Abstract
Cu2ZnSnS4 (CZTS) thin films in 61 nm, 112 nm, 242 nm and 313 nm thickness which have been produced by Pulsed Laser Deposition (PLD) on Soda Lime Glass substrates as a function of the number of laser pulses. As the deposition of ablated material has been augmented with increasing number of laser pulses, it has been noticed that CZTS-ultrathin film’s thicknesses and particle sizes have been increased, their crystalline structures have been improved. Larger particles limit the transmission of light and cause thin films to absorb photons. The band gaps of CZTS (61 nm), CZTS (112 nm), CZTS (242 nm) and CZTS (313 nm) thin film which have been determined to be 1.95 eV, 1.90 eV, 1.50 eV and 1.45 eV, respectively. CZTS (61 nm) ultrathin film with the thinnest one among the thin films produced in this work, which is Cu and S poor but Sn and Zn rich. By increasing the thickness of the film, it has been observed that the amount of Cu and S were increased, and the ratio of Sn and Zn were decreased. In addition, it has been systematically investigated that the photocatalytic activity of the ultra-thin CZTS films coated in different thicknesses by PLD method. Among all the photocatalysts, the CZTS (in 242 nm thickness) photocatalyst has exhibited the highest photocatalytic performance, managing to remove 96.1% of methylene blue (MB) in 240 min. Furthermore, the mechanism that performs photocatalysis has been investigated by scavenger experiments, and it was observed that \({}^{ \cdot }O_{2}^{ - }\) radical ions have an important role in the reaction, while holes have little effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
While the industrial revolution has rapidly improved living standards, the global pollution has brought with it poses a serious threat to the environment as well as to all living creatures. Wastewater arising as a result of industrial developments as well as growth that contains organic pollutants such as dyes (Pérez-González and Tomás 2021), phenols (Hu et al. 2020), pesticides (Vaya and Surolia 2020), bacteria (Baaloudj et al. 2021), etc., and therefore, it is necessary to eliminate these pollutants in the wastewater before discharging it into nature. For the treatment of pollutants from industrial wastewater, various methods such as ion exchange (Feng et al. 2019), ozonation (Wu et al. 2016) and photo Fenton (Yang et al. 2020) which have been applied. However, due to the high cost of these methods, it is necessary and critical to develop more effective and cheaper alternative approaches that require fewer chemicals and energy.
Researchers have recently focused on semiconductor photocatalysis which uses sunlight to degrade organic contaminants and is a promising approach for solving environmental problems in wastewater treatment (Han et al. 2022; Xiao-Wei et al. 2021). A number of studies on the development of various semiconductor materials such as TiO2 (Marcelino et al. 2019), ZnO (Singha and Patra 2020), SiO2 (Fateh et al. 2013), WO3 (Tahir et al. 2019), BiOCl (Yao et al. 2021), have been increased after the pioneering work presented by Fujishima and Honda (1972). In the semiconductor photocatalysis process, when the material is exposed to external light, electron hole-pairs (e−–h+) are formed on its surface. Through photodegradation, oxidation and reduction processes, electrons react with contaminants, and as a result of this reaction, they move to conduction band by generating a hole in the valence band (Saravanan et al. 2017). It is desirable that the semiconductor material to be used in this process has some properties such as being chemically stable, photoactive, nontoxic and low cost as well as absorbing solar energy as high as possible (Zhu and Zhou 2019). Semiconductor oxides such as n-type TiO2 and ZnO are commonly utilized in photocatalytic studies, but they have limitations owing to their wide band gap allowing only UV light to pass through (Dong et al. 2015; Ong et al. 2018). For this reason, semiconductor materials with a narrower band gap as CZTS material have recently attracted some great attention as photocatalysts by researchers.
Cu2ZnSnS4 (CZTS) has attracted a lot of attention recently because it has a high absorption coefficient (104 cm−1) and optimal fundamental band gap energy (1.5 eV) (Sarswat and Free 2012). CZTS films are highly appealing in a variety of applications, including solar cells (Dong et al. 2015; Ong et al. 2018; Sarswat and Free 2012), CO2 reduction (Wang et al. 2018; Zhang et al. 2016), water splitting (Atwee et al. 2019), organic pollutant degradation (Wadhene et al. 2020; Madiraju et al. 2016; Phaltane et al. 2017; Xia et al. 2015), and so on, due to their favorable characteristics such as non-toxicity, abundance of its components in soil and low cost, as well as their above-mentioned unique properties. It has also been reported that it exhibits stability better than some n-type semiconductor materials such as TiO2 and CdS (Wang et al. 2018; Zhang et al. 2016). CZTS has been produced using a variety of techniques, including sol–gel (Atwee et al. 2019), electrodeposition (Wadhene et al. 2020), microwave (Madiraju et al. 2016), hydrothermal (Phaltane et al. 2017), hot injection (Xia et al. 2015), SILAR (Apostolopoulou et al. 2018), sputtering (Inamdar et al. 2013), thermal evaporation (Peksu and Karaagac 2021) and PLD (Beres et al. 2018; Elhmaidi et al. 2020), following Katagiri initially reported.
PLD is a very effective thermal evaporation technique. By easily adjusting the parameters (laser fluence, laser pulse number, laser wavelength, substrate temperature, backgorund gas pressure etc.) during or before thin film production, and thus crystalline and epitaxial growth of homogeneous and smooth thin films can be easily produced by transferring stoichiometry of sputtering target materials even at low substrate temperature (Gezgin and Kılıç 2019; Gezgin et al. 2021a). Since thin films can be produced in a high ultra-vacuum environment, almost free of pollution and impurities in the thin film. Controlling laser intensity and number of laser pulses, in particular, allows for the production of qualified multicomponent ultra-thin films with a thickness of less than 500 nm (Rajan 2018).
In this study, it is aimed to examine the photocatalyst properties of CZTS thin films, which are low cost and environmentally friendly, absorbing photons in the visible region from the sun in high amounts. In the photocatalyst application area, it is of great importance to determine the ideal film thickness for the use of CZTS thin film. That is, as the thickness of some thin films significantly raise, their crystal structures develop, but photo-excited charge carriers can not respond well to the photocatalyst due to their short diffusion length compared to the film thickness. Therefore, in this study, to determine the best-performing CZTS film thickness for photocatalyst application, CZTS thin films with various thicknesses were successfully fabricated on sodalime glass substrates (SLG) by PLD technique. These films obtained in this work have been employed to investigate photocatalytic degradation of organic dye contaminants. The first, the compositional, morphological, optical, and electrical properties of CZTS films as a function of film thickness have been examined. Then, CZTS films have also been tested as a photocatalyst using the degradation of MB under visible light. Here, the thickness-dependent photocatalytic performance of CZTS thin films produced by PLD has been studied for the first time and it was observed that thin films have exhibited high photocatalytic activity. In addition, the radicals that enable photocatalysis to take place have been identified by scavenger experiments and the mechanism for photocatalysis has been proposed. As a result, this study, which examines the photocatalyst property depending on the thickness of the complex CZTS thin film produced by the PLD technique, has not been studied in the literature, and it can show an effective way to the photocatalyst studies, especially using semiconductor thin films with quaternary or triple absorber complex structure.
2 Experimental procedure
2.1 Preparation of CZTS thin films using PLD technique
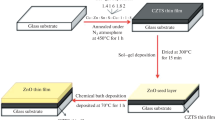
The PLD technique was used to prepare CZTS thin films. CZTS target with %99.99 purity was supplied commercially by GoodWill-Chine company. The sputtering target used for the production of thin films that has elemental Cu:Zn:Sn:S ratios of 2:1:1:4. CZTS thin film has been produced on SLG substrate by deposition of the laser formed CZTS plasma from the target. PLD system was incorporated into nanosecond (ns) Nd:YAG (Continuum, Surelite, NY, USA) laser system, which produces laser pulses with a main wavelength of 1064 nm and a duration of 5 ns at a repetition rate of 10 Hz. PLD system is one of the most powerful systems among the clean, thin film preparation methods with the possibility of producing thin films in vacuum conditions (~ 10–6 mbar). During the experimental performance, both the substrate and the target are spinning under the control of a step motor driver in the vacuum environment which is very important process to control homogeneous grow of thin films without damaging the sputtering target. The control of both either time duration of ablation process or laser power per pulse are the essential for obtaining thin films in desired thickness. In this study, thin films at four different thicknesses were obtained by varying laser ablation time duration by keeping laser power per pulse is constant, and then characterization of these four thin films were performed. The thicknesses of these thin films produced were measured to be 61, 112, 242, and 313 nm which have been prepared by applying 2400, 4800, 9600, and 12,000 number of laser pulses, respectively, and therefore these thin films were produced as a function of laser ablation time. The experimental system has been given in Fig. 1 schematically and procedures followed are described elsewhere in literature (Gezgin and Kılıç 2019; Gezgin et al. 2021a; Gezgin et al. 2021; Kılıç et al. 2021; Gezgin and Kiliç 2019).
The PLD technique was used to create CZTS thin films, which were then placed in a tube furnace with 50 mg of sulphide powder and heated to 375 °C for 45 min while under vacuum. (Fig. 1).
2.2 Photocatalytic activity of CZTS thin films in methylene blue
The degradation activity of CZTS thin films produced in 61, 112, 242 and 313 nm thicknesses is a new phenomenon and therefore, they need to be investigated in organic waste dyes such as MB (Merck). In this study, classical UV–Vis absorption spectroscopy was used to carry out an interpretation of MB absorbance characteristics. The maximum absorption peak for MB, was obtained and recorded at ~ 664 nm wavelength as discussed below.
The photocatalytic activity was investigated by taking UV–Vis spectroscopy (Biochrom Libra S22) into account for CZTS thin films depending on their thicknesses. The experimental process was performed in aqueous solution with MB to investigate the photocatalytic activity of CZTS thin film at room themperature conditions. Visible light sources (250 W metal halide lamp (GE ARC250)) were used to irradiate CZTS thin films depending on time in water with MB. It was studied by irradiating the pollutant solution for a total time of 240 min by taking samples at every 30 min.
3 Result and disscussion
3.1 Characterization of CZTS-ultrathin film
Figure 2A indicates that the thicknesses of CZTS-ultrathin films deposited by ablating the target with 2400, 4800, 9600 and 12,000 laser pulses and then thicknesses of deposited thin films have been measured to be 61 nm, 112 nm, 242 nm, and 313 nm, respectively. It has been determined that the thicknesses of CZTS-ultrathin films have been increased as the laser pulse numbers increase (Đekić et al. 2017; Vakulov et al. 2018). CZTS-ultrathin films in polycrystalline tetragonal structre, which have been crystallised in (112), (200) and (220) crystal planes appeared at 2θ = 28°, 33o and 47° angles (Gezgin and Kiliç 2019; Camara et al. 2013) (JCPDS Card No: 01-075-4122), respectively. In the lowest thickness, CZTS-ultrathin film (61 nm) has the poorest crystal structure. As laser pulse number applied for ablation is increased, the amount of ablated and then deposited material are increased. Therefore, the thickness of thin film increases, so in this case, atoms of CZTS strucrue have reached on the substrate that fill the appropriate vacancies in crystal plane and make an ideal nucleation, thereby the crystal structure is improved. Thus, it was observed that some increase in the thickness of thin film causes an improvement in the crystal structure of thin film to increase the main (112) peak density (Tao et al. 2015). Therefore, CZTS-ultrathin film (313 nm) for the case of the highest thickness presents the most advanced crystal structure.
The Scherrer Equation (Eq. (1)) is used to calculate the main crystal size of CZTS-ultrathin films:
S is the crystalline size, λ is x-ray wavelength, θ is Bragg diffraction angle, β is the full width at half-maximum of diffraction peak. The main crystalline sizes of ultrathin CZTS films of 61 nm, 112 nm, 242 nm and 313 nm in thicknesses have been detemined to be 7.34 nm, 10.71 nm, 17.79 nm, and 22.25 nm, respectively. It can be indicated that as thin film thickness increases, the crystal size developes (Gezgin et al. 2021a).
Figure 3a shows that the absorption capacity of CZTS-ultrathin films expands from UV region to Vis region. So, as some increases in film thickness causes particle formation in thin films to produce enlarged particles in size and denser film. Thus, the absorptivity of thin films increases with augmenting thickness.
The Tauc law stated in Eq. (2) is used to calculate the band gap of CZTS-ultrathin films:
A is a constant, hv is photon energy, \({E}_{g}\) is band gap. \({E}_{g}\) is determined by a straight line on photon energy \((h\nu )\) in Tauc-plot presented in Fig. 3b. CZTS thin films have a direct band gap. \({E}_{g}\) values of CZTS (61 nm), CZTS (112 nm), CZTS (242 nm) and CZTS (313 nm) ultrathin films were obtained to be 1.95 eV, 1.90 eV, 1.50 eV and 1.45 eV, respectively. Some increase in particle size with augmenting thickness reduces the transmission of light, and thus higher photon absorption of CZTS-ultrathin films causes some decreases in the band gap value (Gezgin and Kiliç 2019). Among ultrathin films, it has been determined that CZTS (242 nm) ultrathin film has an ideal band gap value of 1.5 eV (Gezgin et al. 2021a).
AFM images in Fig. 4a and b show the morphological structures of CZTS (61 nm) and CZTS (313 nm) ultrathin films of the lowest and highest thicknesses, respectively. Since the deposition of CZTS material is in the lowest amount as the material is ablated by the lowest laser pulse number, few atoms reach on the substrate. The lower number of atoms were deposited on top of each other and side by side that limiting particle coalescing and thus, particles with smaller size were formed. CZTS (61 nm) ultrathin film produced by ablation performance of 2400 laser pulses consists of small-sized particles as seen in Fig. 4a. On the contrary, with the augment of the laser pulse-numbers, the particles deposited in high density that combine with each other and cause particle growth as seen in Fig. 4b. SEM image in Fig. 5a also confirms the morphology of CZTS (61 nm) ultrathin film, which is composed of particles in low density and small sizes. When the laser pulse number is increased from 2400 to 12,000, the number of atoms deposited on the substrate also increases. This situation leads to an enlarged the particle size and increased the particle density (Đekić, et al. 2017; Vakulov, et al. 2018), as indicated in AFM and SEM images of CZTS (313 nm) ultrathin film in Figs. 4b and 5c, respectively.
The tables given as an inset of EDX spectra in Fig. 5b and d give the atomic weight ratios of the elements that form CZTS (61 nm) and CZTS (313 nm) ultrathin films, respectively. CZTS (61 nm) ultrathin film of low thickness contains low amount of Copper (Cu) and Sulfur (S), and high rate of Tin (Sn) and Zinc (Zn) elements. VCu (Copper vacancy)—ZnCu (Zn replace Cu vacancy) acceptor defects and SnCu (Sn replace Cu vancancy)—VS (Sulfur vacancy) donor defects may occur in CZTS (61 nm) ultrathin film (Gupta et al. 2019). With an augment in thickness of CZTS-ultrathin film, Cu and S amounts increased while Sn and Zn rations decreased. Thus, CZTS (313 nm) ultrathin film components tended to provide stoichiometric transfer. However, even in the latter case, CZTS (313 nm) ultrathin films were observed to be somewhat Cu-poor, Zn and Sn-rich (Gezgin et al. 2021a).
3.2 Photocatalytic properties of CZTS thin films
Photocatalysis is one of the most important methods used in the solution of many problems, especially in the removal/reduction of environmental pollution in waste water (Ferreira et al. 2020; Pedanekar et al. 2020). Organic dyes compose an important part of industrial pollutants. These dyes doesnt only cause environmental pollution, but also negatively affects the health of living things (Rathi et al. 2021). In this study, ultrathin CZTS films produced by PLD method were used to remove MB dye, which is one of the organic pollutants. Taking into account our team's previous research as well as works reported in literature, the pH effect was the first investigated parameter in photocatalysis studies (Dursun et al. 2021). One of the reasons for photocatalysis studies have been carried out at different pH values is that pH is one of parameters that significantly affect the efficiency and kinetics of photo-oxidation processes. In this way, separation of the e−–h+ pairs in thin films from the photocatalyst surface can be facilitated (Dursun et al. 2018). Another reason is the sensitivity of the components used in photocatalysis to different pH values. An example of this situation is changes in the activity of MB, which is a cationic dye, at different pH values (Dursun et al. 2020). Therefore, the photocatalyst effect of pH on the photocatalytic activity of CZTS films has been examined first time in literature and the results obtained is given in Fig. 6a. Ultrathin film CZTS (242 nm) with the best photocatalytic efficiency was used to examine pH effects. As seen from Fig. 6a, the photocatalytic efficiency has been increased significantly by some increase in pH. In the photocatalysis application at pH = 4, only 40.1% of MB was removed in 540 min, while this value was measured as 77.2% for pH = 7 at the same time. However, at pH = 10, CZTS film has exhibited an impressive degradation efficiency, removing 96.1% of MB dye within 240 min. Some increase in photocatalytic activity as a function of pH change that can probably be associated with a variation of surface chargesand some increase in surface adsorption. In addition, higher dye degradation is anticipated because of the electrostatic contact that the basic environment causes between the photocatalyst's negative surface and the MB cations. (Tamjidi et al. 2021).
a The effect of pH on degradation in the presence of CZTS (242 nm) film photocatalysts, b the effect on degradation of CZTS films coated with different thicknesses by the PLD method, c First-order kinetic data of thin film photocatalysts of different thicknesses, and d Real-time absorption spectrum of the dye in the presence of ultrathin CZTS film (MB degradation mechanism is given as inset in d)
In Fig. 6b, there was systematically studied that the percentage of degradation values occurring due to the initial concentration of MB in the presence of ultrathin CZTS films coated in different thicknesses (61, 112, 242, and 313 nm) by PLD method. In these tests, firstly, the photocatalyst and dye solution were kept in the dark for 30 min to ensure the adsorption–desorption equation, and it was observed that there was no significant change in MB concentration as a result of the measurement. This suggests that dye adsorption by thin sheets is hardly noticeable. In addition, it was investigated whether only MB dye has exhibited degradation under visible light without using photocatalysis or not, and it was determined that only 4.2% of MB dye degraded during 240 min. The photocatalysis studies have been carried out in the presence of CZTS film photocatalyst in different thicknesses, it has been determined that the efficiency of photocatalysis increases as a function of thin film thickness up to a certain thickness value, and it has started to decrease with further increase in the thickness value. In other words, it was observed that as thickness of CZTS photocatalyst increases from 61 to 242 nm, the photocatalytic activity of the photocatalyst increases, and, in oppositely, decreases beyond 242 nm thickness value such as 313 nm. This variation in photocatalytic activity is in agreement with what reported in literature (Dundar et al. 2020; Wu et al. 2013). Some increases in the photocatalytic activity of CZTS with increasing thickness; (i) some increament in surface area due to some increament in surface roughness and pore size, and thus the ability of the photocatalyst to interact with extra light and dye, and (ii) some decrease in the band gap of the thin film with some increase in thickness, resulting in e−–h+ can be explained by the formation of the pair. However, when thickness is further increased, the photocatalytic activity decreases with some increase in recombination that taken place in the system at high thicknesses. Actually, as the film thickness increases, it can be expected that the film will give better photocatalyst performance as the crystal structure of the film improves. But, the charge carriers formed by the excitation of the incoming photons can undergo recombination within the film before they have received the necessary path to interact with the dye solution (Gezgin et al. 2019; Gezgin et al. 2021b). So, while the photo-excited charge carriers close to the surface of the thick film interact more effective with the dye solution, the diffusion length of the photo-excited charge carriers produced near the bottom surface of the film remains shorter than the thickness of the film and can recombine at the trap points in the film without interacting with the dye solution (Azeez et al. 2018; Kumar et al. 2011). For this reason, the 313 nm thin film could not show better photocatalyst properties. Among all the photocatalysts, the photocatalyst with a thin film catalisor of CZTS in thickness of 242 nm has exhibited the highest photocatalytic performance by showing the success of removing 96.1% of MB in 240 min.
The Langmuir–Hinshelwood first-order kinetic model (ln (Ct/C0) = kt) was used to compare the photocatalytic activity of ultrathin CZTS films generated in various thicknesses by PLD technique shown in Fig. 6(c). In the graph, linear gradients of CZTS films in different thickness values show that the degradation is in accordance with the first-order kinetic model. The reaction rate constant of MB dye degradation was calculated to be only 0.0962 h−1 by providing all photocatalysis conditions (pH = 10) without using any catalyst. When CZTS (242 nm) thin film photocatalyst with the highest photocatalytic activity was used, the rate constant value was 0.816 h−1. The rate constant values exhibited by thin-film photocatalysts with 61, 112 and 313 nm thickness, which were calculated to be 0.438, 0.607 and 0.484 h−1, respectively.
In Fig. 6d, there has been given that the real degradation graph of the photocatalyst with the highest photocatalytic efficiency (CZTS (242 nm)) among ultrathin CZTS film photocatalysts produced in different thicknesse. As seen in Fig. 6d, it has been observed that the characteristic peak of MB formed at ~ 664 nm, gradually and almost linearly decreases over time and completely disappears at the end of process as it has been exposed to visible light. The disappearance of this peak over time that indicates to us that the degradation of the dye by thin film photocatalyst and visible light that is its conversion to degraded products (Khan et al. 2022; Wang, et al. 2018). For a better understanding of this situation, the reaction mechanism is given as an inset of Fig. 6d.
Scavengers were used to determine which radicals in CZTS film photocatalysts play an active role in the degradation of organic dyes. These chemicals are IPA, EDTA-2Na and BQ are used to detect \({}^{ \cdot }OH\), h+ and \({}^{ \cdot }O_{2}^{ - }\) radicals, respectively. The dye degradation values obtained in the photocatalysis experiments performed in the presence and absence of IPA, EDTA-2Na and BQ scavengers that have been given in Fig. 7. Also, during the time until the end of 240 min, the total dye degradation scavengers are given as an inset of Fig. 7. While 96.1% degradation was observed when no scavenger was used, this value remained constant at 58% in the presence of BQ scavenger. The significant decrease in dye degradation in the presence of BQ shows that \({}^{ \cdot }O_{2}^{ - }\) radicals have a very important role in photocatalysis studies in the case of usage of CZTS photocatalysts. In the presence of IPA as a scavenger, the rate of degradation was found to be higher (80%) than in the presence of BQ. This means that \({}^{ \cdot }OH\)’s is partially effective in the degradation. Finally, in the presence of EDTA-2Na, a result (92%) has been obtained very close to the photocatalysis efficiency obtained without using any scavenger. This result means that h+’s has the least effect on the degradation mechanism compared to other radicals.
Based on the above discussion, a representative schematic illustration is given to show through which process and how organic dyes can be removed under visible light in the presence of ultrathin CZTS film photocatalyst (Fig. 8). For a briefly explanation, the photochemical process over CZTS film photocatalysts is that photons with an energy equal to or higher than the band gap of CZTS semiconductor are absorbed by CZTS film, and thus the generation of photo-induced e−–h+ pairs can be obtained. However, these pairs either lose energy and recombine, or they reach the photocatalyst surface and participate in reduction–oxidation reactions. The related equation is given by Eq. 3 below. The resulting holes, oxidize water molecules and allow the formation of hydroxyl (\({}^{ \cdot }OH\)) radicals, as shown in Eq. 4. At the same time, electrons reduce oxygen molecules attached to the surface of the photocatalyst to superoxide anions (\({}^{ \cdot }O_{2}^{ - }\)) (Eq. 5). These radicals provide the formation of hydroperoxyl radicals (Eq. 6) and hydroperoxyls (Eq. 7), respectively. As a result, these \({}^{ \cdot }OH\), \(H{O}_{2}\) and \({}^{ \cdot }O_{2}^{ - }\) radicals play an important role in the conversion of organic pollutants into degraded products (Eq. 9) (Amakiri et al. 2022).
4 Conclusion
CZTS-ultrathin films in 61 nm, 112 nm, 242 nm, and 313 nm thicknesses have been produced as a function of laser pulse number, employing PLD technique. An increase in the number of ablating laser pulses has resulted in a rise in the deposition amount and thus an increment in particle size as well as in film thickness. CZTS-ultrathin films in the higher thicknesses absorb photons and their band gaps decrease. CZTS (61 nm) ultrathin film in the lowest thickness contains low amounts of Cu–S elements and high amounts of Zn-Sn elements. The highest thickness of CZTS (313 nm) ultrathin film approximated the resultant structure produced by ideal stoichiometric transfer. Thin films, which were successfully produced and characterized, were used in photocatalysis applications. In photocatalitic applications, the effect of pH on photocatalysis has been first investigated and the changes in the photocatalytic activity have been discussed. Then, the %degradation values occurring at the initial concentration of MB in the presence of ultra-thin CZTS films coated in different thicknesses by PLD method have been systematically investigated. It has been observed and reported that the photocatalytic activity of the photocatalyst, devoleps as the thickness of CZTS photocatalyst increases from 61 to 242 nm and decreases at the highest thickness value such as 313 nm investigated in this work. This enhancement in the photocatalytic activity, increases with an increase in the thickness and can be explained by the increase in surface roughness and pore size and/or the decrease in the band gap, with the photocatalyst interacting with extra light and dye. However, when the thickness is further increased, in addition to the difficulty of the interaction of the thin film with the dye solution and light, and photon absorption, the photocatalytic activity decreases due to the recombination that occurs in the system at high thicknesses. As a result, among the photocatalysts, the CZTS photocatalyst with the thin film in thickness of 242 nm that showed the highest photocatalytic performance by removing 96.1% of MB in 240 min. In addition, scavenger experiments were carried out to determine which radicals play an active role in the photocatalysis study. As a result of these experiments, it has been determined that the most active radical is the \({}^{ \cdot }O_{2}^{ - }\) radicals, and the \({}^{ \cdot }OH\) radicals are less active than the \({}^{ \cdot }O_{2}^{ - }\) radical, and the h+ are almost inactive. Considering the obtained results, the mechanism of the CZTS thin film has been proposed.
References
Amakiri, K.T., Angelis-Dimakis, A., Ramirez Canon, A.: Recent advances, influencing factors, and future research prospects using photocatalytic process for produced water treatment. Water Sci. Technol. 85(3), 769–788 (2022)
Apostolopoulou, A., et al.: Novel development of nanocrystalline kesterite Cu2ZnSnS4 thin film with high photocatalytic activity under visible light illumination. J. Phys. Chem. Solids 112, 37–42 (2018)
Atwee, T., et al.: Effect of film thickness on structural, morphological, and optical properties of Cu2ZnSnS4 thin films prepared by sol–gel spin coating. Appl. Phys. A 125(4), 1–10 (2019)
Azeez, F., et al.: The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 8(1), 1–9 (2018)
Baaloudj, O., et al.: Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: a review. J. Water Process Eng. 42, 1–11 (2021)
Beres, M., et al.: Stoichiometry control in Cu2ZnSnS4 thin films grown by pulsed laser deposition. Mater. Chem. Phys. 205, 90–96 (2018)
Camara, S.M., Wang, L., Zhang, X.: Easy hydrothermal preparation of Cu2ZnSnS4 (CZTS) nanoparticles for solar cell application. Nanotechnology 24(49), 495401 (2013)
Đekić, M., et al.: Influence of deposition parameters on pulsed laser deposition of K0. 3MoO3 thin films. Bull. Chem. Technol. Bosnia Herzeg. 48(1), 1–4 (2017)
Dong, H., et al.: An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 79, 128–146 (2015)
Dundar, I., et al.: Thickness effect on photocatalytic activity of TiO2 thin films fabricated by ultrasonic spray pyrolysis. Catalysts 10(9), 1–13 (2020)
Dursun, S., et al.: UV/visible light active CuCrO 2 nanoparticle–SnO 2 nanofiber p–n heterostructured photocatalysts for photocatalytic applications. Dalton Trans. 47(41), 14662–14678 (2018)
Dursun, S., et al.: Production of CuO–WO3 hybrids and their dye removal capacity/performance from wastewater by adsorption/photocatalysis. J. Water Process Eng. 36, 1–10 (2020)
Dursun, S., Akyildiz, H., Kalem, V.: PMN-PT nanoparticle/SnO2 nanofiber heterostructures: enhanced photocatalytic degradation performance by ultrasonic wave induced piezoelectric field. J. Alloy. Compd. 889, 161769 (2021)
Elhmaidi, Z., et al.: In-situ tuning of the zinc content of pulsed-laser-deposited CZTS films and its effect on the photoconversion efficiency of p-CZTS/n-Si heterojunction photovoltaic devices. Appl. Surf. Sci. 507, 1–10 (2020)
Fateh, R., Dillert, R., Bahnemann, D.: Preparation and characterization of transparent hydrophilic photocatalytic TiO2/SiO2 thin films on polycarbonate. Langmuir 29(11), 3730–3739 (2013)
Feng, Y., et al.: In-situ ion exchange electrocatalysis biological coupling (i-IEEBC) for simultaneously enhanced degradation of organic pollutants and heavy metals in electroplating wastewater. J. Hazard. Mater. 364, 562–570 (2019)
Ferreira, M.A., et al.: Fabrication of SrTiO3/g-C3N4 heterostructures for visible light-induced photocatalysis. Mater. Sci. Semicond. Process. 108, 1–10 (2020)
Fujishima, A., Honda, K.: Electrochemical photolysis of water at a semiconductor electrode. Nature 238(5358), 37–38 (1972)
Gezgin, S.Y., Kiliç, H.Ş: The electrical characteristics of ITO/CZTS/ZnO/Al and ITO/ZnO/CZTS/Al heterojunction diodes. Optik 182, 356–371 (2019)
Gezgin, S.Y., Kılıç, H.Ş: The electrical characteristics of ITO/CZTS/ZnO/Al and ITO/ZnO/CZTS/Al heterojunction diodes. Optik 182, 356–371 (2019)
Gezgin, S.Y., Houimi, A., Kılıç, H.Ş: Production and photovoltaic characterisation of n-Si/p-CZTS heterojunction solar cells based on a CZTS ultrathin active layers. Optik 199, 1–11 (2019)
Gezgin, S.Y., et al.: The effect of CZTS ultrathin film thickness on the electrical characteristic of CZTS/Si heterojunction solar cells in the darkness and under the illumination conditions. SILICON 13(10), 3555–3567 (2021a)
Gezgin, S.Y., et al.: Determination of photovoltaic parameters of CIGS hetero junction solar cells produced by PLD technique, using SCAPS simulation program. Vacuum 192, 1–9 (2021b)
Gupta, G.K., Reddy, V., Dixit, A.: Impact of excess and disordered Sn sites on Cu2ZnSnS4 absorber material and device performance: a 119Sn Mössbauer study. Mater. Chem. Phys. 225, 410–416 (2019)
Han, S., et al.: Construction of ZnIn2S4-CdIn2S4 microspheres for efficient photo-catalytic reduction of CO2 with visible light. CHIN. J. STRUCT. CHEM., 41(1), (2022)
Hu, J., et al.: High-efficiency removal of phenol and coking wastewater via photocatalysis-Fenton synergy over a Fe-g-C3N4 graphene hydrogel 3D structure. J. Ind. Eng. Chem. 84, 305–314 (2020)
Inamdar, A., et al.: Optimized fabrication of sputter deposited Cu2ZnSnS4 (CZTS) thin films. Sol. Energy 91, 196–203 (2013)
Khan, I., et al.: Review on methylene blue: its properties, uses, toxicity and photodegradation. Water 14(2), 1–30 (2022)
Kılıç, H.Ş, et al.: Nonlinear optical properties of Cu 2 ZnSnS 4 nanocrystal thin films and its constituents thin films. Opt. Quant. Electron. 53(1), 1–11 (2021)
Kumar, K.J., Raju, N.R.C., Subrahmanyam, A.: Thickness dependent physical and photocatalytic properties of ITO thin films prepared by reactive DC magnetron sputtering. Appl. Surf. Sci. 257(7), 3075–3080 (2011)
Madiraju, V.A., et al.: CZTS synthesis in aqueous media by microwave irradiation. J. Mater. Sci. Mater. Electron. 27(4), 3152–3157 (2016)
Marcelino, R.B., et al.: Novel and versatile TiO2 thin films on PET for photocatalytic removal of contaminants of emerging concern from water. Chem. Eng. J. 370, 1251–1261 (2019)
Ong, C.B., Ng, L.Y., Mohammad, A.W.: A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 81, 536–551 (2018)
Pedanekar, R., Shaikh, S., Rajpure, K.: Thin film photocatalysis for environmental remediation: a status review. Curr. Appl. Phys. 20(8), 931–952 (2020)
Peksu, E., Karaagac, H.: Characterization of Cu2ZnSnS4 thin films deposited by one-step thermal evaporation for a third generation solar cell. J. Alloy. Compd. 862, 1–9 (2021)
Pérez-González, M., Tomás, S.: Surface chemistry of TiO2-ZnO thin films doped with Ag. Its role on the photocatalytic degradation of methylene blue. Catal. Today 360, 129–137 (2021)
Phaltane, S.A., et al.: Photocatalytic degradation of methylene blue by hydrothermally synthesized CZTS nanoparticles. J. Mater. Sci. Mater. Electron. 28(11), 8186–8191 (2017)
Rajan, G., et al.: Characterization and analysis of ultrathin CIGS films and solar cells deposited by 3-Stage process. J. Spectrosc. 2018, 1–9 (2018)
Rathi, B.S., Kumar, P.S., Vo, D.-V.N.: Critical review on hazardous pollutants in water environment: occurrence, monitoring, fate, removal technologies and risk assessment. Sci. Total Environ. 797, 1–22 (2021)
Saravanan, R., Gracia, F., Stephen, A.: Basic principles, mechanism, and challenges of photocatalysis. In: Nanocomposites for Visible Light-Induced Photocatalysis, pp. 19–40. Springer, Berlin (2017)
Sarswat, P.K., Free, M.L.: A study of energy band gap versus temperature for Cu2ZnSnS4 thin films. Physica B 407(1), 108–111 (2012)
Singha, M.K., Patra, A.: Highly efficient and Reusable ZnO microflower photocatalyst on stainless steel mesh under UV–Vis and natural sunlight. Opt. Mater. 107, 1–9 (2020)
Tahir, M., Ali, S., Rizwan, M.: A review on remediation of harmful dyes through visible light-driven WO3 photocatalytic nanomaterials. Int. J. Environ. Sci. Technol. 16(8), 4975–4988 (2019)
Tamjidi, S., et al.: Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: a review study. Process Saf. Environ. Prot. 148, 775–795 (2021)
Tao, J., et al.: A sputtered CdS buffer layer for co-electrodeposited Cu 2 ZnSnS 4 solar cells with 6.6% efficiency. Chem. Commun. 51(51), 10337–10340 (2015)
Vakulov, Z., et al.: Size effects in LiNbO3 thin films fabricated by pulsed laser deposition. J. Phys. Conf. Series 1124, 022032 (2018)
Vaya, D., Surolia, P.K.: Semiconductor based photocatalytic degradation of pesticides: an overview. Environ. Technol. Innov. 20, 1–25 (2020)
Wadhene, R., et al.: Electrodeposition of Cu2ZnSnS4 thin films onto TiO2 nanorods for photocatalytic application: effect of deposition time. Inorg. Chem. Commun. 122, 1–11 (2020)
Wang, X., et al.: Ag clusters anchored conducting polyaniline as highly efficient cocatalyst for Cu2ZnSnS4 nanocrystals toward enhanced photocatalytic hydrogen generation. ACS Sustain. Chem. Eng. 6(9), 11424–11432 (2018)
Wang, X.-Q., et al.: Photocatalytic oxidation degradation mechanism study of methylene blue dye waste water with GR/iTO2. In MATEC Web of Conferences. 2018. EDP Sciences
Wu, C.-Y., et al.: Thickness-dependent photocatalytic performance of nanocrystalline TiO2 thin films prepared by sol–gel spin coating. Appl. Surf. Sci. 280, 737–744 (2013)
Wu, J., et al.: Catalytic ozonation of organic pollutants from bio-treated dyeing and finishing wastewater using recycled waste iron shavings as a catalyst: removal and pathways. Water Res. 92, 140–148 (2016)
Xia, D., et al.: Synthesis and characterization of Cu2ZnSnS4 nanocrystals by hot-injection method. J. Mater. Sci. Mater. Electron. 26(7), 5426–5432 (2015)
Xiao-Wei, M., et al.: Dramatically enhanced visible-light-responsive H-2 evolution of Cd1-xZnxS via the synergistic effect of Ni2P and 1T/2H MoS2 cocatalysts. Chin. J. Struct. Chem. 40(1), 7–22 (2021)
Yang, J., et al.: Enhanced catalytic activation of photo-Fenton process by Cu0· 5Mn0· 5Fe2O4 for effective removal of organic contaminants. Chemosphere 247, 1–9 (2020)
Yao, L., et al.: Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater. Chemosphere 273, 1–19 (2021)
Zhang, K., et al.: An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation. Energy Environ. Sci. 9(2), 499–503 (2016)
Zhu, D., Zhou, Q.: Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: a review. Environ. Nanotechnol. Monit. Manag. 12, 1–11 (2019)
Acknowledgements
Author kindly would like to thank, Selcuk University Scientific Research Project (BAP) Coordination for the support with the number 18401124 and project, Selçuk University, High Technology Research and Application Center (İL-TEK) and SULTAN Center for infrastructures.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
S.D.: Formal analysis, Software, Visualization, Writing—Original Draft, Writing—Review & Editing, Resources, Methodology; F.B.S.: Conceptualization, Visualization, Writing—Original Draft, Writing—Review& Editing, Resources, Methodology; S.K.: Methodology, Writing—Original Draft, Writing—Review& Editing, Resources; S.Y.G.: Conceptualization, Visualization, Writing—Original Draft, Writing—Review& Editing, Resources, Methodology, Formal analysis, Software; Y.G.: Visualization, Writing—Original Draft, Writing—Review & Editing, Resources, Methodology, Formal analysis, Software; H.Ş.K.: Project administration, Supervision, Visualization. Writing—Original Draft, Writing—Review & Editing, Resources, Conceptualization, Methodology.
Corresponding author
Ethics declarations
Confict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
We declare that the manuscript entitled “Investigation of photocatalytic activity (under visible light) of ultrathin CZTS films produced in different thicknesses by PLD method” is original, has not been fully or partly published before, and is not currently being considered for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dursun, S., Sarıipek, F.B., Kılıç, S. et al. Investigation of photocatalytic activity (under visible light) of ultrathin CZTS films produced in different thicknesses by PLD method. Opt Quant Electron 55, 166 (2023). https://doi.org/10.1007/s11082-022-04417-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-022-04417-w