Abstract
The effect of stage number of multistage AC gliding arc discharge reactors on the process performance of the combined reforming and partial oxidation of simulated CO2-containing natural gas having a CH4:C2H6:C3H8:CO2 molar ratio of 70:5:5:20 was investigated. For the experiments with partial oxidation, either pure oxygen or air was used as the oxygen source with a fixed hydrocarbon-to-oxygen molar ratio of 2/1. Without partial oxidation at a constant feed flow rate, all conversions of hydrocarbons, except CO2, greatly increased with increasing number of stages from 1 to 3; but beyond 3 stages, the reactant conversions remained almost unchanged. However, for a constant residence time, only C3H8 conversion gradually increased, whereas the conversions of the other reactants remained almost unchanged. The addition of oxygen was found to significantly enhance the process performance of natural gas reforming. The utilization of air as an oxygen source showed a superior process performance to pure oxygen in terms of reactant conversion and desired product selectivity. The optimum energy consumption of 12.05 × 1024 eV per mole of reactants converted and 9.65 × 1024 eV per mole of hydrogen produced was obtained using air as an oxygen source and 3 stages of plasma reactors at a constant residence time of 4.38 s.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, natural gas has become a versatile energy resource due to both environmental reason and due to its lower price as compared to petroleum. Therefore, the consumption of natural gas as an alternative fuel is currently increasing [1]. Generally, natural gas mostly contains a large amount of methane with lesser amounts of ethane, propane, and carbon dioxide, and its composition varies according to sub-geological conditions. Because methane is an inexpensive fuel, attempts at converting methane to more valuable hydrogen and higher hydrocarbons with new alternative technologies have been increasingly carried out. Moreover, the direct utilization of methane and carbon dioxide has been an area of great interest due to its environmentally friendly concept of decreasing greenhouse gas emissions. Nevertheless, methane reforming with carbon dioxide, as shown in Eq. 1, using conventional catalytic methods often encounters two main problems; it is a highly endothermic reaction consuming a large amount of energy, and the deactivation of catalysts due to coke deposition on the catalyst surface commonly occurs under various reaction conditions [2].
Methane reforming with carbon dioxide:
The second route for methane conversion to syngas is the steam reforming process, as shown in Eq. 2. This reaction is also highly endothermic, resulting in high energy consumption. Steam reforming reactors generally run with excess amounts of water in order to prevent coke deposition on the catalyst surface [3]. Methane reforming with steam has poor selectivity to CO and produces syngas with a high H2/CO ratio, while methane reforming with carbon dioxide gives a higher CO selectivity and a lower H2/CO ratio [4].
Steam reforming of methane:
Another route used to produce syngas is the catalytic partial oxidation of methane, as shown in Eq. 3. This process gives high activity and selectivity, but cannot be easily controlled because of the generation of hot spots in the catalytic bed due to its exothermic nature [4].
Partial oxidation of methane:
A combination of these reactions can potentially provide good and synergistic performance. For example, the combination of carbon dioxide reforming and steam reforming of methane can reduce coke deposition on the catalyst surface, resulting in a controlled and limited coke formation [5]. In addition, the combination of carbon dioxide reforming and partial oxidation of methane has a benefit in terms of balancing the heat load and reducing energy consumption [6, 7].
An attractive alternative for reforming hydrocarbon compounds is to use non-thermal plasma processes. The plasma contains highly active species (such as electrons, ions, and radicals), which can react with methane to produce various valuable products under ambient conditions. The electrical discharge produced also provides heat to the system, apart from generating radicals and excited species to initiate and enhance the plasma chemical reactions [8]. Furthermore, the plasma reforming processes can be operated at mild conditions with less energy consumption, and they have been employed for many applications [9–16].
Our previous works have addressed the reforming of simulated natural gas, which contained a CH4:C2H6:C3H8:CO2 molar ratio of 70:5:5:20, with and without partial oxidation, using a single gliding arc discharge plasma reactor [17, 18]. The addition of air was found to be superior to pure oxygen for hydrogen production with an optimum hydrocarbon-to-oxygen molar ratio of 2/1. In this present work, a multistage gliding arc plasma system comprising four gliding arc discharge plasma reactors connected in series was used for the investigation of a combined reforming and partial oxidation of the simulated natural gas to produce hydrogen and higher hydrocarbons. Initially, the effect of stage number of plasma reactors was systematically investigated to evaluate the process performance under two series of experiments with a fixed feed flow rate and a fixed residence time. Both pure oxygen and air were comparatively used as oxygen sources. A comparison of process performance between the systems, with and without partial oxidation, was finally made. The optimum stage number with corresponding low energy consumption was obtained.
Experimental
Reactant Gases
Simulated natural gas (containing methane, ethane, propane, and carbon dioxide, with a CH4:C2H6:C3H8:CO2 molar ratio of 70:5:5:20) was specially produced for this work by Thai Industry Gas (Public) Co., Ltd. Ultra-high purity oxygen and air zero used for performing the combined plasma reforming and partial oxidation of the simulated natural gas were also supplied by Thai Industry Gas (Public) Co., Ltd.
Setup of the Multistage Gliding Arc Discharge System
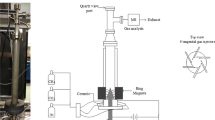
The experimental setup of the multistage AC gliding arc discharge system with 4 stages in series and the configuration of each reactor are shown in Figs. 1 and 2, respectively. The gliding arc reactors were made of a glass tube with 9 cm OD and 8.5 cm ID. Each reactor had two diverging knife-shaped electrodes that were fabricated from stainless steel sheets with a 1.2 cm width for each electrode. The gap distance between the pair of electrodes was fixed at 6 mm. Two Teflon sheets were placed at the top and bottom of the electrodes to force the feed gas to pass through the reaction zone. The flow rates of the reactant gases were regulated by a set of mass flow controllers and transducers supplied by SIERRA® Instrument, Inc. Stainless steel (7 μm) filters were placed upstream of all mass flow controllers in order to trap any solid particles in the reactant gases. The check valves were also placed downstream of the mass flow controllers to prevent any backflow. The simulated natural gas was well mixed with either pure oxygen or air to obtain a hydrocarbon-to-oxygen molar ratio of 2/1, which was the optimum ratio found in our previous work [18]. The mixed reactant gases were introduced upward into the first reactor at ambient temperature and atmospheric pressure. The compositions of the feed gas mixture and the effluent gases were analyzed by an on-line gas chromatograph (HP, 5890), equipped with a Carboxen 1000 packed column connected to a thermal conductivity detector (TCD) and a PLOT Al2O3 “S” capillary column connected to a flame ionization detector (FID).
For any studied conditions, the feed gas mixture was first introduced into the gliding arc system without turning on the power supply unit. After the composition of outlet gas was invariant with time, the power supply unit was turned on. The outlet gas composition was analyzed every 30 min by the on-line GC. After the system reached steady state, an analysis of the outlet gas composition was taken at least few times every hour. The experimental data were averaged to evaluate the process performance. During the experiments, the temperature at the reactor wall was found to be in the range of 150–200°C. Since the volume of the reactor outlet zone was rather large, the outlet gas was cooled close to room temperature. It was observed that a small amount of water droplets appeared on the surrounding inner wall of the gliding arc reactor during the experiment. In the case of using air, a NO X analyzer was employed to determine if there were any nitrogen oxides present in the effluent gas; none were detected. Therefore, under the studied conditions, nitrogen oxides were not produced by the plasma system. The flow rates of both the feed and the outlet gases were measured by using a digital bubble flow meter because of the gas volume change after the reaction.
The power supply unit used in this work was operated in three steps. For the first step, the domestic AC input of 220 V and 50 Hz was converted to a DC output of 70 V by a DC power supply converter. For the second step, a 500 W power amplifier with a function generator was used to convert the DC to AC with a sinusoidal waveform. For the final step, the output voltage was stepped up by using two transformers in series. The output voltage and frequency were controlled by the function generator. Since the plasma generated in each plasma reactor is non-equilibrium in nature, it is not possible to measure the voltage across the electrodes of the reactor (high-side voltage). Therefore, the low-side voltage and current were measured instead, and the high-side voltage and current were then calculated by multiplying and dividing by a factor of 130, respectively. A power analyzer was used to measure power, frequency, and voltage at the low side of the power supply unit. Based on our previous work on the combined reforming and partial oxidation of CO2-containing natural gas using a single-stage gliding arc discharge system [18], the obtained optimum operating conditions (an applied voltage of 17.5 kV, an input frequency of 300 Hz, and a hydrocarbon-to-oxygen molar ratio of 2/1) were used as the base conditions to operate the multistage gliding arc discharge system in this work.
Reaction Performance Evaluation
The reactant conversion is defined as:
The selectivities for C-containing products are defined on the basis of the amount of C-containing reactants converted into any specified product, as stated in Eq. 5 below. In the case of the hydrogen product, its selectivity is calculated based on H-containing reactants converted, as stated below in Eq. 6:
where
[P] = moles of product in the outlet gas stream
[R] = moles of each reactant in the feed stream to be converted
CP = number of carbon atoms in the product molecule
CR = number of carbon atoms in each reactant molecule
where
HP = number of hydrogen atoms in the product molecule
HR = number of hydrogen atoms in each reactant molecule
The product yields are formulated as follows:
The specific energy consumption is calculated in a unit of electron (eV) per mole of C-containing reactants converted or per mole of hydrogen produced (eV/mol) using the following equation:
where
P = power (W)
M = rate of converted carbon in the feed or rate of produced hydrogen molecules (g mol min−1)
1 eV = 1.602 × 10−19 W s
Results and discussion
Results of Natural Gas Reforming without Partial Oxidation
Effect of Stage Number on Reactant Conversion and Product Yield
Constant Residence Time
The reactant conversions and product yields as a function of stage number of plasma reactors when varying feed flow rate at a constant residence time of 4.38 s are illustrated in Fig. 3a. For each stage of plasma reactors, the feed flow rate was controlled at 31.25, 62.50, 93.75, and 125 cm3/min, respectively, in order to maintain the same residence time (4.38 s). The results show that only the conversion of C3H8 gradually increased with increasing stage number, whereas the conversions of the rest of the reactant components remained almost unchanged. It was also confirmed experimentally by the concentrations of outlet gases that the concentration of C3H8 sharply decreased, whereas the CH4 concentration was nearly unchanged. The explanation is that the conversion of reactant gases depends upon the collision between the highly energetic electrons and the reactant gases, which is governed by both residence time and stage number of plasma reactors. Since the plasma operation in this experimental part was performed at a constant residence time, the process performance must be governed by the stage number. The results suggest that the formations of CH4 and C2H6 are approximately equal to the conversions of these two reactants, leading to unchanged CH4 and C2H6 conversions. Another reason is due to their different bond dissociation energies, which are 4.33, 4.35, 4.55, and 5.52 eV, for propane, ethane, methane, and CO2, respectively [19]. Moreover, the H2 and C2 yields only slightly increased with increasing stage number of plasma reactors, whereas the CO yield remained almost unchanged (Fig. 3a). The results suggest that the coupling reactions of the active species and oxidative dehydrogenation reactions may not be significantly affected by the stage number.
Effect of stage number of plasma reactors on reactant conversions and product yields for the reforming of natural gas without O2 or air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
The reactant conversions and product yields as a function of stage number of plasma reactors at a constant feed flow rate of 125 cm3/min are illustrated in Fig. 3b. The residence time of the single stage, 2, 3, and 4 stages was controlled at 1.09, 2.19, 3.29, and 4.38 s, respectively. The conversions of all hydrocarbons, except CO2, increased considerably with increasing stage number because of the longer residence time. Beyond 3 stages, only the propane conversion increased, but the other reactant conversions remained almost unchanged. Comparatively, the propane conversion increased rapidly with increasing stage number of plasma reactors, whereas the methane conversion tended to slightly increase. The conversions of all reactant gases were in the following order: propane > ethane > methane > CO2. The same reasons as mentioned above can be used to explain the different conversions of all reactants. For the H2 and C2 yields, the increase in residence time due to the increase in the stage number of plasma reactors enhances the conversion of all reactants, as aforementioned, and consequently leads to increasing the production yields. However, the CO yield only slightly increased with increasing stage number of plasma reactors, possibly because the system was operated at an oxygen-deficient condition (a hydrocarbon-to-oxygen molar ratio of 2/1).
Effect of Stage Number on Product Selectivity and Product Molar Ratio
Constant Residence Time
The effect of stage number of plasma reactors on the selectivities for H2, C2H2, C2H4, C4H10, and CO at a constant residence time of 4.38 s is depicted in Fig. 4a. The selectivities for H2 and C2H4 tended to slightly increase with increasing stage number from 1 to 3 stages, and the selectivities for CO and C4H10 remained almost unchanged. The results are relevant to the molar ratios of H2/C2H2 and C2H4/C2H2, as also shown in Fig. 4a, while the molar ratio of H2/C2H4 gradually decreases. This implies that the increase in the C2H4 selectivity exceeds the increase in the H2 selectivity, resulting in the gradually decreasing H2/C2H4 ratio. It should be noted, therefore, that the hydrogenation of C2H2 more favorably occurs than the coupling reaction of hydrocarbon active species with increasing stage number of plasma reactors from 1 to 3 stages, leading to the consumption of H2 to some extent to produce C2H4. At 3 stages of plasma reactors, the maximum selectivity for H2 was obtained at approximately 38.2%. From this point of view, it could be concluded that the increase in stage number of plasma reactors from 1 to 3 stages assists in improving the H2 selectivity.
Effect of stage number of plasma reactors on product selectivities and product molar ratios for the reforming of natural gas without O2 or air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
The effect of stage number of plasma reactors on the product selectivities at a constant feed flow rate of 125 cm3/min is shown in Fig. 4b. The results reveal that the selectivities for H2 and C2H2 markedly increased with increasing stage number of plasma reactors, whereas those for C2H4, C4H10, and CO remained almost unchanged. This implies that the oxidative dehydrogenation preferably occurs at a high residence time. The H2/C2H4 ratio increased with increasing stage number of plasma reactors from 1 to 3 stages, whereas the molar ratio of C2H4/C2H2 slightly decreased, suggesting that the dehydrogenation of C2H4 is likely to occur when the stage number of plasma reactors is increased. Moreover, the coupling reaction of hydrogen radicals can occur, and results in the great increase in the selectivity for H2 at 3 stages of plasma reactors. In contrast, the selectivities for C2H4, C4H10, and CO remained almost unchanged. From these experimental results, it can be concluded that the rate of dehydrogenation increases with increasing stage number of plasma reactors when the multistage system is operated at a constant feed flow rate.
Effect of Stage Number on Energy Consumption
Constant Residence Time
The effect of stage number of plasma reactors on energy consumption per mole of reactants converted and per mole of hydrogen produced at the constant residence time is shown in Fig. 5a. The energy consumption per mole of hydrogen produced sharply declined from 1 to 2 stages of plasma reactors and then gradually decreased with increasing stage number from 2 to 4 stages, while the energy consumption per mole of reactants converted also decreased in the same manner as the energy consumption per mole of hydrogen produced when the stage number increased from 1 to 2 stages but became almost unchanged with further increasing stage number up to 4 stages. The minimum energy consumption was about 1.03 × 1025 eV per mole of reactants converted and 1.28 × 1025 eV per mole of hydrogen produced at 4 stages of plasma reactors.
Effect of stage number of plasma reactors on energy consumption (EC = energy consumption per mole of reactants converted and EH2 = energy consumption per mole of hydrogen produced) for the reforming of natural gas without O2 or air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
The effect of stage number of plasma reactors on energy consumption per mole of reactants converted and per mole of hydrogen produced at the constant feed flow rate is depicted in Fig. 5b. The energy consumption per mole of hydrogen produced substantially decreased when the stage number of plasma reactors increased from 1 to 3 stages, but the energy consumption per mole of reactants converted was insignificantly changed. At 3 stages, a minimum energy consumption of about 1.07 × 1025 eV per mole of hydrogen produced was achieved.
Results of Natural Gas Reforming with Partial Oxidation
For the combined plasma reforming and partial oxidation of the simulated natural gas, the effects of residence time and feed flow rate were also systematically investigated to determine whether or not the addition of oxygen to the natural gas feed could improve the system performance by using two different oxygen sources: pure oxygen and air.
Results of Natural Gas Reforming with Partial Oxidation Using Pure Oxygen
Effect of Stage Number on Reactant Conversion and Product Yield
Constant Residence Time
The effect of stage number of plasma reactors of the system operated at a constant residence time of 4.38 s and a hydrocarbon-to-oxygen ratio of 2/1 is shown in Fig. 6a. All of the reactant conversions only slightly increased with increasing stage number from 1 to 3 stages. Beyond 3 stages, they remained almost unchanged. The H2, C2, and CO yields gradually increased with increasing stage number of plasma reactors; however, the H2 and CO yields reached the maximum values at 3 stages. The values of H2 and CO yields were somewhat greater than 100% due to the complexity of the reactions, of which both forward and backward reactions simultaneously occurred in the plasma reactors. From the experimental observation, the outlet concentrations of all reactants decreased with increasing stage number of plasma reactors except that the outlet concentration of CO2 remained unchanged, which might result from its high bond dissociation energy, as mentioned above.
Effect of stage number of plasma reactors on reactant conversions and product yields for the reforming of natural gas with pure O2 addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
As shown in Fig. 6b, at the constant feed flow rate, the conversion of all reactants, except CO2, considerably increases when the stage number of plasma reactors increases from 1 to 3 stages, and all the reactant conversions remain almost unchanged with further increasing stage number to 4 stages. This is probably due to a small amount of oxygen molecules left in the fourth plasma reactor, as indicated by the oxygen conversion profile. For the CO2 conversion, a minus value was observed. This can be explained in that the formation rate of CO2 by the hydrocarbon oxidation is higher than the CO2 consumption rate by the reforming reactions with partial oxidation [18]. The H2 and CO yields also significantly increased with increasing stage number of plasma reactors to reach the maximum values at 3 stages, and after that they tended to decrease, as also observed in the case of the constant residence time. In the meantime, the C2 yield slightly increased with increasing stage number. Moreover, the H2 yield was found to be much higher than the C2 yield. The most significant difference between H2 and C2 yields was noticed at 3 stages of plasma reactors. This implies that at a higher stage number of plasma reactors, up to 3 stages, the H2 production via the dehydrogenation reactions occurs more favorably than the C2 production via the coupling reactions. However, at 4 stages, less H2 production due to less reactant conversion, as well as higher hydrogen consumption for the hydrogenation reactions, may simultaneously occur instead.
Effect on Product Selectivity and Product Molar Ratio
Constant Residence Time
Most of the product selectivities do not significantly change when the stage number of plasma reactors increases at the constant residence time, except that the selectivity for H2 tends to increase (Fig. 7a). The molar ratio of H2/C2H4 increased as the number of plasma reactors increased from 1 to 2 stages and then remained almost unchanged with further increasing stage number, whereas the other product molar ratios were almost constant. This can be explained in that both the dehydrogenation reactions and the coupling reaction of hydrogen radicals to produce H2 have a high possibility of occurring due to the greater opportunity to collide with the active species present at the early stages of plasma reactors at the residence time of 4.38 s.
Effect of stage number of plasma reactors on product selectivities and product molar ratios for the reforming of natural gas with pure O2 addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
All of the product selectivities gradually decrease with increasing stage number of plasma reactors at the constant feed flow rate, especially from single stage to two stages, as illustrated in Fig. 7b. The decreased selectivities for all the products are due to the fact that with an increasing stage number of plasma reactors, the residence time is also increased. The sharp decline of all product selectivities, except for CO2, when increasing stage number from 1 to 2 stages results from a large quantity of oxygen-active species available in the system. However, the outlet concentration of CO2 was experimentally found to be almost unchanged, probably because of the equivalent rates of formation and reduction of CO2. The molar ratio of H2/C2 products increased with increasing stage number from 1 to 3 stages and then decreased when the stage number was further increased, whereas the H2/CO molar ratio did not vary much. This also implies that the dehydrogenation and coupling reactions were favorable to take place at a higher residence time and to reach maximum levels at a very high residence time.
Effect of Stage Number on Energy Consumption
Constant Residence Time
As shown in Fig. 8a, the sharp decreases in the energy consumption per mole of both reactants converted and hydrogen produced with increasing stage number of plasma reactors were observed at the constant residence time, especially from 1 to 2 stages, as observed above. The minimum energy consumption was 7.58 × 1024 eV per mole of reactants converted and 6.07 × 1024 eV per mole of hydrogen produced at 4 stages of plasma reactors operated with partial oxidation, which were both significantly lower than those of the system without partial oxidation (Fig. 5a).
Effect of stage number of plasma reactors on energy consumption (EC = energy consumption per mole of reactants converted and EH2 = energy consumption per mole of hydrogen produced) for the reforming of natural gas with pure O2 addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
For the multistage plasma system operated at the constant feed flow rate, the energy consumption per mole of reactants converted rapidly declines with increasing stage number of plasma reactors from 1 to 2 stages, and after that it remains almost constant, as depicted in Fig. 8b. Moreover, the energy consumption per mole of hydrogen produced decreased until reaching 3 stages, and then it slightly increased at 4 stages. The minimum energy consumption was observed to be about 6.58 × 1024 eV per mole of reactants converted at 2 stages of plasma reactors and 4.90 × 1024 eV per mole of hydrogen produced at 3 stages of plasma reactors, also being much lower than those of the system without partial oxidation (Fig. 5b).
Results of Natural Gas Reforming with Partial Oxidation Using Air
Effect of Stage Number on Reactant Conversion and Product Yield
Constant residence time
As shown in Fig. 9a, similar to the case of pure oxygen, the conversions of all reactants and the yields of H2 and C2 slightly increase with increasing stage number from 1 to 3 stages. The same explanation as in the case of pure oxygen can be used for the case of air for the partial oxidation at the constant residence time. However, at 4 stages, the conversions remained almost constant in the case of air, whereas they adversely decreased in the case of pure oxygen (Fig. 6a). It may be proposed that the backward reactions of some products and/or the coupling reactions may take place. Interestingly, the CO2 conversion also increased with increasing stage number of plasma reactors when using air as an oxygen source, which is in contrast with the use of pure oxygen, as discussed later.
Effect of stage number of plasma reactors on reactant conversions and product yields for the reforming of natural gas with air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
As depicted in Fig. 9b, at the constant feed flow rate, all the reactant conversions and the product yields markedly increase as the stage number of plasma reactors increases up to 4 stages. It can be hypothesized that the higher stage number of plasma reactors, or longer residence time, allows more opportunity for highly energetic electrons to collide with reactant molecules, especially oxygen molecules in air, for subsequent reactions, leading to higher reactant conversions and product yields. It can be noticed that the H2 yield became much higher than the C2 yield at a higher stage number of plasma reactors, indicating that the dehydrogenations and oxidative dehydrogenations to produce hydrogen are more favorable to occur than the coupling reactions at a longer residence time.
Effect of Stage Number on Product Selectivity and Product Molar Ratio
Constant Residence Time
For the constant residence time, the selectivities for C2H2, C2H4, and C4H10 remain nearly unchanged, while the selectivity for CO slightly declines with increasing stage number of plasma reactors, as shown in Fig. 10a. This can be explained in that increasing the stage number of plasma reactors results in a greater opportunity for CO oxidation. The molar ratio of H2/C2H4 increased with increasing stage number of plasma reactors from 1 to 3 stages; however, after that it tended to decrease. This suggests that oxidative dehydrogenation of C2H4 is likely to occur, leading to an increase in the H2 selectivity with increasing stage number of plasma reactors from 1 to 3 stages.
Effect of stage number of plasma reactors on product selectivities and product molar ratios for the reforming of natural gas with air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
The selectivities for CO, H2, and C2H2 increase with increasing stage number of plasma reactors, whereas the selectivity for C2H4 tends to decrease, and the selectivity for C4H10 remains unchanged for the constant feed flow rate, as shown in Fig. 10b. Figure 10b also shows the rapid increase in the molar ratio of H2/C2H4, while the molar ratios of the others were nearly unchanged. These results can be explained in that with increasing stage number of plasma reactors, the dehydrogenation reactions more favorably occur, as mentioned above.
Effect of Stage Number on Energy Consumption
Constant Residence Time
The same tendency of the energy consumption per mole of reactants converted and per mole of hydrogen produced for the constant residence time was found (Fig. 11a), as also observed in the previous case of pure oxygen addition, that they tended to rapidly decline from 1 to 2 stages, but after that only gradually decreased.
Effect of stage number of plasma reactors on energy consumption (EC = energy consumption per mole of reactants converted and EH2 = energy consumption per mole of hydrogen produced) for the reforming of natural gas with air addition: a at a constant residence time of 4.38 s and b at a constant feed flow rate of 125 cm3/min (hydrocarbon-to-oxygen molar ratio, 2/1; applied voltage, 17.5 kV; frequency, 300 Hz; and electrode gap distance, 6 mm)
Constant Feed Flow Rate
For the constant feed flow rate, the energy consumption both per mole of hydrogen produced and per mole of hydrocarbons converted decreases with increasing stage number of plasma reactors from 1 to 3 stages, but beyond 3 stages, it conversely increases, as shown in Fig. 11b. The minimum energy consumption was 7.38 × 1024 eV per mole of hydrogen produced and 8.87 × 1024 eV per mole of reactants converted at 3 stages of plasma reactors. At 4 stages, the higher energy consumption was observed due to less reactant conversions, despite higher H2 production, as compared to the 3 stages of plasma reactors.
Comparisons of Reforming of Natural Gas with and without Partial Oxidation
Two systems with a constant residence time and a constant feed flow rate were so far investigated. From an engineering point of view, the systems operating at a constant residence time under different feed flow rates with and without oxygen addition are more meaningful for making a comparison. Figures 12, 13, 14, and 15 show the comparative results of the CO2-containing natural gas reforming with and without the addition of either pure oxygen or air using the multistage plasma system under the operating conditions of a constant residence time of 4.38 s, an applied voltage of 17.5 kV, a frequency of 300 Hz, an electrode gap distance of 6 mm, and a hydrocarbon-to-oxygen molar ratio of 2/1.
Comparisons of conversions of a CH4, b C2H6, c C3H8, d CO2, and e O2 of the reforming of natural gas with and without partial oxidation at different stage numbers of plasma reactors (applied voltage, 17.5 kV; frequency, 300 Hz; electrode gap distance, 6 mm; residence time, 4.38 s; and hydrocarbon-to-oxygen molar ratio, 2/1)
Comparisons of selectivities for a H2, b C2H2, c C2H4, d CO, and e C4H10 of the reforming of natural gas with and without partial oxidation at different stage numbers of plasma reactors (applied voltage, 17.5 kV; frequency, 300 Hz; electrode gap distance, 6 mm; residence time, 4.38 s; and hydrocarbon-to-oxygen molar ratio, 2/1)
Comparisons of energy consumption for the reforming of natural gas with and without partial oxidation at different stage numbers of plasma reactors: a energy consumption per mole of reactants converted (EC) and b energy consumption per mole of hydrogen produced (EH2) (applied voltage, 17.5 kV; frequency, 300 Hz; electrode gap distance, 6 mm; residence time, 4.38 s; and hydrocarbon-to-oxygen molar ratio, 2/1)
The conversions of all reactants, except CO2, increase substantially in the presence of oxygen compared to that without the partial oxidation (Fig. 12). This may be because oxygen molecules assist in improving the performance of the reactions, especially via the oxidative dehydrogenation to produce hydrogen [18]. For a comparison between the two oxygen sources, the air system provided a higher process performance in terms of reactant conversions than the pure oxygen system. The results reveal that the addition of air in the feed potentially contributes the positive effect to the activation of reactant gases for the reforming of CO2-containing natural gas. Surprisingly, the CO2 conversion in the case of adding pure oxygen exhibited comparatively lower values that those of the air system at all stages of plasma reactors. This can be explained in that the nitrogen molecules in air possibly act as the third body to facilitate chemical reactions by altering the dielectric properties of the system and/or by reducing the activation energies of some chemical reactions [20]. From the results, the highest reactant conversions were attained at 3 stages of plasma reactors when using air as an oxygen source.
As shown in Fig. 13, the system with oxygen addition provided much higher H2 and C2 yields than the system without oxygen addition because the oxygen molecules can be easily activated by the plasma to form several oxygen active species for extracting the H atoms from the hydrocarbon molecules, as mentioned above. Interestingly, for the case of oxygen addition, the use of air as an oxygen source also provided significantly higher product yields than that of pure oxygen. These can be explained in that nitrogen molecules in air can possibly act as the third body to facilitate the reactions, as also mentioned above. Moreover, 3 stages of plasma reactors were clearly found to provide the highest yields of H2 and C2, in addition to the highest reaction conversions, for both systems (i.e. with pure oxygen and air addition).
The product selectivities are shown comparatively in Fig. 14. Most of the product selectivities, except for the C2H4 and C4H10 selectivities, significantly increased, especially for the CO selectivity, when adding both oxygen sources to the system. However, the addition of oxygen provided negative effect on the C2H4 and C4H10 selectivities because the oxygen active species generated by the plasma are responsible for the oxidative dehydrogenations of both reactants and intermediates to form H2 and C2H2 products instead of more saturated C2H4 and C4H10 products. Moreover, when comparing the ability of pure oxygen and air to induce the H2 formation, it was clearly observed that using air as an oxygen source provided the superior performance.
The energy consumption of the investigated systems is shown comparatively in Fig. 15. The energy consumption per mole of reactants converted of the systems with oxygen addition tended to become lower than that without oxygen addition at any given stage number of plasma reactors. However, the energy consumption per mole of hydrogen produced of the systems with oxygen addition was clearly lower than that without oxygen addition at all stage numbers of plasma reactors. When considering the energy consumption at high stage numbers in the cases of both the pure oxygen and the air additions, even though using the pure oxygen as an oxygen source consumed less energy for the plasma system operation, the system with the pure oxygen addition showed lower energy consumption both per mole of reactants converted and per mole of hydrogen produced than that with the air addition. Moreover, since no other works have been performed to investigate the reforming of natural gas by using any gliding arc discharge systems, the energy consumption in this present work cannot be evaluated for making a comparison. However, from our previous work [13], the energy consumption of the gliding arc discharge system was found to be lower than that of the corona discharge system for the reforming of methane with partial oxidation. This verifies the high efficiency of the gliding arc discharge system.
From these comparisons, it can be concluded that the addition of oxygen (pure oxygen or air) provided positive effects on the reforming of CO2-containing natural gas with not only enhancing the reactant conversions, the desired product yields, and the desired selectivities, but also on consuming comparatively less energy for producing hydrogen, as compared to the system without the oxygen addition. In addition, the use of air as the oxygen source assists in improving the reforming of CO2-containing natural gas more effectively than pure oxygen in terms of both higher reactant conversions and higher product yields and selectivities, which are more advantageous from an economic viewpoint, even though the energy consumption is slightly higher for the system with air addition. Finally, from the overall results, it can also be reasonably concluded that 3 stages of plasma reactors are sufficient to achieve a satisfactorily high process performance.
Conclusions
The combined reforming and partial oxidation of CO2-containing natural gas was investigated under two systems with a constant residence time and a constant feed flow rate by using a multistage gliding arc discharge system. The major products were mainly hydrogen and C2 hydrocarbons. For the system without partial oxidation operated at a constant feed flow rate, all reactant conversions, except CO2 conversion, as well as product yields and product selectivities, increased with increasing stage number of plasma reactors. For the system operated at a constant residence time, the stage number of plasma reactors only slightly affected the reactant conversions, product yields, and product selectivities. For the system with partial oxidation, the stage number of plasma reactors provided positive effects on the reactant conversions, product yields, and product selectivities, and also energy consumption was also lower compared to the system without partial oxidation. The addition of air as the oxygen source provided better process performance for the reforming of CO2-containing natural gas than that of pure oxygen in terms of higher selectivities and yields, with reasonably low energy consumption. Moreover, the plasma system with 3 stages of plasma reactors led to good process performance with acceptably high reactant conversions and desired product yield.
References
Jeong HK, Kim SC, Han C, Lee H, Song HK, Na BK (2001) Korean J Chem Eng 18:196–201
Li MW, Xu GH, Tian YL, Chen L, Fu HF (2004) J Phys Chem A 108:1687–1693
Hwang BB, Yeo YK, Na BK (2003) Korean J Chem Eng 20:631–634
Xu H, Shi K, Shang Y, Zhang Y, Xu G, Wei Y (1999) J Mol Catal A: Chem 147:41–46
Effendi A, Zhang ZG, Hellgardt K, Honda K, Yoshida T (2002) Catal Today 77:181–189
Supat K, Chavadej S, Lobban LL, Millinson RG (2003) Ind Eng Chem Res 42:1654–1661
Supat K, Kruapong A, Chavadej S, Lobbon LL, Mallinson RG (2003) Energ Fuel 17:418–474
Paulmier T, Fulcheri L (2005) Chem Eng J 106:59–71
Eliasson B, Hirth M, Kogeischatz U (1987) J Appl Phys 20:1421–1437
LA Rosacha, GK Anderson, LA Bechtold, JJ Coogan, HG Heck, M Kang, WH McCulla, RA Tennant, PJ Wantuck (1993) NATO ASI Series, Part B 34
Krawczyk K, Młotek M (2001) Appl Catal B: Environ 30:233–245
Chavadej S, Kiatubolpaiboon W, Rangsunvigit P, Sreethawong T (2007) J Mol Catal A: Chem 263:128–136
Sreethawong T, Thakonpatthanakun P, Chavadej S (2007) Int J Hydrogen Energ 32:1067–1079
Chavadej S, Saktrakool K, Rangsunvigit P, Lobban LL, Sreethawong T (2007) Chem Eng J 132:345–353
Sreethawong T, Suwannabart T, Chavadej S (2008) Plasma Chem Plasma Process 28:629–642
Chavadej S, Tansuwan A, Sreethawong T (2008) Plasma Chem Plasma Process 28:643–662
Rueangjitt N, Akarawitoo C, Sreethawong T, Chavadej S (2007) Plasma Chem Plasma Process 27:559–576
Rueangjitt N, Sreethawong T, Chavadej S (2008) Plasma Chem Plasma Process 28:49–67
Dean JA (1999) Lange’s handbook of chemistry, 5th edn. McGraw-Hill, New York
Indarto A, Choi JW, Lee H, Song HK (2006) Energy 31:2650–2659
Acknowledgments
The authors would like to thank the Ratchadapisek Somphot Endowment Fund, Chulalongkorn University, Thailand; the Commission on Higher Education, Thailand; the National Research Council of Thailand (NRCT); the Sustainable Petroleum and Petrochemicals Research Unit, Center for Petroleum, Petrochemicals, and Advanced Materials, Chulalongkorn University, Thailand; and the Petrochemical and Environmental Catalysis Research Unit under the Ratchadapisek Somphot Endowment Fund, Chulalongkorn University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rueangjitt, N., Jittiang, W., Pornmai, K. et al. Combined Reforming and Partial Oxidation of CO2-Containing Natural Gas Using an AC Multistage Gliding Arc Discharge System: Effect of Stage Number of Plasma Reactors. Plasma Chem Plasma Process 29, 433–453 (2009). https://doi.org/10.1007/s11090-009-9191-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-009-9191-1