply evaluated as \(\Gamma _{ + } \approx J_{ + } /e\). The applicability of both modeling algorithm and reaction schemes for the given range of process conditions have been confirmed in our previous works [18, 19] by the decent agreement between model-predicted and measured densities for F atoms and CF2 radicals.
Results and Discussion
When the fluorocarbon-based plasmas are used for the etching process, the changes of the etching rate versus operating parameters depend on two main factors, and namely on (1) the fluxes of plasma active species (chemically active neutrals, energetic ions) and (2) the steady-state structure and thickness of the deposited FC polymer film [4, 5]. According to Refs. [28, 29], the growth of the FC polymer on the etched surface is mainly provided by the fluorocarbon radicals with more than one free bonds while both oxygen atoms and positive ions etch the FC polymer film by chemical and physical pathways, respectively. Therefore, the species of principal interest which determine the dry etching mechanisms in CF4 + O2 + Ar plasma are F atoms, O atoms, CFx (x = 1, 2) radicals and positive ions.
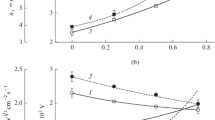
Figures 1 and 2 represent measured and model-predicted plasma parameters and densities of charged species as functions of CF4/O2 and Ar/O2 gas mixing ratios in CF4 + O2 + Ar gas mixture. From Fig. 1, it can be seen that the substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const results in decreasing both T e (3.6–3.4 eV for 0–50% O2) and J + (1.5–1.3 mA cm−2 for 0–50% O2) that corresponds to n + = 5.7 × 1010–4.4 × 1010 cm−3. Oppositely, the substitution of CF4 for O2 at y Ar = const causes an increase in electron temperature (T e = 3.6–4.6 eV for 0–50% O2) and ion current density (J + = 1.5–2.3 mA cm−2 for 0–50% O2). Accordingly, an increase in J + provides the same behavior of total positive ion density (n + = 5.7 × 1010–6.2 × 1010 cm−3 for 0–50% O2). In both cases, the model-predicted electron density follows the behavior n + and changes as 4.4 × 1010–2.8 × 1010 cm−3 for 0–50% O2 at \(y_{{CF_{4} }}\) = const and 4.4 × 1010–5.1 × 1010 cm−3 for 0–50% O2 at y Ar = const (Fig. 2). From Fig. 2, it can be seen also that the total density of negative ions in the case of \(y_{{CF_{4} }}\) = const monotonically increases in the range of 1.3 × 1010–1.6 × 1010 cm−3 for 0–50% O2 that provides n −/n e = 0.28–0.58. At the same time, the change in CF4/O2 mixing ratio at y Ar = const slightly lowers both n - (1.3 × 1010–1.1 × 1010 cm−3 for 0–50% O2) and n −/n e (0.28–0.23 for 0–50% O2). The analysis of these data allows one to explain the differences in plasma parameters influencing the kinetics of electron impact reactions in given gas systems as follows:
-
The behavior of T e is determined by the changes in the electron energy losses in inelastic collisions with plasma species. The substitution of Ar by O2 at \(y_{{CF_{4} }}\) = const increases the overall electron energy loss because the molecular gas has more excitation pathways than the noble one. Really, the first excitation potential for Ar atoms is ~11.6 eV while the O2 molecules provide the continuous election energy loss starting from ~0.2 eV [5, 6, 13]. The last feature is provided by the vibrational excitation of ground-state O2 (ɛ th = 0.16 eV, where ɛ th is the threshold energy) as well by the formation of metastable electronically excited states O2(a1Δ) with ɛ th = 0.98 eV and O2(b1Σ) with ɛ th = 1.64 eV [30]. Oppositely, an increase in O2 fraction at y Ar = const decreases the overall electron energy loss for vibrational and low-threshold electronic excitations. The reason is that the corresponding cross-sections (and thus, the partial electron energy losses determined by the parameter ɛ th k, where k is the process rate coefficient) for CF4-related species are higher compared with those for O2 molecules [30, 31].
-
The behavior of n e (and thus, of n + and n − with accounting for the quasi-neutrality condition) is determined by the change in the formation-decay balance for charged species. Particularly, Fig. 3 shows the influence of CF4/O2 and Ar/O2 mixing ratios on the ionization frequencies for dominant neutral species (ν iz ) determines as the multiplications of their densities and corresponding ionization rate coefficients (k iz ) [12, 13, 17]. In the non-oxygenated 50% CF4 + 50% Ar plasma, the main contribution to the total ionization rate belongs to ground-state Ar atoms. Such situation appears due to k iz,Ar > \(k_{{iz,CF_{4} }}\) (~6.9 × 10−10 cm3 s−1 and ~3.3 × 10−10 cm3 s−1, respectively, at T e = 3.6 eV). Since the values of k iz are highly sensitive to electron temperature (due to \(\varepsilon_{iz}\) ≈ 12–15 eV > (3/2)T e , where ɛ iz is the threshold energy for ionization [5, 16], and (3/2)T e is the mean electron energy), the change in T e (and thus, in gas mixing ratios) noticeably influences the ionization efficiency in both gas systems. The substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const lowers both k iz,Ar and n Ar while a decrease in ν iz,Ar is not compensated by the impacts from the oxygen-containing species (Fig. 3a). Therefore, the change in Ar/O2 mixing ratio toward more oxygenated plasmas suppresses the ionization (total ν iz = 4.3 × 104–2.0 × 104 s−1, and ν iz n e = 1.9 × 1015–5.6 × 1014 cm−3 s−1 for 0–50% O2) and thus, lowers the formation rates for both electrons and positive ions. Simultaneously, the substitution of the electropositive component for the electronegative one increases the formation rate for negative ions, lifts up plasma electronegativity as well as accelerates decay of positive ions and electrons in bulk plasma. In the case of y Ar = const, one can obtain an opposite situation. An increasing O2 fraction in a feed gas lifts up the total ionization frequency through both increasing ν iz,Ar (k iz,Ar = 6.9 × 10−10–2.2 × 10−9 cm3 s−1 for 0–50% O2) and the noticeable contribution from \(\nu_{{iz,O_{2} }}\) at \(y_{{O_{2} }}\) > 40% (Fig. 3b). The last effect is provided by the condition \(k_{{iz,O_{2} }}\) > \(k_{{iz,CF_{4} }}\) due to the corresponding differences in their ionization cross-sections [31, 32]. As a result, one can obtain a monotonic increase in both total ionization frequency (4.3 × 104–1.1 × 105 s−1 for 0–50% O2) and ionization rate (1.9 × 1015–5.7 × 1015 cm−3 s−1 for 0–50% O2). Simultaneously, the lower electronegativity of O2 (\(k_{{da,O_{2} }}\) = 2.3 × 10−11–3.4 × 10−11 cm3 s−1 for 0–50% O2) compared with CF4 (\(k_{{da,CF_{4} }}\) = 3.9 × 10−11–4.2 × 10−11 cm3 s−1 for 0–50% O2) provides the weak monotonic decrease in both formation rate and density of negative ions. This slightly reduces the decay rates of both electrons and positive ions through dissociative attachment and ion–ion recombination, respectively.
Measured positive ion density (1, 2) as well as model-predicted electron density (3, 4) and relative density of negative ions (5, 6) in CF4 + O2 + Ar plasma as functions of both O2/Ar mixing ratio at \(y_{{CF_{4} }}\) = const (1, 3 and 5) and CF4/O2 mixing ratio at y Ar = const (2, 4 and 6). The input process conditions correspond to Fig. 1
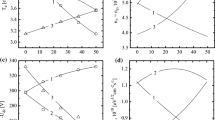
Figures 4 and 5 illustrate kinetics and densities in neutral species in CF4 + O2 + Ar plasmas with various CF4/O2 and Ar/O2 gas mixing ratios. As can be seen from Fig. 4, in the non-oxygenated 50% CF4 + 50% Ar plasma, the main source of F atoms are the electron-impact dissociations of CF4 (R1, R3) and CF3 (R5). These processes constitute approximately 85% of the total F atom formation rate while the contribution from the CF2 and CF radicals through R6 and R8 does not exceed 5%. The remaining 10% comes from R9, which is supported by the high F → F2 recombination rate on the reactor walls in R51. Accordingly, the decay of F atoms is mainly caused by their heterogeneous recombination in R51–R55 while the rate of the fastest bulk process R24 is about 10 times less.
Fluorine atom formation kinetics in CF4 + O2 + Ar plasma at \(y_{{CF_{4} }}\) = const (a) and \(y_{Ar}\) = const (b). The labels on the curves correspond to reaction numbers in Table 1. The term “total F” means the total formation rate for fluorine atoms
The substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const noticeably reduces the rates of R1, R3 and R5 even in the low-oxygenated (\(y_{{O_{2} }}\) < y Ar ) gas mixtures (Fig. 4a) due to the simultaneous decrease in n e , \(n_{{CF_{4} }}\) (3.5 × 1013–1.9 × 1013 cm−3 for 0–12% O2), and \(n_{{CF_{3} }}\) (5.2 × 1012–1.9 × 1012 cm−3 for 0–12% O2) (Fig. 5a). The density of CF3 radicals decreases because of their decomposition in R25, R26, R40 and R41 with the participation of O, O(1D), and CFO. The behavior of \(n_{{CF_{4} }}\) follows that of \(n_{{CF_{3} }}\) because the latter represent the main source of CF4 molecules in the plasma chemical reactions. At the same time, the addition of O2 introduces new channels for the formation of F atoms involving CFO (R16) and CF2O (R17), while also accelerating R9. The high formation rate for the CFO species is provided by R17 and R50, while CF2O is effectively formed in R41, R46 and R47. The acceleration of R9 is due to the rapidly increasing F2 density (\(n_{{F_{2} }}\) = 9.2 × 1011–9.0 × 1012 cm−3 for 0–12% O2) because of the formation of these species in R48 and heterogeneous recombination of F atoms. As a result, the total F atom formation rate increases compared with the 50% CF4 + 50% Ar plasma, which causes an increase in F atom density (n F = 5.8 × 1012–2.6 × 1013 cm−3 for 0–12% O2). The transition to the high-oxygenated (\(y_{{O_{2} }}\) > y Ar ) gas mixtures maintains all the previously mentioned tendencies for reaction rates while also introducing additional mechanisms for the formation of F atoms. Particularly, the electron impact dissociation rate of the FO species (R15) reaches the levels of R16 and R17, and the total effect of R15–R17 becomes greater than the sum of R1, R3 and R5. The high formation rate and density of FO molecules (8.2 × 1010–6.3 × 1012 cm−3 for 12–50% O2) are provided mainly by R22 and the heterogeneous interaction between F and O atoms in R51 and R56. Additionally, the rates of the atom-molecular processes R22, R36–R38 and R44 increase together with the increasing O2 content in the feed gas and, finally, appear to be comparable with R15–R17. Therefore, the substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const provides a continuous increase in the F atom formation rate (Fig. 4a) and thus, the F atom density (Fig. 5a).
The substitution of CF4 for O2 at y Ar = const is escorted by the decreasing amount of CF4 in a feed gas and thus, results in much faster decrease in the rates of R1, R3 and R5 (Fig. 4b) as well as in deeper falls in the densities of fluorocarbon radicals (Fig. 5b). However, until the condition \(y_{{O_{2} }}\) < \(y_{{CF_{4} }}\) is valid, the kinetics of F atoms is quite similar to the described above. Here, and increase in F atom formation rate and density is provided by the rapidly increasing rates of R15–R17 and R36 with a participation of FO, CFO and CF2O. At one and the same \(y_{{O_{2} }}\), the densities of these species appears to be noticeably higher than in previous case because of the higher densities of both O and O(1D). The last effect is connected with the higher formation rate for oxygen atoms in R10, R11 and R14 due to increasing n e as well as with their lower consumption in R25, R26, R28 and R29. At the same time, the transition to the high-oxygenated (\(y_{{O_{2} }}\) > \(y_{{CF_{4} }}\)) gas mixtures changes the situation due to the lack of fluorine-containing species. Particularly, the deep decrease in n CF , \(n_{{CF_{2} }}\) and \(n_{{CF_{3} }}\) limits the formation of CFO and CF2O species through both volume (R40, R41, R50) and heterogeneous (R53, R54, R56) processes. That is why the rates of R15–R17 and R44 exhibit a maximum at about 25–30% O2. Simultaneously, an increasing consumption of F2 in R22 limits the formation of F atoms through R9 which also losses an increasing tendency after 25% O2. Since R22 represents the sufficient source of FO, the maximums on the density of these species as well as on the rates of R36–R38 are shifted toward more oxygenated plasmas and appear at about 35% O2. All these result in the non-monotonic changes in both total F atom formation rate (Fig. 4b) and F atom density (Fig. 5b). In fact, the difference between CF4 + O2 + Ar with y Ar = const and binary CF4 + O2 plasmas under one and the same operating conditions is only in the absolute values of species densities due to the different values of T e and n e in the presence of Ar. From Fig. 5, it can be understood also that the highest values of F atom density, n max F , in the gas systems with \(y_{{CF_{4} }}\) = const and y Ar = const are different and obtained at different fractions of O2 in a feed gas. Particularly, when the substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const takes place, n max F = 4.9 × 1013 cm−3 at 50% O2. At the same time, the substitution of CF4 for O2 at y Ar = const results in n max F = 3.1 × 1013 cm−3 at 25% O2. The remarkable thing is that, in both cases, n max F corresponds to \(y_{{O_{2} }}\) ≈ \(y_{{CF_{4} }}\). This is because the near-to-equal amounts of CF4 and O2 in a feed gas provide the best conditions for both homogeneous and heterogeneous reactions between their dissociation products.
Since the most of dry etching processes in fluorocarbon-based plasmas are provided by the ion-assisted chemical reaction [3,4,5], the issue of key interest is to compare how the changes in CF4/O2 and Ar/O2 mixing ratios influence physical and chemical etching pathways. In the case if the chemical etching pathway is driven by F atoms, the rate of heterogeneous chemical reaction may be taken as \(\gamma_{R}\Gamma _{F}\) [33,34,35], where \(\Gamma _{F}\) is the flux of fluorine atoms, and γ R is the reaction probability. Then, assuming γ R ≈ const at constant surface temperature, the changes in the chemical etching pathway with variations of CF4/O2 and Ar/O2 mixing ratios may be characterized by the changes in \(\Gamma _{F}\) only. The data of Fig. 6 indicate that the fluxes of F atoms follow the behaviors of theirs densities and show the same differences with increasing fraction of O2 in a feed gas. Accordingly, taking in mind the points corresponding to n max F , one can say that the CF4 + O2 + Ar plasma with \(y_{{CF_{4} }}\) = const provides by ~60% higher efficiency of the chemical etching pathway for one and the same etched material. The role of ion bombardment in the CF4 + O2 + Ar plasma may include the sputtering of the native surface atoms, the ion-stimulated desorption of low-volatility reaction products, and the destruction of the FC polymer film. From published works [27, 33,34,35,36], it can be understood that the rate of any mentioned physical etching pathway is given by \(Y_{S}\Gamma _{ + }\), where Y S is the ion-type-averaged sputtering yield. For the ion bombardment energy ɛ i < 500 eV, one can assume Y S to be proportional to the energy transferred by the incident ion to the surface atom [28]. Therefore, the efficiency of the physical etching pathway can be characterized by the ion energy flux \(\varepsilon_{i}\Gamma _{ + }\), where ɛ i ≈ e| − U f − U dc |, and U f is the floating potential. The data of Fig. 7 shows that the substitution of Ar for O2 at \(y_{{CF_{4} }}\) = const results in increasing −U dc in the range of 137–153 V. The increasing ɛ i compensates for the fall of \(\Gamma _{ + }\) (9.0 × 1015–8.1 × 1015 cm−2 s−1 for 0–50% O2), so that the parameter \(\varepsilon_{i}\Gamma _{ + }\) keeps a near-to-constant value (~1.3 × 1018 eV cm−2 s− for 0–50% O2). As such, the Ar/O2 mixing ratio has no noticeable influence on the efficiency of the physical etching pathway. The substitution of CF4 for O2 at y Ar = const causes the slightly decreasing −U dc = 137–125 V for 0–50% O2. This effect does not overcompensate an increasing tendency of \(\Gamma _{ + }\) (9.0 × 1015–1.4 × 1016 cm−2 s−1 for 0–50% O2), so that the parameter \(\varepsilon_{i}\Gamma _{ + }\) also increases in the range of 1.3 × 1018–1.8 × 1018 eV cm−2 s−1, or by 1.5 times. Therefore, one can obtain the intensification of the physical etching pathway in more oxygenated gas mixtures. The difference in \(\varepsilon_{i}\Gamma _{ + }\) between the gas systems with \(y_{{CF_{4} }}\) = const and y Ar = const increases together with \(y_{{O_{2} }}\) and reaches ~1.4 times at 50% O2 (Fig. 6). However, in the points corresponding to n max F such difference appears to be less than 15%. In fact, this means the near-to-equal efficiencies of the physical etching pathways in both gas systems.
Model-predicted fluxes (solid lines) and flux-to-flux ratios (dashed lines) in CF4 + O2 + Ar plasma: a as functions of O2/Ar mixing ratio at \(y_{{CF_{4} }}\) = const; b as functions of CF4/O2 mixing ratio at y Ar = const. The label “ions” means the total positive ion flux while the label “pol” relates to the flux of polymerizing radicals
Another important thing is to compare the parameters, which determine the formation and destruction kinetics for the FC polymer film. From Fig. 7, it can be seen that the gas systems with \(y_{{CF_{4} }}\) = const and y Ar = const exhibit the similar monotonic decrease in the fluxes of polymerizing radicals toward more oxygenated plasmas. Such effects are provided by the decreasing densities of both CF2 and CF shown in Fig. 5. The main quantitative differences in \(\Gamma _{pol}\) appear after 40% O2 where in the case of y Ar = const this parameters falls down to zero. In the points corresponding to n max F , the flux of polymerizing radicals resulted from the variation of CF4/O2 mixing ratio appears to be about 7 times higher compared then that obtained by the change in Ar/O2 mixing ratio (\(\Gamma _{pol}\) = 8.2 × 1013 cm−2 s−1 for 50% O2 at \(y_{{CF_{4} }}\) = const and 6.4 × 1014 cm−2 s−1 for 25% O2 at y Ar = const). At the same time, the higher value of \(\Gamma _{pol}\) in the CF4 + O2 + Ar gas system with variable CF4/O2 mixing ratio does not mean directly the higher polymerizing ability. Earlier, it was found that the FC polymer film growths better in the fluorine-poor plasmas. In such conditions, the polymer surface is composed by the less saturated fluorocarbon groups and thus, appears to be more reactive for the radicals coming from the bulk plasma [29, 37]. That is why the more relevant parameter for evaluation of polymerizing ability in fluorocarbon-based plasmas is the \(\Gamma _{F} /\Gamma _{pol}\) ratio. From Fig. 6, it can be seen that the CF4 + O2 + Ar gas system with variable Ar/O2 mixing ratio shows the systematically higher \(\Gamma _{F} /\Gamma _{pol}\) ratios in the range of 0–50% O2 while in the points corresponding to n max F the difference between two gas systems reaches an order of magnitude (\(\Gamma _{F} /\Gamma _{pol}\) = 13,000 for 50% O2 at \(y_{{CF_{4} }}\) = const and 1100 for 25% O2 at y Ar = const). Therefore, when the composition of CF4 + O2 + Ar gas system is optimized for obtaining the highest F atom density, the variation of Ar/O2 mixing ratio at \(y_{{CF_{4} }}\) = const simultaneously provides the much worse conditions for the deposition of the FC polymer film. However, the FC film thickness (and thus, the FC film effect on the etching kinetics) depends not only on the deposition rate, but also on the rates of FC film destruction through both etching by O atoms and physical sputtering. In our opinion, the FC film formation/destruction balance by chemical and physical pathways may be characterized by \(\Gamma _{O} /\Gamma _{pol}\) and \(\Gamma _{ + } /\Gamma _{pol}\) ratios, respectively. The data of Fig. 6 allow one to conclude that an increase in O2 fraction in a feed gas always shifts the FC film formation/destruction balance toward the destruction. Again, taking in mind the points corresponding to n max F , one can see that variation of Ar/O2 mixing ratio at \(y_{{CF_{4} }}\) = const provides the higher \(\Gamma _{O} /\Gamma _{pol}\) (795 for 50% O2 at \(y_{{CF_{4} }}\) = const vs. 111 for 25% O2 at y Ar = const) and \(\Gamma _{ + } /\Gamma _{pol}\) (98 for 50% O2 at \(y_{{CF_{4} }}\) = const vs. 18 for 25% O2 at y Ar = const) ratios and thus, better suppresses the polymerization. Therefore, one can definitely conclude that the CF4 + O2 + Ar gas system with \(y_{{CF_{4} }}\) = const gives the ability to combine the higher F atom density together with lower amount of FC polymer on the etched surface. Probably, such feature may be useful for the optimization of the etching processes where the key issues are the maximum etching rate and the minimum etching residues.
Conclusion
In this work, we investigated how the changes of CF4/O2 (at constant fraction of Ar) and Ar/O2 (at constant fraction of CF4) mixing ratios in the three-component CF4 + O2 + Ar gas mixture influence plasma parameters (electron temperature, electron and total positive ion densities, ion energy flux, densities and fluxes of neutral species) determining the dry etching kinetics. The investigation combined plasma diagnostics by Langmuir probes and zero-dimensional plasma modeling. It was shown that the variations of CF4/O2 and Ar/O2 mixing ratios at constant fractions of corresponding third component have the opposite effects on T e , n e and n + due to different changes in the electron energy loss channels and formation-decay kinetics for charged species. It was found also that the substitution of CF4 for O2 produces the non-monotonic (with a maximum at 25% O2) changes in both F atom density and flux. At the same time, the substitution of Ar for O2 causes the monotonic growth of these parameters as well as provides their higher values under one and the same operating conditions. Being optimized to highest n F , both gas systems are characterized by the quite close ion energy fluxes and thus, have no principal differences in the efficiencies of physical etching pathway. However, the variation of Ar/O2 mixing ratio at constant fraction of CF4 better suppresses the polymerization and thus, allows one to obtain lower amount of FC polymer on the etched surface.
References
Rossnagel SM, Cuomo JJ, Westwood WD (1990) Handbook of plasma processing technology. Noyes Publications, Park Ridge
Rooth JR (1995) Industrial plasma engineering. IOP Publishing, Philadelphia
Roosmalen AJ, Baggerman JAG, Brader SJH (1991) Dry etching for VLSI. Plenum Press, New York
Wolf S, Tauber RN (2000) Silicon processing for the VLSI era. Process technology, vol 1. Lattice Press, New York
Lieberman MA, Lichtenberg AJ (1994) Principles of plasma discharges and materials processing. Wiley, New York
Kimura T, Ohe K (2002) J Appl Phys 92:1780
Rao MVVS, Sharma SP, Cruden BA, Meyyappan M (2002) Plasma Sources Sci Technol 11:69
Kim J-H, Chung K-H (2005) J Korean Phys Soc 47:249
Jenq J, Ding J, Taylor JW, Hershkowitz N (1994) Plasma Sources Sci Technol 3:154
Efremov AM, Kim D-P, Kim C-I (2004) Vacuum 75:133
Efremov AM, Kwon K-H, Shabadarova DA (2016) Izv Vyssh Uchebn Zaved Khim Khim Tekhnol 59:12
Kimura T, Ohe K (1999) Plasma Sources Sci Technol 8:553
Kimura T, Noto M (2006) J Appl Phys 100:063303
Plumb IC, Ryan KR (1986) Plasma Chem Plasma Proc 6:205
Grigoryev YuN, Gorobchuk AG (2007) Russ Microlectron 36:321
Kawata H, Murata K, Nagami K (1986) J Vac Sci Technol B4:6
Kimura T, Hanaki K (2008) Jpn J Appl Phys 47:8546
Chun I, Efremov A, Yeom GY, Kwon K-H (2015) Thin Solid Films 579:136
Efremov A, Kim JH, Kwon K-H (2014) Plasma Chem Plasma Proc 34:239
Son J, Efremov A, Yun SJ, Yeom GY, Kwon K-H (2014) J Nanosci Nanotechnol 14:9534
Johnson EO, Malter L (1950) Phys Rev 80:58
Sugavara M (1998) Plasma etching: fundamentals and applications. Oxford University Press, New York
Efremov A, Min N-K, Choi B-G, Baek K-H, Kwon K-H (2008) J Electrochem Soc 155:D777
Kwon K-H, Efremov A, Kim M, Min NK, Jeong J, Kim K (2010) J Electrochem Soc 157:H574
Li X, Ling L, Hua X, Fukasawa M, Oehrlein GS, Barela M, Anderson HM (2003) J Vac Sci Technol A 21:284
Gogolides E, Stathakopolous M, Boudouvis A (1994) J Phys D Appl Phys 27:1878
NIST Chemical Kinetica Database. http://kinetics.nist.gov/kinetics/
Chapman B (1980) Glow discharge processes: sputtering and plasma etching. Wiley, New York
Stoffels WW, Stoffels E, Tachibana K (1998) J Vac Sci Technol A 16:87
Christophorou LG, Olthoff JK, Rao MVVS (1996) J Phys Chem Ref Data 25:1341
Krishnakumar E, Srivastava SK (1992) Int J Mass Sectr Ion Proc 113:1
Christophorou LG, Olthoff JK (2004) Fundamental electron interactions with plasma processing gases. Springer, New-York
Lee C, Graves DB, Lieberman MA (1996) Plasma Chem Plasma Proc 16:99
Efremov AM, Kim DP, Kim CI, Trans IEEE (2004) Plasma Sci 32:1344
Efremov AM, Kim DP, Kim CI (2005) Thin Solid Films 474:267
Jin W, Vitale SA, Sawin HH (2002) J Vac Sci Technol A 20:2106
Kokkoris G, Gogolides E, Boudouvis AG (2000) Microelectron Eng 53:395
Acknowledgements
This work was supported by the MOTIE (Ministry of Trade, Industry and Energy) and KSRC (Korea Semiconductor Research Consortium) support program for the development of the future semiconductor device (No. 2016-10052890).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Efremov, A., Lee, J. & Kim, J. On the Control of Plasma Parameters and Active Species Kinetics in CF4 + O2 + Ar Gas Mixture by CF4/O2 and O2/Ar Mixing Ratios. Plasma Chem Plasma Process 37, 1445–1462 (2017). https://doi.org/10.1007/s11090-017-9820-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9820-z