Abstract
Ultraviolet (UV) light with a wavelength of 254 nm was applied to a double dielectric barrier discharge (DDBD) system to decompose of gaseous xylene. The results show that a significantly synergistic effect can be achieved with the introduction of UV light into the DDBD system. When UV light is applied, the system show a 21.8 % increase in its removal efficiency for xylene at 35 kV with an ozone concentration close to 971 ppmv. The CO x (x = CO2 and CO) selectivity of outlet gas rises from 6.54 to 76.2 %. The optimal synergetic effect between UV light and DDBD can be obtained at a peak voltage of 30 kV. The system is robust for humidity, which only slightly reduces the xylene removal efficiency at a high peak voltage (30–35 kV). With the increase of gas flow rate, the removal efficiency for xylene decreases due to a reduced residence time. In addition, the products of xylene degradation were also analyzed. The major products of the degradation were found to be CO2 and H2O while byproducts such as O3 and HCOOH were observed as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution due to volatile organic compounds (VOCs) is receiving more and more attention from the public, especially in developing countries such as China and India. The photochemical smog and aerosol originated from VOCs can cause seriously issues to the environment and human health [1]. As a typical VOC, xylene is often released during the production of fiber, films, or resins. Painting industry is another major source which generates a significant amount of xylene emission. Xylene can harm human being when it is breathed in, ingested, or contacting skin. High-level exposure to xylene can cause dizziness, faint, and even death. Repeated exposure to xylene may cause the damage of bone marrow, leading to a low blood cell count. Therefore, it is necessary to develop technologies to destruct xylene to protect human health and the environment.
As one of alternative treatments [2], dielectric barrier discharge (DBD) plasma has been widely used in the treatment of VOCs [3]. There are two types of DBD reactors: single DBD (SDBD) and double DBD (DDBD). The former has one electrode separated from the working gas flow by a dielectric barrier while the latter has both of two electrodes separated by dielectric barriers. For the DDBD reactors, the two electrodes do not emit or absorb a significant amount of free electrons, the charge transfer across the gap between the dielectric barriers through microdischarges eventually reduces the “net” electric field at the location of each discharge, causing them to extinguish. The DDBD reactors do not rely on the width of imposed voltage pulse to limit the duration of the microdischarges, which makes it possible for them to be driven by a simple high voltage AC power supply. Another advantage of the DDBD reactors is that they have no electrodes exposed to the gas flow, and the material properties of the electrodes cannot affect the microdischarges [4, 5].
Often time, the non-thermal plasma (NTP) discharge process in a DBD or DDBD reactor uses photocatalysts to enhance the removal efficiency of gas pollutants [6–9]. In recent years, the degradation of the gas pollutants by using a combination of NTP and UV attracts lots of attention. Researchers reported that the gas pollutants such as SO2, NO, CS2, and H2S can be directly degraded by combining NTP and UV without using any photocatalysts [10–14]. Robert et al. [15] showed that a synergistic effect can be achieved when toluene was degraded by integrating dielectric barrier discharge (DBD) with UV. Compared with a case using only DBD or UV, the removal efficiency of toluene was significantly improved. Ye et al. [6] adopted DBD to excite a Kr/I2 gas mixture to generate UV light, which was then combined with DBD for benzene degradation. The removal efficiency of benzene was improved by 15.9 % when compared with a DBD only setting. Zhu et al. [16] reported that the decomposition of ethanethiol can be improved with a 185 nm UV light assisted DBD system by 10.4 %, as compared with a DBD only system. These studies suggest that the decomposition of xylene can also benefit a lot from a combination of UV light and NTP.
Up to date, most treatments of xylene with NTP do not involve UV light. For instance, Lee and Chang achieved the best energy efficiency of 7.1 g kWh−1 for the gas streams containing 500 ppmm p-xylene, 5 % O2, 1.6 % H2O(g), and balance N2 at an applied voltage of 18 kV. Further product analysis shows that around 70 or 95 % of the carbons in p-xylene molecules are converted into carbon dioxide for the gas streams without or with water vapor, respectively [17]. In order to examine the possible effect of UV light on NTP, the degradation of a mixed xylene, composed of o-xylene, m-xylene, and p-xylene was investigated in a DDBD system with an optional 254 nm UV light. This mixed xylene is common in the spray paint waste gas. The CO2 selectivity, the role of O3, the impact of gas flow rate on the removal efficiency of xylene were discussed, respectively. The synergy between UV light and DDBD was also analyzed. In addition, the analysis on byproducts generated during the xylene degradation in the UV–DDBD system was carried out as well.
Experimental Section
Experimental Setup
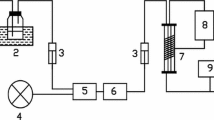
The setup of experiments is shown in Fig. 1. Xylene (ortho-xylene:meta-xylene:para-xylene=2:11:2) was purchased from Sinopharm Chemical Reagent Co., Ltd. The standard air (O2:N2 = 21:79) was used as carrier gas, which was obtained from Hangzhou Jingong Co., Ltd. They were used as received. An air stream was generated from a compressed gas cylinder. After passing a buffer tank, the air stream was divided into three streams. Three mass flow controllers were used to regulate the flow rates of each stream. Gaseous xylene was generated by passing part of the air stream through liquid of xylene which was kept in a controlled-temperature water bath (25 ± 1 °C). Water vapor was introduced into feed gas by passing part of the air stream through a bubbler filled with deionized water. Then the two streams were mixed in a buffer tank with the other stream which was directly from the previous buffer tank. By adjusting the flow rate of the three streams, the concentration of xylene or water vapor were varied to obtain a simulated gas. The simulated gas was then degraded by a UV–DDBD reactor designed in house. The concentrations of xylene and CO2 were simultaneously measured by two gas chromatographs (Fuli 9790, column T = 100 °C, P = 0.1 MPa) whereas ozone concentration was monitored by an ozone analyzer (Eco Sensor UV-100). The flow rate of the gas stream passing through the column of chromatographs is about 2–3 mL min−1. The analysis of chemical composition for the gas streams was accomplished by a Fourier Transform Infrared Spectroscope (FTIR, Thermo Fish IS10), which was used to identify main byproducts after being treated with the UV–DDBD reactor. The humidity of the simulated gas was measured by a hygrometer (Rotronic A1H). All experiments were conducted at atmospheric pressure.
Schematic diagram of the experimental setup (1 gas cylinder; 2 buffer tank; 3 mass flow controller; 4 xylene bubbler; 5 water vapor bubbler; 6 thermo hydrometer; 7 UV–DDBD reactor; 8 high pulse voltage source; 9 high voltage probe; 10 digital storage oscilloscope; 11 current probe 12 gas analytical system)
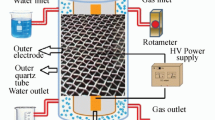
The UV–DDBD reactor was shown in Fig. 2. It is composed of an airtight stainless steel shell, two UV light lamps (Cnlight ZW10D15W-Z212, 254 nm, 15 W), and a DDBD plasma tube. The two UV light lamps are on the top and the bottom of the tube, respectively. There are two electrodes in the tube. The distance between the electrodes is 11 mm. The inner electrode is a copper wire (0.5 mm in diameter), which is in a quartz tube with an outer diameter of 8 mm and an inner diameter of 3 mm. The external electrode is a layer of wire mesh which is wrapped on the exterior of another quartz tube with an outer diameter of 25 mm and an inner diameter of 20 mm. The external electrode is grounded. A Blumlein pulse forming network (BPFN) type of narrow pulse generator was described in detail in previous work [18]. The pulse frequency adopted in this study is 100 pps (pulse second) and the range of peak voltage is 15–35 kV. The pulse voltage and current waveforms were measured by a four channel digital storage oscilloscope (Tektronix TDS2014B 350 MHZ) capable of sampling 1 GS s−1 (Giga Sample per second), a high voltage passive probe (Texas HVP-3020), and a current probe (CT4 TCP 202).
Analysis Methods
The xylene removal efficiency (η), carbon dioxide selectivity (Sco2), specific input energy (SIE), and energy yield (EY) are defined as follows (Eqs. 1, 2, 3, 4):
Xylene removal efficiency (η):
Carbon dioxide selectivity (Sco2):
Specific input energy (SIE, J/L):
Energy yield (EY, mg kJ−1):
where C 1 and C 2 are the inlet and outlet concentrations of xylene (mg m−3), respectively. 8 in Eq. (2) is the number of carbon atoms in a xylene molecule. [CO2] is the CO2 concentration of outlet gas (mg m−3). U and I are the input voltage (kV) and current (mA), respectively. Q is the gas flow rate (L s−1).
Results and Discussion
Synergetic Effect of UV Light and DDBD
In order to determine whether there is a synergetic effect existing in the UV–DDBD system, three types of settings were examined. The first one had only UV light applied to the gas stream of xylene, while the second one had only DDBD applied. For the third one, both UV light and DDBD were applied. The xylene removal efficiency of all the three settings were investigated at an inlet gas flow rate of 200 mL min−1 and a xylene concentration of 45 mg m−3. The peak voltage was varied from 15 to 35 kV.
As shown in Table 1, the direct photolysis of xylene in the UV light only setting was found to be ineffective. This is consistent with other studies. For instances, Alapi et al. [19] observed that UV irradiation at a wavelength of 254 nm showed no significant effects for the direct photolysis of methyl chloride. Due to the generation of a large number of active species during NTP discharge [17], the DDBD only setting was found to be much more effective than the UV light only setting in a peak voltage range of 15–35 kV.
When both UV light and DDBD were used, the removal efficiency of xylene was further enhanced. At a peak voltage ranging from 15 to 25 kV, the xylene removal efficiency of the UV–DDBD setting is about twice than that of the DDBD one. At a peak voltage of 30 kV, the former is more than four times larger than the latter. The results indicate that there is a strong synergetic effect between UV light and DDBD. UV light may greatly enhance the generation of active species and promote effective collision among active species, xylene, and intermediate products, which leads to the significant improvement in the removal efficiency of xylene. Table 1 also shows that the strong synergetic effect does not apply to the whole range of peak voltage examined in this study. For instance, the effect of UV light and DDBD are simply additive at the peak voltage ranging from 15 to 25 kV.
In order to quantify the synergetic effect of UV light and DDBD for the xylene removal efficiency, cofactor R was introduced as follows [20]:
where ηhybrid represents the removal efficiency of xylene in the UV–DDBD setting, ηtheory is the theoretical value of xylene removal efficiency, ηUV is the xylene removal efficiency in the UV light only system, and ηDDBD is the xylene removal efficiency in the DDBD only system. As shown in the Table 1, UV light and DDBD had a synergetic effect on the R value, which was greater than 1, at the peak voltage of 15–35 kV. Moreover, the strength of the synergetic effect is relatively low for most of the peak voltages examined. The strongest synergetic effect between UV light and DDBD was obtained at the voltage of 30 kV, where R was equal to 4.4. Applying UV light to the DDBD setting not only reduced the breakdown voltage but also accelerated the decomposition of xylene [6].
Role of Ozone
O3 is one of the important active species generated by UV light and high energy electrons due to discharging [21]. It is directly related to the decomposition of xylene. Therefore, three types of settings were also used to examine their capabilities in generating O3, respectively. One had UV light only, one had DDBD only, and another one had both UV light and DDBD applied to a gas stream. In order to obtain a clear picture about how these settings affected O3 generation, the gas stream contained no xylene. The O3 concentration in all the three settings were investigated at an inlet gas flow rate of 200 mL min−1. The peak voltage was in the range of 15–35 kV.
As shown in Fig. 3, with the UV light only setting, the concentration of O3 is 5 ppm. As a comparison, the DDBD setting is able to generate O3 similarly at the peak voltage of 15 kV but a greater amount than the UV light setting when the peak voltage is beyond 20 kV.
The production of O3 is at a high level for the DDBD only setting at large peak voltages (30–35 kV) and increases as the peak voltage gets larger. In a related study, Chang et al. [10] reported that the amount of O3 produced in a DBD system reaches 425 ppmv at the peak voltage of 25 kV. When the peak voltage is 35 kV, the O3 concentrations is 971 and 952 ppmv for UV–DDBD and DDBD systems, respectively. With the presence of UV light, O3 is partially consumed (Eqs. 7, 8) [22, 23].
This explains the slower increase of O3 concentration for larger peak voltages.
In order to obtain the direct relationship between xylene degradation and O3 consumption, a factor called consumption quantity which reflects the ozone utilization efficiency (CQ) was defined as follows:
\(\Delta {\text{C}}_{{{\text{O}}_{3} }}\) represents the difference of the amount of O3 between in the inlet and outlet of a DDBD reactor when there is no xylene in the inlet gas stream. \(\Delta {\text{C}}_{{{\text{C}}_{8} {\text{H}}_{10} }}\) is the difference of the amount of xylene between the inlet and outlet of the DDBD reactor. CQ for both the DDBD and UV–DDBD settings were compared with an inlet gas flow rate of 200 mL min−1 and a xylene concentration of 45 mg m−3. The results in Table 2 show that there are optimal values of CQ for both the DDBD and UV–DDBD settings due to the existence of competing reactions such as the ones shown in Eqs. 7 and 10. The optimal value of CQ is 38.48 at a peak voltage of 30 kV for the DDBD setting. For the UV–DDBD setting, the optimal value of CQ is 71.66 at a peak voltage of 25 kV instead.
Effect of Humidity
The effect of water vapor on a UV–DDBD system cannot be neglected because water vapor is almost ubiquitous in the organic waste gases treatments for industrial applications. Water vapor can significantly affect VOCs degradation in NTP systems [24–26]. Figure 4 shows that the xylene removal efficiency as a function of peak voltage in the UV–DDBD reactor in dry or humid conditions with 80 % relative humidity, respectively. The system was operated at a simulated gas flow rate of 200 mL min−1 and a xylene inlet concentration of 45 mg m−3.
No significant difference in xylene removal efficiency was observed in either the dry or humid conditions. The xylene removal efficiency is less than 20.0 % when the peak voltage ranges from 15 to 25 kV. The xylene removal efficiency increases rapidly after the peak voltage reaches 30 kV. This rapid increase can be explained by the break of the weakest C–C (332 kJ mol−1) bond in xylene when the peak voltage is greater than 25 kV [18]. Higher voltage leads to a larger energy input which generates many more high-energy active species and enhances xylene degradation [27]. This result is also consistent with other related studies. For instance, Zhang et al. [14] observed a similar trend when decomposing H2S gas with a combined plasma photolysis reactor.
The results also suggests that the effect of water is twofolded. When the peak voltage ranges from 15 to 25 kV, the xylene removal efficiency measured in humid condition is slightly higher (by 3.0–6.5 %) compared with the dry condition, which can be explained by the formation of ·OH and ·HO2 radicals from H2O (Eqs. 10, 11) [28]. The mechanism can also be used to explain the relatively low O3 concentration in the humid condition compared with the dry condition, as shown Fig. 5.
When the peak voltage is larger than 25 kV, the break of the bonds in xylene became more and more dominant. As the existence of H2O in the DBD reactor also restricts the electrons density and consumed active species, water vapor shows an overall inhibitory effect on xylene degradation, suggested by Guo et al. [29].
Effect of Peak Voltage
Energy yield (EY) is an important performance indicator in industrial application of UV–DDBD, therefore, the effect of peak voltage on EY in both DDBD and UV–DDBD settings was investigated with a simulated waste gas flow rate of 200 mL min−1 and an inlet xylene concentration of 45 mg m−3. As shown in Fig. 6, EY of xylene obtained in the UV–DDBD setting is significantly higher than the yield of the DDBD setting. When the peak voltage is larger than 25 kV, EY gradually decreases with the increase of peak voltage, which is in accordance with the results of xylene degradation by DBD-photocatalysis systems [30, 31]. The highest EY is achieved when the peak voltage reduces to the minimum value and the obtained EY in the UV–DDBD and DDBD setting at the peak voltage of 15 kV are 0.086 and 0.037 mg kJ−1, respectively. When the peak voltage is larger than 30 kV, the degradation of xylene becomes efficient. EY in UV–DDBD and DDBD are about 0.03 and 0.02 mg kJ−1, respectively.
Effect of Gas Flow Rate
The flow rate of a xylene gas stream affects two key operation parameters, the retention time and the energy density in a UV–DDBD reactor. The xylene removal efficiency in the reactor was examined in a dry condition with various flow rates of the gas stream (200–1200 mL min−1) and a xylene inlet concentration of 45 mg m−3. As showed in Fig. 7, the removal efficiency of xylene declines with the increase in the flow rate of the gas stream. The decline is similar for low peak voltages, ranging from 15 to 25 kV. At high voltages, the decline gets pretty significant. For instance, at a peak voltage of 35 kV, the removal efficiency of xylene decreases from 88.4 to 38.1 % when the flow rate rises from 200 to 1200 mL min−1. The decline is due to the difference in the retention time of waste gas in the reactor at different flow rates. As the flow rate of gas flow rises from 200 to 1200 mL min−1, the retention time of gas in the UV–DDBD reactor is down from 28.5 to 4.8 s. Meanwhile, the energy density in UV–DDBD reactor decreases as well. The result is consistent with other similar studies. For example, Zhu et al. [16] observed that the removal efficiency of ethanethiol decreases from 96.5 to 68.5 % as the inlet gas flow rate increases from 0.5 to 1.8 m3 h−1 in a UV–DBD reactor.
Carbon Balance
CO x selectivity accounts for the proportion of CO and CO2 converted from xylene after a treatment. Higher CO2 selectivity suggests that less byproducts are produced during the treatment. The CO and CO2 selectivity of the DDBD only or UV–DDBD setting was compared in Fig. 8. The experiments for CO x selectivity were performed with a simulated waste gas flow rate of 200 mL min−1 and an inlet xylene concentration of 45 mg m−3 in dry condition. CO x selectivity is low (less than 25.0 %) with either the DDBD or UV–DDBD setting when the peak voltage ranges from 15 to 25 kV. When the peak voltage rises from 25 to 35 kV, the CO x selectivity for the UV–DDBD setting quickly rises to 76.2 % (CO2 selectivity is 47.0 % and CO selectivity is 29.2 %), which is significantly larger than that for the DDBD only setting. When the peak voltage is 30 kV, the UV–DDBD reactor exhibits a greater enhancement in CO x selectivity as compared to the DBBD reactor. This suggests that UV light not only improves the removal efficiency of xylene, but also promotes the degradation of intermediate products, leading to less byproducts in the outlet gas stream. Strong oxidizing active species such as O3 and ·OH, generated by UV light, are believed to be the major reason for the enhancement of CO x selectivity [6].
Product Analysis
To investigate the effects of UV and different operating parameters, FTIR was used to analyze byproducts. The FTIR spectrum of the byproducts of xylene degradation in either dry or humid (80 % RH) conditions with the DDBD only or UV–DDBD settings are shown in Fig. 8. The primary products of xylene degradation are CO2, H2O and HCOOH for these cases. Although N2O and N2O5 are among reaction products, their amounts are really low. There is also a small quantity of CO observed in the outlet gas stream. The amount of CO is significantly decreased when a humid condition exists. The highest amount of HCOOH was obtained in the DDBD setting in a dry condition. In this case, CO2 selectivity was low compared with the other two cases (Fig. 9).
Generally, O3 is considered to be a byproduct which poses risks to the environment. With UV light, the concentration of ozone decreases because more ozone are utilized to decompose xylene. Results also show that O3 generated in the reactor can be effectively removed with the existence of water vapor. In a humid condition, the amount of O3 in reaction products is greatly reduced compared to dry condition. The reason for this reduction is twofolded. One is that the existence of H2O leads to the reduction of ·O, which is a precursor of O3 (Eq. 12) [32]. The other is that O3 can directly react with H2O in the reactor (Eqs. 13, 14) [20].
Conclusions
In this work, the degradation of gaseous xylene in a DDBD reactor combined with UV light at a wave length of 254 nm was studied. Results show that the xylene removal efficiency reaches 88.4 % when UV light is applied to the DDBD reactor. Compared with DDBD only setting, the removal efficiency is increased by 21.8 %. UV light also significantly promotes the CO2 selectivity when peak voltage is larger than 30 kV. At 35 kV, the CO2 selectivity for UV–DDBD reactor is as high as 76.2 %. The maximum synergistic effect of UV and DDBD was observed to be at a peak voltage of 30 kV. Moreover, the performance of UV–DDBD system is pretty robust in a humid condition.
References
Duane M, Poma B, Rembges D, Astorga C, Larsen BR (2002) Isoprene and its degradation products as strong ozone precursors in Insubria, Northern Italy. Atmos Environ 36:3867–3879
Sultana S, Vandenbroucke AM, Leys C, De Geyter N, Morent R (2015) Abatement of VOCs with alternate adsorption and plasma-assisted regeneration: a review. Catalysts 5:718–746
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46
Chae JO, Moon SI, Sun HS, Kim KY, Vassiliev VA, Mikholap EM (1999) A study of volatile organic compounds decomposition with the use of non-thermal plasma. KSME Int J 13:647–655
Zhang HB, Li K, Shu CH, Lou ZY, Sun TH, Jia JP (2014) Enhancement of styrene removal using a novel double-tube dielectric barrier discharge (DDBD) reactor. Chem Eng J 256:107–118
Ye ZL, Shen Y, Zhang RX, Hou HQ (2008) Destruction of benzene in an air stream by the outer combined plasma photolysis method. J Phys D Appl Phys 41:025201
Assadi AA, Bouzaza A, Merabet S, Wolbert D (2014) Modeling and simulation of VOCs removal by nonthermal plasma discharge with photocatalysis in a continuous reactor: synergetic effect and mass transfer. Chem Eng J 258:119–127
Palau J, Assadi AA, Penya-Roja JM, Bouzaza A, Wolbert D, Martinez-Soria V (2015) Isovaleraldehyde degradation using UV photocatalytic and dielectric barrier discharge reactors, and their combinations. J Photochem Photobiol A 299:110–117
Zhang HB, Li K, Sun TH, Jia JP, Lou ZY, Yao SA, Wang G (2015) The combination effect of dielectric barrier discharge (DBD) and TiO2 catalytic process on styrene removal and the analysis of the by-products and intermediates. Res Chem Intermed 41:175–189
Chang MB, Balbach JH, Rood MJ, Kushner MJ (1991) Removal of SO2 from gas streams using a dielectric barrier discharge and combined plasma photolysis. J Appl Phys 69:4409–4417
Chang MB, Kushner MJ, Rood MJ (1993) Removal of SO2 and no from gas streams with combined plasma photolysis. J Environ Eng ASCE 119:414–423
Fang HH, Hou HQ, Xia LY, Shu XH, Zhang RX (2007) A combined plasma photolysis (CPP) method for removal Of CS2 from gas streams at atmospheric pressure. Chemosphere 69:1734–1739
Huang L, Xia LY, Ge XX, Jing HY, Dong WB, Hou HG (2012) Removal of H2S from gas stream using combined plasma photolysis technique at atmospheric pressure. Chemosphere 88:229–234
Zhang H, Ji TY, Zhang RX, Hou HQ (2012) Destruction of H2S gas with a combined plasma photolysis (CPP) reactor. Plasma Sci Technol 14:134–139
Robert P, Sung KJ, Chul A, Soon YY, Demidyuk V (2006) Experimental study of toluene decomposition by combination of barrier discharge plasma and UV light. WIT Trans Ecol Environ 86:401–410 (J. W. S. Longhurst and C. A. Brebbia)
Zhu CZ, Liu Y, Lu J, Yang Z, Li YX, Chen TH (2015) Decomposition of ethanethiol using dielectric barrier discharge combined with 185 nm UV-light technique. Plasma Chem Plasma Process 35:355–364
Lee HM, Chang MB (2003) Abatement of gas-phase p-xylene via dielectric barrier discharges. Plasma Chem Plasma Process 23:541–558
Ruan JJ, Li W, Shi Y, Nie Y, Wang X, Tan TE (2005) Decomposition of simulated odors in municipal wastewater treatment plants by a wire-plate pulse corona reactor. Chemosphere 59:327–333
Alapi T, Dombi A (2007) Direct VUV photolysis of chlorinated methanes and their mixtures in an oxygen stream using an ozone producing low-pressure mercury vapour lamp. Chemosphere 67:693–701
Pan H, Su QF, Wei JW, Jian YF (2015) Promotion of nonthermal plasma on the SO2 and H2O tolerance of Co–In/Zeolites for the catalytic reduction of NOx by C3H8 at low temperature. Plasma Chem Plasma Process 35:831–844
Assadi AA, Bouzaza A, Vallet C, Wolbert D (2014) Use of DBD plasma, photocatalysis, and combined DBD plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—synergetic effect and byproducts identification. Chem Eng J 254:124–132
Taranto R, Frochot D, Pichat P (2007) Combining cold plasma and TiO2 photocatalysis to purify gaseous effluents: a preliminary study using methanol-contaminated air. Ind Eng Chem Res 46:7611–7614
Huang HB, Ye DQ (2009) Combination of photocatalysis downstream the non-thermal plasma reactor for oxidation of gas-phase toluene. J Hazard Mater 171:535–541
Van Durme J, Dewulf J, Sysmans W, Leys C, Van Langenhove H (2007) Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere 68:1821–1829
Chen J, Yang JT, Pan H, Su QF, Liu YM, Shi Y (2010) Abatement of malodorants from pesticide factory in dielectric barrier discharges. J Hazard Mater 177:908–913
Fan X, Zhu TL, Wan YJ, Yan XA (2010) Effects of humidity on the plasma-catalytic removal of low-concentration BTX in air. J Hazard Mater 180:616–621
Assadi AA, Palau J, Bouzaza A, Penya-Roja J, Martinez-Soriac V, Wolbert D (2014) Abatement of 3-methylbutanal and trimethylamine with combined plasma and photocatalysis in a continuous planar reactor. J Photochem Photobiol A 282:1–8
Chen J, Su QF, Pan H, Wei JW, Zhang XM, Shi Y (2009) Influence of balance gas mixture on decomposition of dimethyl sulfide in a wire–cylinder pulse corona reactor. Chemosphere 75:261–265
Guo YF, Ye DQ, Chen KF, Tian YF (2006) Humidity effect on toluene decomposition in a wire-plate dielectric barrier discharge reactor. Plasma Chem Plasma Process 26:237–249
Ye ZP, Wang CX, Shao ZH, Ye Q, He Y, Shi Y (2012) A novel dielectric barrier discharge reactor with photocatalytic electrode based on sintered metal fibers for abatement of xylene. J Hazard Mater 241:216–223
Wei BL, Chen YP, Ye MJ, Shao ZH, He Y, Shi Y (2015) Enhanced degradation of gaseous xylene using surface acidized TiO2 catalyst with non-thermal plasmas. Plasma Chem Plasma Process 35:173–186
Chen JH, Wang PX (2005) Effect of relative humidity on electron distribution and ozone production by DC coronas in air. IEEE Trans Plasma Sci 33:808–812
Acknowledgments
This work was supported by the National Natural Science Foundation of China through (Grant Numbers 21450110411, 21476191, 91434110).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Y., Shao, Z., Shou, T. et al. Abatement of Gaseous Xylene Using Double Dielectric Barrier Discharge Plasma with In Situ UV Light: Operating Parameters and Byproduct Analysis. Plasma Chem Plasma Process 36, 1501–1515 (2016). https://doi.org/10.1007/s11090-016-9741-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9741-2